Spasmolytic, Antimicrobial, and Antioxidant Activities of Spray-Dried Extracts of Gentiana asclepiadea L. with In Silico Pharmacokinetic Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
2.2. Spasmolytic Activity
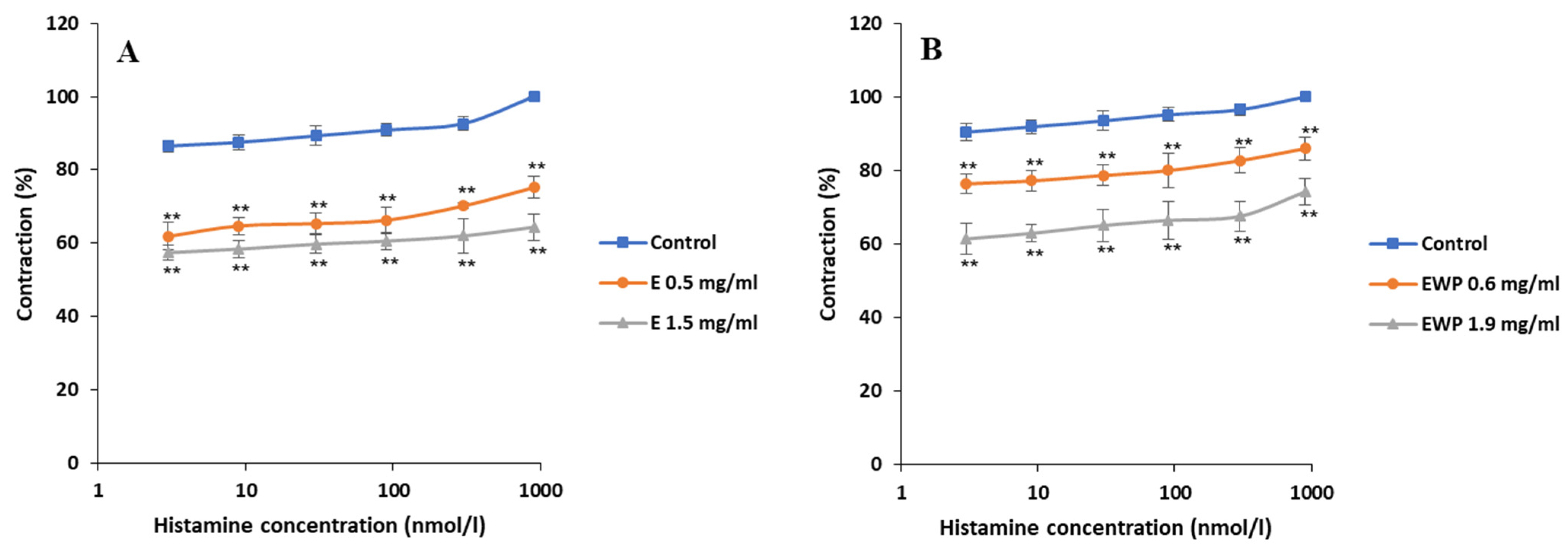
2.2.1. Spasmolytic Activity on Spontaneous Contractions

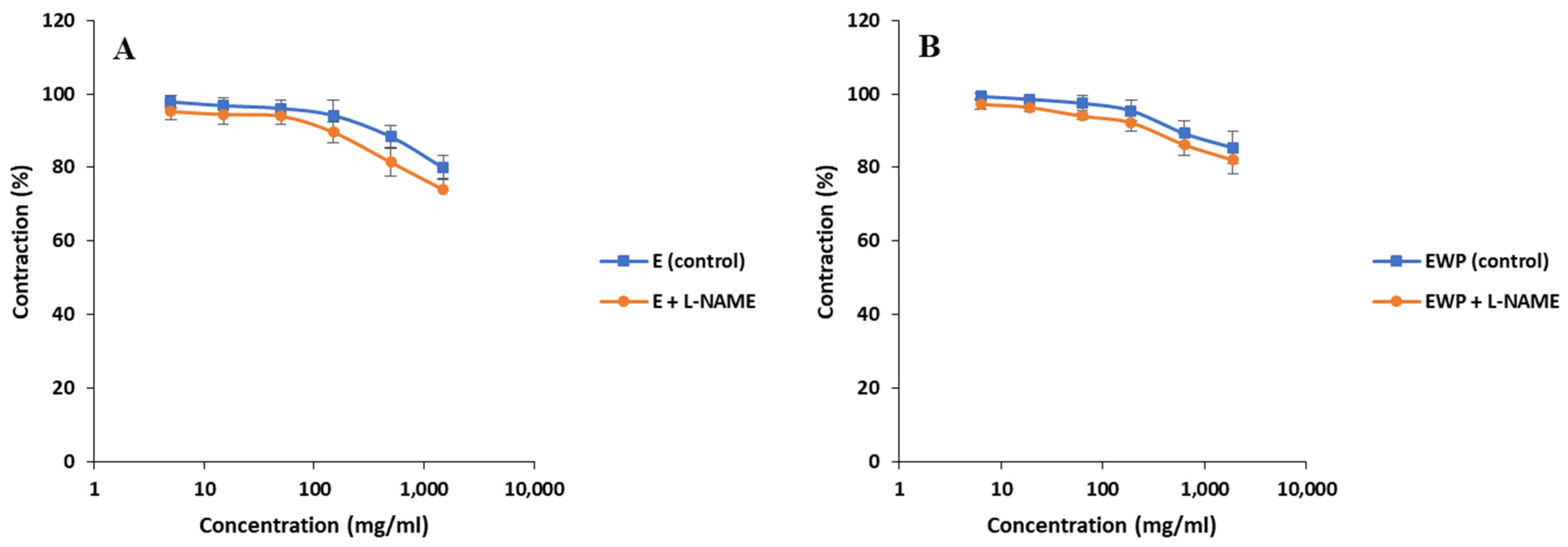
2.2.2. Mechanism of Action Involved in the Spasmolytic Effect
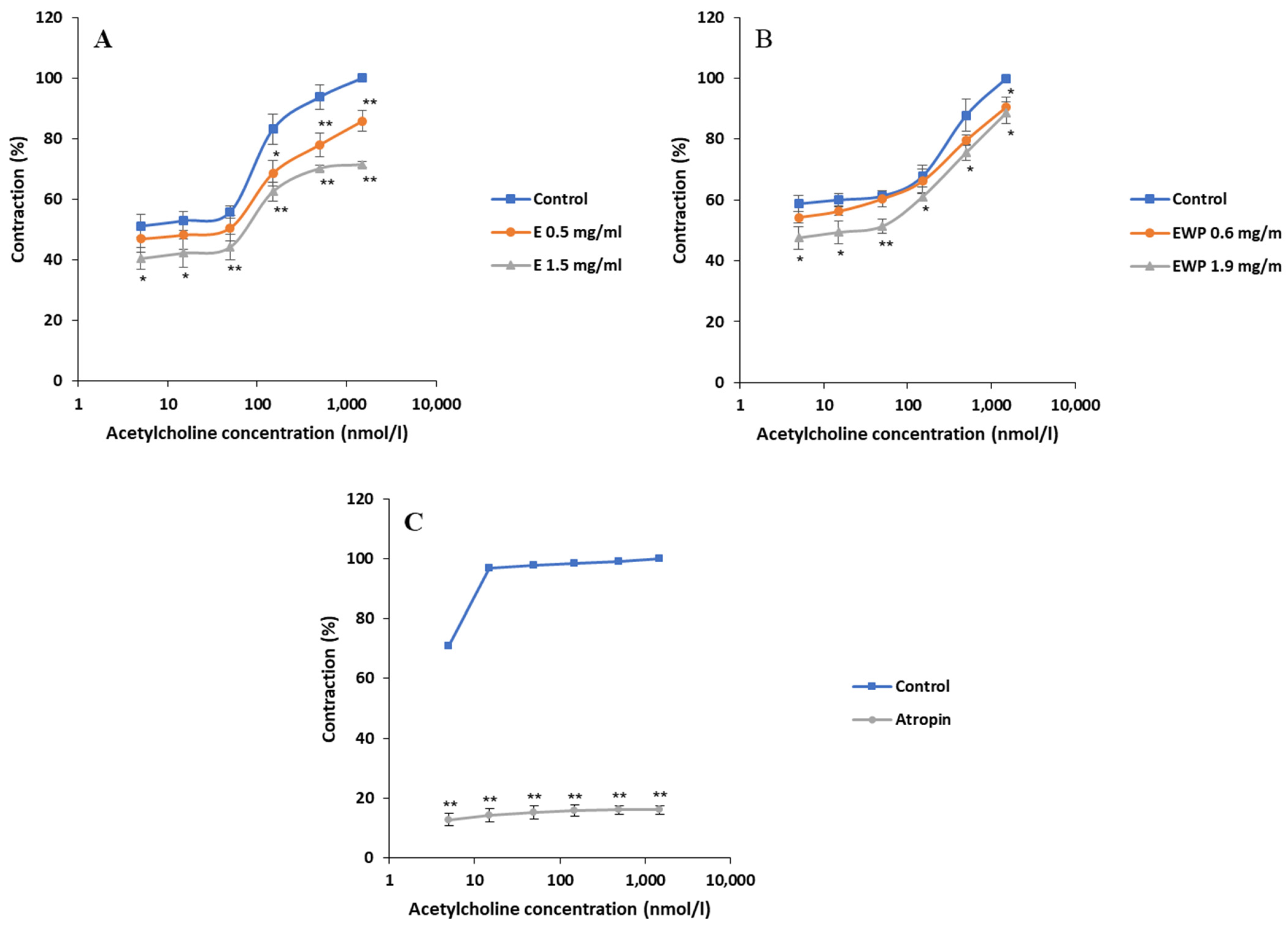
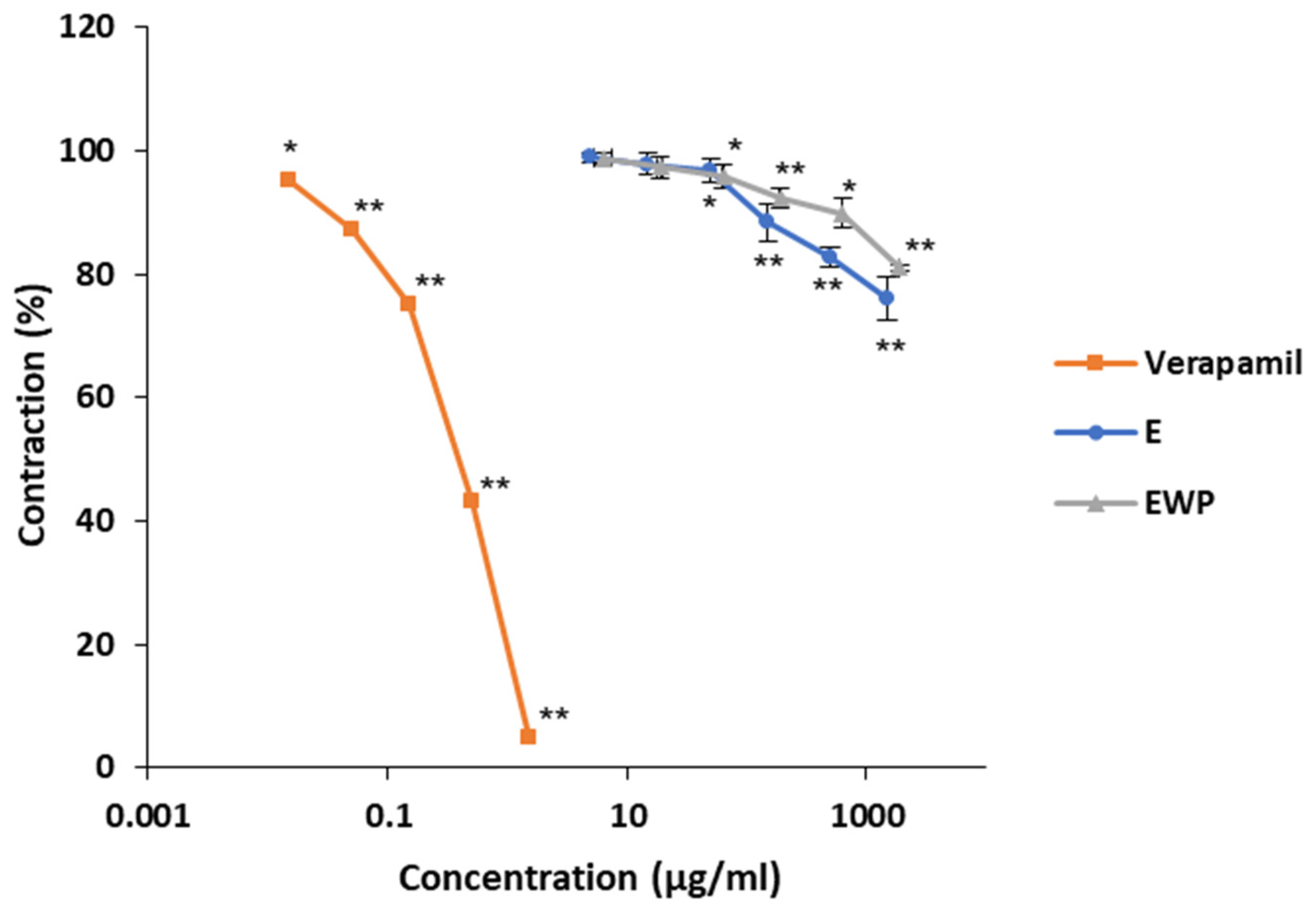
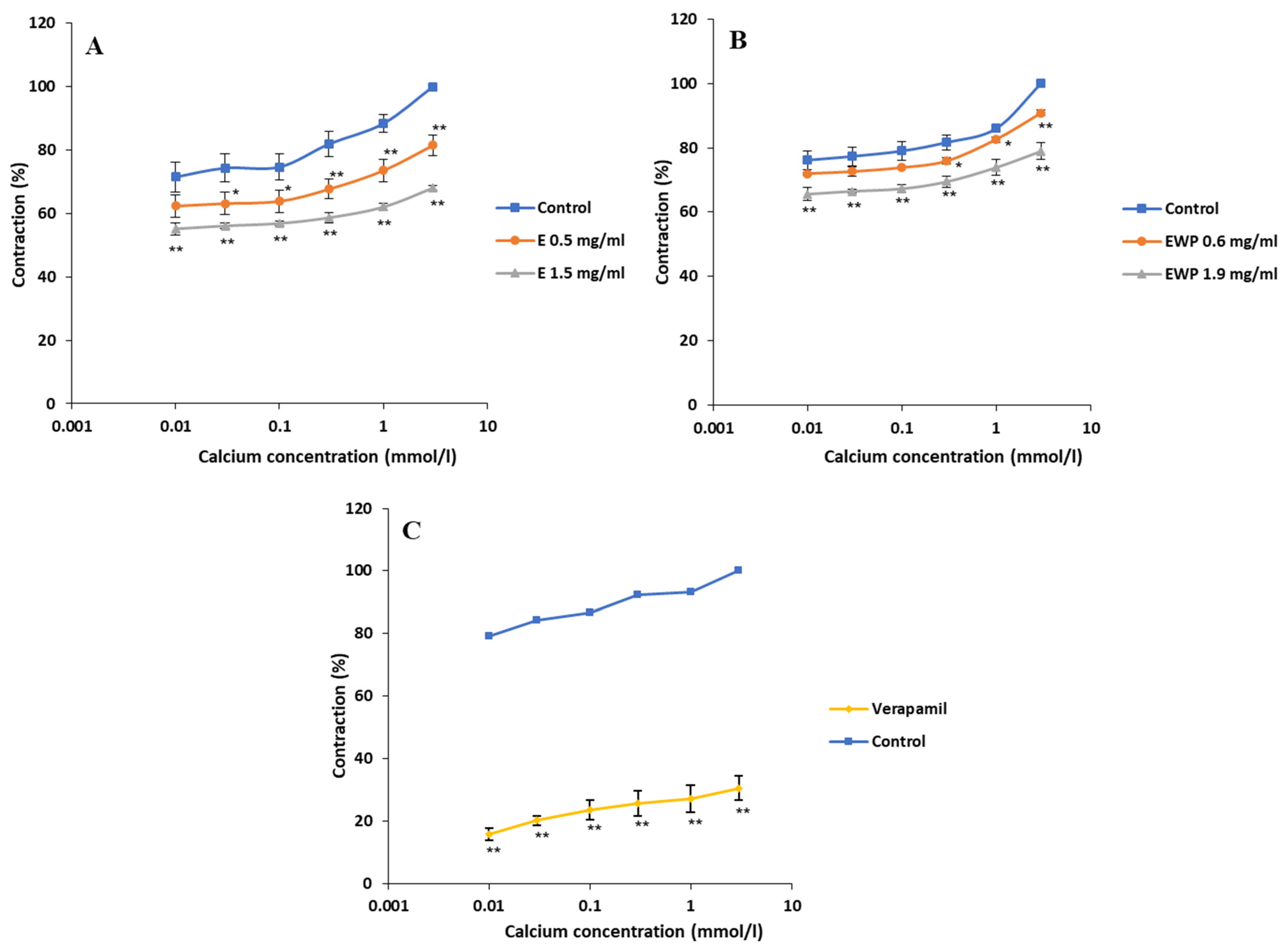
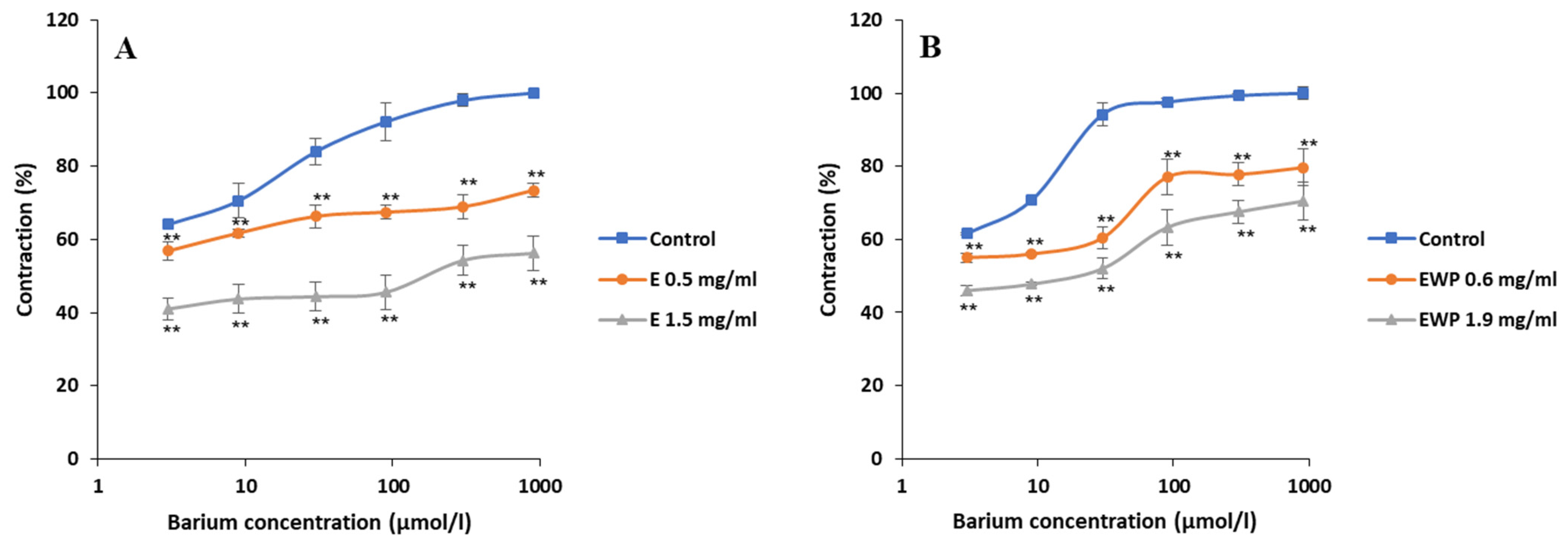

2.3. Antimicrobial Activity
2.4. Antioxidant Activity
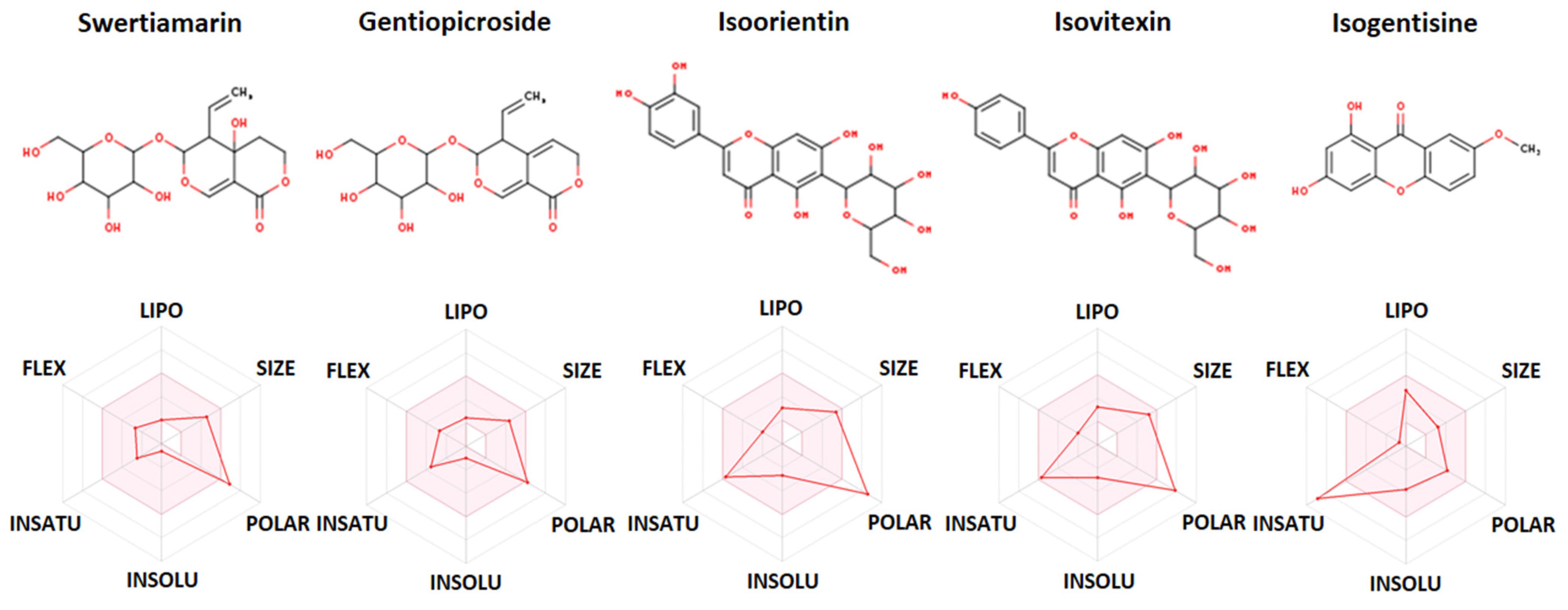
2.5. In Silico ADME and Drug-Likeness Study
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. Extract Encapsulation
3.3. Phytochemical Characterization
3.3.1. HPLC Analysis
3.3.2. Total Polyphenol Content
3.4. Spasmolytic Activity Assay
3.4.1. Experimental Animals and Housing
3.4.2. Preparation of Rat Ileum
3.4.3. Experimental Design
3.5. Antioxidant Activity Assay
3.5.1. DPPH Radical Scavenging Assay
3.5.2. Linoleic Acid/β-Carotene Bleaching Assay
3.6. Antimicrobial Activity Assay
3.7. In Silico ADME Profiling
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McMullen, M.K.; Whitehouse, J.M.; Whitton, P.A.; Towell, A. Bitter tastants alter gastric-phase postprandial haemodynamics. J. Ethnopharmacol. 2014, 154, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Cui, B.-W.; Wu, Y.-L.; Nan, J.-X.; Lian, L.-H. Genus Gentiana: A review on phytochemistry, pharmacology and molecular mechanism. J. Ethnopharmacol. 2021, 264, 113391. [Google Scholar] [CrossRef]
- Mihailović, V.; Mihailović, M.; Uskoković, A.; Arambašić, J.; Mišić, D.; Stanković, V.; Katanić, J.; Mladenović, M.; Solujić, S.; Matić, S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2013, 52, 83–90. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Menković, N.; Šavikin, K.; Tasić, S.; Zdunić, G.; Stešević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Matejić, J.S.; Stefanović, N.; Ivković, M.; Živanović, N.; Marin, P.D.; Džamić, A.M. Traditional uses of autochthonous medicinal and ritual plants and other remedies for health in Eastern and South-Eastern Serbia. J. Ethnopharmacol. 2020, 261, 113186. [Google Scholar] [CrossRef]
- Popović, Z.; Krstić-Milošević, D.; Marković, M.; Vidaković, V.; Bojović, S. Gentiana asclepiadea L. from Two High Mountainous Habitats: Inter- and Intrapopulation Variability Based on Species’ Phytochemistry. Plants 2021, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Tankhaeva, L.M. Iridoids and Flavonoids of Four Siberian Gentians: Chemical Profile and Gastric Stimulatory Effect. Molecules 2015, 20, 19172–19188. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Kataeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana Species: Untargeted LC-MS Metabolic Profiling, Antioxidant and Digestive Enzyme Inhibiting Activity of Six Plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef]
- Hudecová, A.; Kusznierewicz, B.; Hašplová, K.; Huk, A.; Magdolenová, Z.; Miadoková, E.; Gálová, E.; Dušinská, M. Gentiana asclepiadea exerts antioxidant activity and enhances DNA repair of hydrogen peroxide- and silver nanoparticles-induced DNA damage. Food Chem. Toxicol. 2012, 50, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, O.; Ličina, B.; Vasić, S.; Radojević, I.; Čomić, L. Bioactive extracts of Gentiana asclepiadea: Antioxidant, antimicrobial, and antibiofilm activity. Bot. Serb. 2018, 42, 223–229. [Google Scholar] [CrossRef]
- Buza, V.; Cătană, L.; Andrei, S.M.; Ștefănuț, L.C.; Răileanu, Ș.; Matei, M.C.; Vlasiuc, I.; Cernea, M. In vitro anthelmintic activity assessment of six medicinal plant aqueous extracts against donkey strongyles. J. Helminthol. 2020, 94, e147. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef] [PubMed]
- Adsersen, A.; Adsersen, H. Plants from Reunion Island with alleged antihypertensive and diuretic effects—An experimental and ethnobotanical evaluation. J. Ethnopharmacol. 1997, 58, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Mudrić, J.; Drinić, Z.; Matejić, J.; Kitić, D.; Bigović, D.; Šavikin, K. Optimization of ultrasound-assisted extraction of bitter compounds and polyphenols from willow gentian underground parts. Sep. Purif. Technol. 2022, 281, 119868. [Google Scholar] [CrossRef]
- Aberham, A.; Pieri, V.; Croom, E.M.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC–MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Jovanović, M.; Ćujić-Nikolić, N.; Drinić, Z.; Janković, T.; Marković, S.; Petrović, P.; Šavikin, K. Spray drying of Gentiana asclepiadea L. root extract: Successful encapsulation into powders with preserved stability of bioactive compounds. Ind. Crops Prod. 2021, 172, 114044. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Whey proteins and peptides in health-promoting functions—A review. Int. Dairy J. 2022, 126, 105269. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Karaca, A.C.; Deng, L.; Wang, Y.; Li, H.; Askari, G.; Rostamabadi, H. Insights into whey protein-based carriers for targeted delivery and controlled release of bioactive components. Food Hydrocoll. 2022, 133, 108002. [Google Scholar] [CrossRef]
- Baba, W.N.; McClements, D.J.; Maqsood, S. Whey protein–polyphenol conjugates and complexes: Production, characterization, and applications. Food Chem. 2021, 365, 130455. [Google Scholar] [CrossRef]
- de Morais, F.P.R.; Pessato, T.B.; Rodrigues, E.; Peixoto Mallmann, L.; Mariutti, L.R.B.; Netto, F.M. Whey protein and phenolic compound complexation: Effects on antioxidant capacity before and after in vitro digestion. Food Res. Int. 2020, 133, 109104. [Google Scholar] [CrossRef] [PubMed]
- Randjelović, M.; Branković, S.; Miladinović, B.; Milutinović, M.; Živanović, S.; Mihajilov-Krstev, T.; Kitić, D. The benefits of Salvia sclarea L. ethanolic extracts on gastrointestinal and respiratory spasms. S. Afr. J. Bot. 2022, 150, 621–632. [Google Scholar] [CrossRef]
- Godfrain, T.; Miller, R.; Wibo, M. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986, 38, 312–416. [Google Scholar]
- Mainente, F.; Piovan, A.; Zanoni, F.; Chignola, R.; Cerantola, S.; Faggin, S.; Giron, M.C.; Filippini, R.; Seraglia, R.; Zoccatelli, G. Spray-drying microencapsulation of an extract from Tilia tomentosa Moench flowers: Physicochemical characterization and in vitro intestinal activity. Plant Foods Hum. Nutr. 2022, 77, 467–473. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Rojas, A.; Bah, M.; Rojas, J.I.; Gutiérrez, D.M. Smooth muscle relaxing activity of gentiopicroside isolated from Gentiana spathacea. Planta Med. 2000, 66, 765–767. [Google Scholar] [CrossRef]
- Khan, A.; Mustafa, M.R.; Khan, A.U.; Murugan, D.D. Hypotensive effect of Gentiana floribunda is mediated through Ca++ antagonism pathway. BMC Complement. Altern. Med. 2012, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Szerb, J.C. Storage and release of labelled acetylcholine in the myenteric plexus of the guinea-pig ileum. Can. J. Physiol. Pharm. 1976, 54, 12–22. [Google Scholar] [CrossRef]
- Ruan, M.; Yu, B.; Xu, L.; Zhang, L.; Long, J.; Shen, X. Attenuation of stress-induced gastrointestinal motility disorder by gentiopicroside, from Gentiana macrophylla Pall. Fitoterapia 2015, 103, 265–276. [Google Scholar] [CrossRef]
- Smolinska, S.; Winiarska, E.; Globinska, A.; Jutel, M. Histamine: A Mediator of Intestinal Disorders-A Review. Metabolites 2022, 12, 895. [Google Scholar] [CrossRef]
- Wölfle, U.; Haarhaus, B.; Schempp, C.M. Amarogentin displays immunomodulatory effects in human mast cells and keratinocytes. Mediat. Inflamm. 2015, 2015, 630128. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.G.; Arif-ullah, K.; Qaiser, J.; Fazal, S.; Rukhsana, G. Antispasmodic and blood pressure lowering effects of Valeriana wallichii are mediated through K+ channel activation. J. Ethnopharmacol. 2005, 100, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kitić, N.; Živković, J.; Šavikin, K.; Randjelović, M.; Jovanović, M.; Kitić, D.; Miladinović, B.; Milutinović, M.; Stojiljković, N.; Branković, S. Spasmolytic Activity of Gentiana lutea L. Root Extracts on the Rat Ileum: Underlying Mechanisms of Action. Plants 2024, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Buza, V.; Niculae, M.; Hanganu, D.; Pall, E.; Burtescu, R.F.; Olah, N.K.; Matei-Lațiu, M.C.; Vlasiuc, I.; Iozon, I.; Szakacs, A.R.; et al. Biological Activities and Chemical Profile of Gentiana asclepiadea and Inula helenium Ethanolic Extracts. Molecules 2022, 27, 3560. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, T.; Hadizadeh, N.; Nikfar, S.; Abdollahi, M. Drug discovery strategies for modulating oxidative stress in gastrointestinal disorders. Expert Opin. Drug Dis. 2020, 15, 1309–1341. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, V.; Matic, S.; Misic, D.; Stankovic, N.; Stanic, S.; Katanić, J.; Mladenovic, M.; Solujic, S. Chemical composition, antioxidant and antigenotoxic activities of different fractions of Gentiana asclepiadea L. roots extract. EXCLI J. 2013, 12, 807–823. [Google Scholar] [PubMed]
- Olennikov, D.; Kashchenko, N.; Chirikova, N.; Koryakina, L.; Vladimirov, L. Bitter Gentian Teas: Nutritional and Phytochemical Profiles, Polysaccharide Characterisation and Bioactivity. Molecules 2015, 20, 20014–20030. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, V.; Misic, D.; Matić, S.; Mihailović, M.; Stanić, S.; Vrvić, M.M.; Katanić, J.S.; Jurić, T.; Srećković, N.; Boroja, T.; et al. Comparative phytochemical analysis of Gentiana cruciata L. roots and aerial parts, and their biological activities. Ind. Crops Prod. 2015, 73, 49–62. [Google Scholar] [CrossRef]
- Mihailović, V.; Katanić Stanković, J.S.; Jurić, T.; Srećković, N.; Mišić, D.; Šiler, B.; Monti, D.M.; Imbimbo, P.; Nikles, S.; Pan, S.; et al. Blackstonia perfoliata (L.) Huds. (Gentianaceae): A promising source of useful bioactive compounds. Ind. Crops Prod. 2019, 145, 111974. [Google Scholar] [CrossRef]
- Adrar, N.S.; Madani, K.; Adrar, S. Impact of the inhibition of proteins activities and the chemical aspect of polyphenols-proteins interactions. PharmaNutrition 2019, 7, 100142. [Google Scholar] [CrossRef]
- Zarev, Y.; Naessens, T.; Theunis, M.; Elgorashi, E.; Apers, S.; Ionkova, I.; Verschaeve, L.; Pieters, L.; Hermans, N.; Foubert, K. In vitro antigenotoxic activity, in silico ADME prediction and protective effects against aflatoxin B1 induced hepatotoxicity in rats of an Erythrina latissima stem bark extract. Food Chem. Toxicol. 2020, 135, 110768. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O.; Mastorakis, N.E. The taste of neuroinflammation: Molecular mechanisms linking taste sensing to neuroinflammatory responses. Pharmacol. Res. 2021, 167, 105557. [Google Scholar] [CrossRef]
- Cruz, J.N.; Oliveira, M.S.D.; Cascaes, M.; Mali, S.N.; Tambe, S.; Santos, C.B.R.D.; Zoghbi, M.D.G.; Andrade, E.H.D.A. Variation in the Chemical Composition of Endemic Specimens of Hedychium coronarium J. Koenig from the Amazon and In Silico Investigation of the ADME/Tox Properties of the Major Compounds. Plants 2023, 12, 2626. [Google Scholar] [CrossRef] [PubMed]
- Ojuka, P.; Kimani, N.M.; Apollo, S.; Nyariki, J.; Ramos, R.S.; Santos, C.B. Phytochemistry of the Vepris genus plants: A review and in silico analysis of their ADMET properties. S. Afr. J. Bot. 2023, 157, 106–114. [Google Scholar] [CrossRef]
- Sánchez-Moya, T.; López-Nicolás, R.; Planes, D.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Frontela-Saseta, C. In vitro modulation of gut microbiota by whey protein to preserve intestinal health. Food Funct. 2017, 8, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, S.; Arai, K.; Kumada, N.; Ishida, Y.; Tanaka, I.; Iwatsuki, S.; Ohwada, T.; Ohnishi, M.; Tokuji, Y.; Kinoshita, M. The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice. Foods 2014, 3, 351–368. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Extraction and chemical quantification. In Analysis of Phenolic Plant Metabolites. Methods in Ecology; Waterman, P.G., Mole, S., Eds.; Blackwell Publishing: Oxford, UK, 1994; pp. 66–103. [Google Scholar]
- Estrada-Soto, S.; Sánchez-Recillas, A.; Navarrete-Vázquez, G.; Castillo-España, P.; Villalobos-Molina, R.; Ibarra-Barajas, M. Relaxant effects of Artemisia ludoviciana on isolated rat smooth muscle tissues. J. Ethnopharmacol. 2012, 139, 513–518. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard; M07-A9; CLSI: Wayne, PA, USA, 2012; Volume 29, pp. 1–63. [Google Scholar]
| Class of Phytocompounds | Phytocompound | Extract | |
|---|---|---|---|
| Without Carrier | Encapsulated with Whey Protein | ||
| Secoiridoids | Swertiamarin | 7.84 ± 0.21 | 7.01 ± 0.19 |
| Gentiopicroside | 145.27 ± 7.33 | 114.59 ± 6.89 | |
| Sweroside | traces | traces | |
| Flavones | Isoorientin | 4.12 ± 0.25 | 4.32 ± 0.24 |
| Isovitexin | 12.24 ± 0.84 | 10.53 ± 0.78 | |
| Xanthones | Isogentisine | 1.43 ± 0.11 | 1.15 ± 0.09 |
| Mangiferin | non detect | non detect | |
| Total polyphenols content * | 34.56 ± 0.26 | 30.27 ± 0.28 | |
| Pathogen | ATCC | E | EWP | WP | Positive Control |
|---|---|---|---|---|---|
| MIC/MMC (mg/mL) | MIC/MMC (µg/mL) | ||||
| Gram (+) bacterial strains | |||||
| Staphylococcus aureus | 6538 | 12.5/25.0 | 25.0/50.0 | >200.0 | 7.81/15.61 |
| Bacillus cereus | 11778 | 12.5/25.0 | 25.0/50.0 | >200.0 | 0.90/15.61 |
| Enterococcus faecalis | 19433 | 3.13/3.13 | 3.13/3.13 | >200.0 | 0.90/1.90 |
| Gram (−) bacterial strains | |||||
| Salmonella enteritidis | 13076 | 12.5/50.0 | 12.5/25.0 | >200.0 | 0.90/1.90 |
| Escherichia coli | 25922 | 12.5/25.0 | 6.25/12.5 | >200.0 | 15.61/15.61 |
| Enterobacter aerogenes | 13048 | 25.0/50.0 | 50.0/>200.0 | >200.0 | 7.81/15.61 |
| Pseudomonas aeruginosa | 9027 | 3.13/12.5 | 6.25/25.0 | >200.0 | 15.61/15.61 |
| Yeast strain | |||||
| Candida albicans | 24433 | 12.5/>200.0 | 50.0/>200.0 | >200.0 | 3.91/7.81 |
| IC50 (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| E | EWP | WP | BHA | BHT | α-Tocopherol | Ascorbic Acid | |
| DPPH assay | 2569.58 ± 111.15 b | 4270.15 ± 198.37 a | / | 2.44 ± 0.09 c | 22.82 ± 2.07 c | 10.40 ± 1.73 c | 4.74 ± 0.34 c |
| β-carotene bleaching assay | 29.5 ± 1.59 a | 31.41 ± 1.73 a | / | 0.04 ± 0.01 c | 0.03 ± 0.00 c | 0.15 ± 0.00 c | 22.95 ± 1.52 b |
| Constituent (PubChem CID) | Swertiamarin (442435) | Gentiopicroside (88708) | Isoorientin (114776) | Isovitexin (162350) | Isogentisine (5281640) |
|---|---|---|---|---|---|
| Physicochemical Properties | |||||
| Molecular weight | 374.34 g/mol | 356.32 g/mol | 448.38 g/mol | 432.38 g/mol | 258.23 g/mol |
| Num. rotatable bonds | 4 | 4 | 3 | 3 | 1 |
| Num. H-bond acceptors | 10 | 9 | 11 | 10 | 5 |
| Num. H-bond donors | 5 | 4 | 8 | 7 | 2 |
| TPSA | 155.14 Å2 | 134.91 Å2 | 201.28 Å2 | 181.05 Å2 | 79.90 Å2 |
| Log Po/w (WLOGP) | −2.48 | −1.67 | −0.53 | −0.23 | 2.37 |
| Consensus Log Po/w | −1.35 | −0.80 | −0.24 | 0.05 | 2.09 |
| Pharmacokinetics | |||||
| GI absorption | Low | Low | Low | Low | High |
| BBB permeant | No | No | No | No | No |
| P-gp substrate | No | Yes | No | No | No |
| CYP1A2 inhibitor | No | No | No | No | Yes |
| CYP2C19 inhibitor | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | Yes |
| CYP3A4 inhibitor | No | No | No | No | Yes |
| Log Kp (skin permeation) | −10.00 cm/s | −9.35 cm/s | −9.14 cm/s | −8.79 cm/s | −5.91 cm/s |
| Drug-likeness | |||||
| Lipinski | Yes; 0 violation | Yes; 0 violation | No; 2 violations: NorO > 10, NHorOH > 5 | Yes; 1 violation: NHorOH > 5 | Yes; 0 violation |
| Ghose | No; 1 violation: WLOGP < −0.4 | No; 1 violation: WLOGP < −0.4 | No; 1 violation: WLOGP < −0.4 | Yes | Yes |
| Veber | No; 1 violation: TPSA > 140 | Yes | No; 1 violation: TPSA > 140 | No; 1 violation: TPSA > 140 | Yes |
| Egan | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 | Yes |
| Muegge | No; 1 violation: TPSA > 150 | Yes | No; 3 violations: TPSA > 150, H-acc > 10, H-don > 5 | No; 2 violations: TPSA > 150, H-don > 5 | Yes |
| Bioavailability Score | 0.11 | 0.56 | 0.17 | 0.55 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, M.S.; Milutinović, M.; Branković, S.; Mihajilov-Krstev, T.; Randjelović, M.; Miladinović, B.; Ćujić Nikolić, N.; Šavikin, K.; Kitić, D. Spasmolytic, Antimicrobial, and Antioxidant Activities of Spray-Dried Extracts of Gentiana asclepiadea L. with In Silico Pharmacokinetic Analysis. Plants 2024, 13, 1445. https://doi.org/10.3390/plants13111445
Jovanović MS, Milutinović M, Branković S, Mihajilov-Krstev T, Randjelović M, Miladinović B, Ćujić Nikolić N, Šavikin K, Kitić D. Spasmolytic, Antimicrobial, and Antioxidant Activities of Spray-Dried Extracts of Gentiana asclepiadea L. with In Silico Pharmacokinetic Analysis. Plants. 2024; 13(11):1445. https://doi.org/10.3390/plants13111445
Chicago/Turabian StyleJovanović, Miloš S., Milica Milutinović, Suzana Branković, Tatjana Mihajilov-Krstev, Milica Randjelović, Bojana Miladinović, Nada Ćujić Nikolić, Katarina Šavikin, and Dušanka Kitić. 2024. "Spasmolytic, Antimicrobial, and Antioxidant Activities of Spray-Dried Extracts of Gentiana asclepiadea L. with In Silico Pharmacokinetic Analysis" Plants 13, no. 11: 1445. https://doi.org/10.3390/plants13111445






