Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Carotenoids Extracted from Pumpkin Peel Using a Central Composite Design
Abstract
:1. Introduction
2. Results and Discussions
2.1. HPLC Analysis for Carotenoid Compounds
2.2. Fitting the Response Surface Models
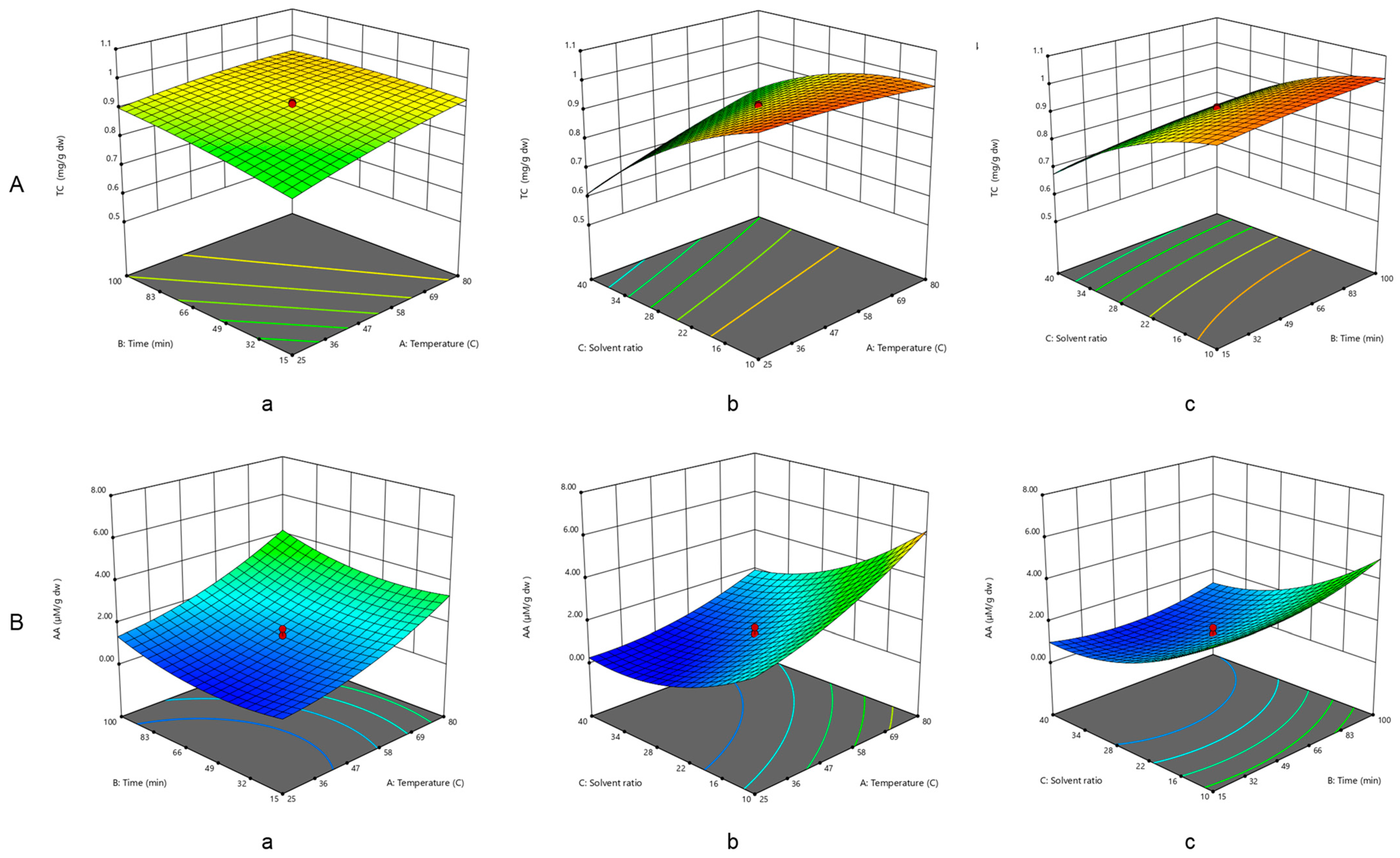
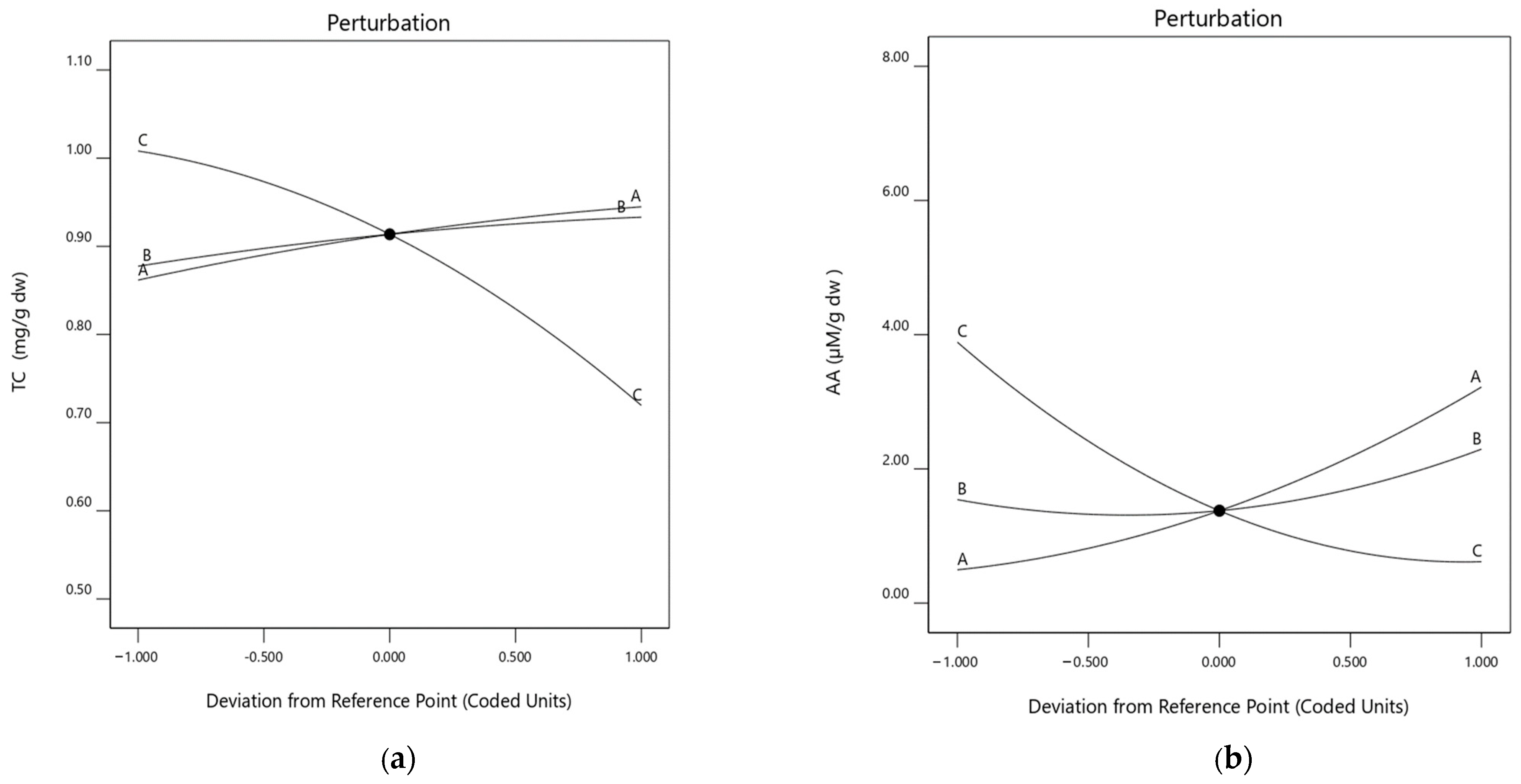
2.3. Influence of the Extraction Parameters on TCs
2.4. Influence of the Extraction Parameters on AA
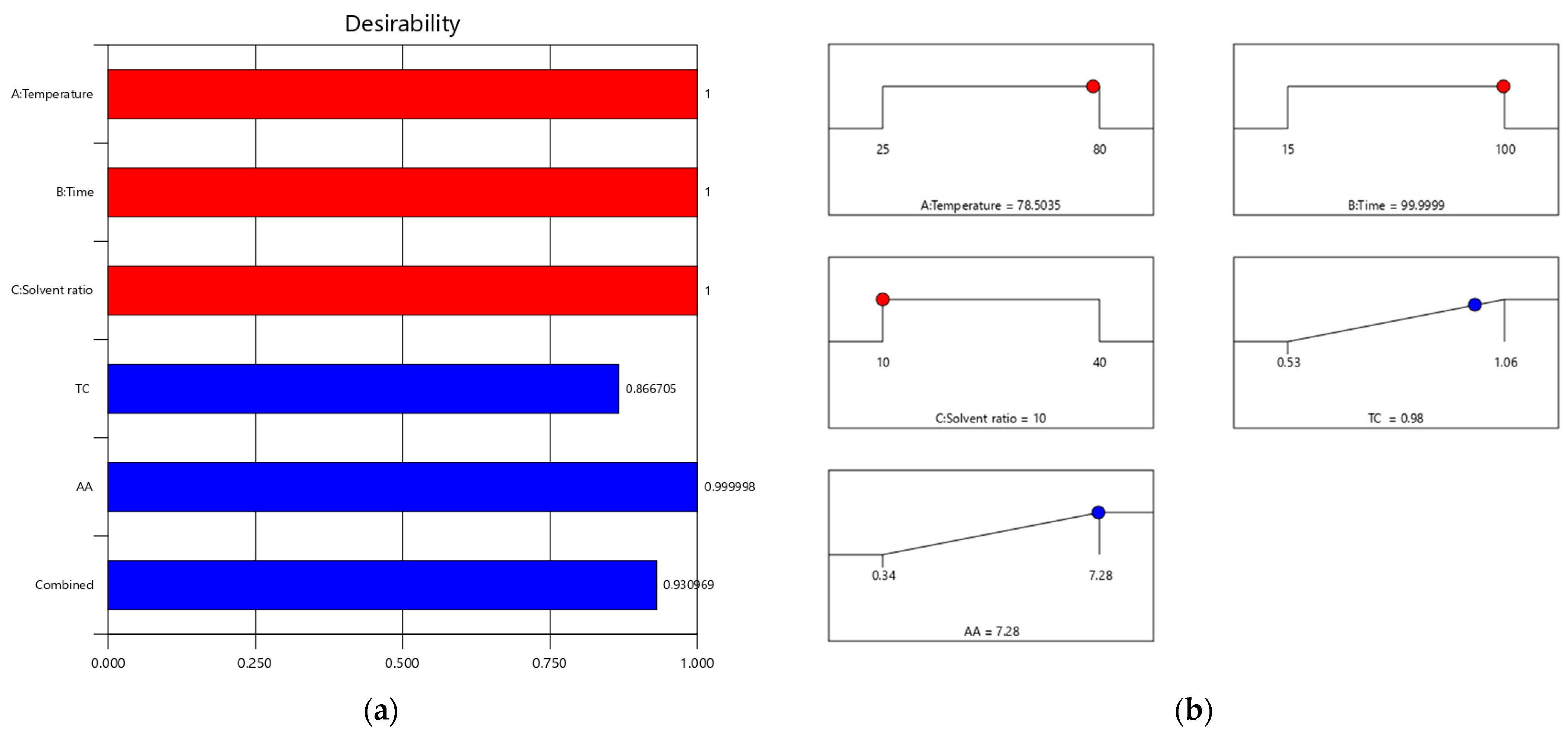
2.5. Optimization and Validation of the Extraction Parameters
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Pumpkin Peel Preparation
3.3. Ultrasound-Assisted Extraction
3.4. Determination of the TC Contents
3.5. Determination of the Antioxidant Activity (AA)
3.6. High-Performance Liquid Chromatography (HPLC) Analysis of the Pumpkin Peel Extract
3.7. Experimental Design
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural Food Additives: Quo Vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of Fruits and Vegetable Wastes and By-Products to Produce Natural Pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources—A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Antioxidant Potential of Phytochemicals in Pumpkin Varieties Belonging to Cucurbita Moschata and Cucurbita Pepo Species. CyTA J. Food 2020, 18, 472–484. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sogi, D.S. Optimization of enzyme aided pigment extraction from pumpkin (Cucurbita maxima Duch) using response surface methodology. J. Food Measurem. Charact. 2022, 16, 1184–1194. [Google Scholar] [CrossRef]
- Hosen, M.; Rafii, M.Y.; Mazlan, N.; Jusoh, M.; Oladosu, Y.; Chowdhury, M.F.N.; Muhammad, I.; Khan, M.M.H. Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges. Horticulturae 2021, 7, 352. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of High Value-Added Compounds from Pineapple, Melon, Watermelon and Pumpkin Processing by-Products: An Overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Arjona, L.P.; Ramírez-Mella, M. Pumpkin Waste as Livestock Feed: Impact on Nutrition and Animal Health and on Quality of Meat, Milk, and Egg. Animals 2019, 9, 769. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Aires, A.; Dias, C.; Almeida, J.A.; De Vasconcelos, M.C.B.M.; Santos, P.; Rosa, E.A. Evaluation of the Potential of Squash Pumpkin By-Products (Seeds and Shell) as Sources of Antioxidant and Bioactive Compounds. J. Food Sci. Technol. 2015, 52, 1008–1015. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Edible Seeds from Cucurbitaceae Family as Potential Functional Foods: Immense Promises, Few Concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae Family and Their Products: Positive Effect on Human Health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef]
- Pereira, A.M.; Krumreich, F.D.; Ramos, A.H.; Krolow, A.C.R.; Santos, R.B.; Gularte, M.A. Physicochemical Characterization, Carotenoid Content and Protein Digestibility of Pumpkin Access Flours for Food Application. Food Sci. Technol. 2020, 40, 691–698. [Google Scholar] [CrossRef]
- Karrar, E.; Sheth, S.; Navicha, W.B.; Wei, W.; Hassanin, H.; Abdalla, M.; Wang, X. A Potential New Source: Nutritional and Antioxidant Properties of Edible Oils from Cucurbit Seeds and Their Impact on Human Health. J. Food Biochem. 2019, 43, e12733. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Barin, J.S.; Binello, A.; Veselov, V.V.; Cravotto, G. Highly Efficient Pumpkin-Seed Extraction with the Simultaneous Recovery of Lipophilic and Hydrophilic Compounds. Food Bioprod. Process. 2019, 117, 224–230. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. The Profile of Carotenoids and Other Bioactive Molecules in Various Pumpkin Fruits (Cucurbita maxima Duchesne) Cultivars. Molecules 2019, 24, 3212. [Google Scholar] [CrossRef] [PubMed]
- Mala, K.S.; Kurian, A.E. Nutritional Composition and Antioxidant Activity of Pumpkin Wastes. Int. J. Pharm. Chem. Biol. Sci. 2016, 6, 336–344. [Google Scholar]
- Norfezah, M.N.; Hardacre, A.; Brennan, C.S. Comparison of Waste Pumpkin Material and Its Potential Use in Extruded Snack Foods. Food Sci. Technol. Int. 2011, 17, 367–373. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Marin, J.; Rivas, J.; Sanhueza, L.; Soto, Y.; Vera, N.; Puente-Díaz, L.; Lemus-Mondaca, R.; Marin, J.; Rivas, J.; et al. Pumpkin Seeds (Cucurbita maxima). A Review of Functional Attributes and by-Products. Rev. Chil. Nutr. 2019, 46, 783–791. [Google Scholar] [CrossRef]
- Lalnunthari, C.; Devi, L.M.; Amami, E.; Badwaik, L.S. Valorisation of Pumpkin Seeds and Peels into Biodegradable Packaging Films. Food Bioprod. Process. 2019, 118, 58–66. [Google Scholar] [CrossRef]
- Öztürk, T.; Turhan, S. Physicochemical Properties of Pumpkin (Cucurbita pepo L.) Seed Kernel Flour and Its Utilization in Beef Meatballs as a Fat Replacer and Functional Ingredient. J. Food Process. Preserv. 2020, 44, e14695. [Google Scholar] [CrossRef]
- Čakarević, J.; Šeregelj, V.; Tumbas Šaponjac, V.; Ćetković, G.; Čanadanović Brunet, J.; Popović, S.; Kostić, M.H.; Popović, L. Encapsulation of Beetroot Juice: A Study on the Application of Pumpkin Oil Cake Protein as New Carrier Agent. J. Microencapsul. 2020, 37, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.M.; Rubio, F.T.V.; Silva, M.P.; Pinho, L.S.; Kasemodel, M.G.C.; Favaro-Trindade, C.S.; Dacanal, G.C. Nutritional Value and Modelling of Carotenoids Extraction from Pumpkin (Cucurbita moschata) Peel Flour By-Product. Int. J. Food Eng. 2019, 15, 20180381. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Norshazila, S.C.N.; Koy, O.; Rashidi, L.H.; Ho, I.; Azrina, R.A.; Nurul Zaizuliana, Z.; Zarinah, Z. The Effect of Time, Temperature and Solid to Solvent Ratio on Pumpkin Carotenoids Extracted Using Food Grade Solvents. Sains Malays. 2017, 46, 231–237. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Montero de Espinosa, V.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and β-Carotene Extraction from Tomato Processing Waste Using Supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Corbu, A.R.; Rotaru, A.; Nour, V. Edible Vegetable Oils Enriched with Carotenoids Extracted from By-Products of Sea Buckthorn (Hippophae rhamnoides ssp. Sinensis): The Investigation of Some Characteristic Properties, Oxidative Stability and the Effect on Thermal Behaviour. J. Therm. Anal. Calorim. 2020, 142, 735–747. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Gungor, K.K.; Torun, M. Pumpkin Peel Valorization Using Green Extraction Technology to Obtain β-Carotene Fortified Mayonnaise. Waste Biomass Valorization 2022, 13, 4375–4388. [Google Scholar] [CrossRef]
- Mansour, B.R.; Falleh, H.; Hammami, M.; Barros, L.; Petropoulos, S.A.; Tarchoun, N.; Ksouri, R. The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita maxima Fruit By-Products. Processes 2023, 11, 1726. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S.; Dotto, G.L. Optimization of Green Extraction for the Recovery of Bioactive Compounds from Brazilian Olive Crops and Evaluation of Its Potential as a Natural Preservative. J. Environ. Chem. Eng. 2021, 9, 105130. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized Liquid Extraction of Bioactive Compounds from Blackberry (Rubus fruticosus L.) Residues: A Comparison with Conventional Methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms Involved in the Therapeutic Properties of Mesenchymal Stem Cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound assisted extraction of lycopene from tomato processing wastes. JFST 2014, 51, 4102–4107. [Google Scholar] [CrossRef]
- Provesi, J.G.; Dias, C.O.; Amante, E.R. Changes in Carotenoids during Processing and Storage of Pumpkin Puree. Food Chem. 2011, 128, 195–202. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Rimac Brnčić, S.; Badanjak Sabolović, M.; Šic Žlabur, J.; Marović, R.; Brnčić, M. Carotenoid Content and Profiles of Pumpkin Products and By-Products. Molecules 2023, 28, 858. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments, 2nd ed.; Productivity Press: New York, NY, USA, 2016. [Google Scholar]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Optimisation of Extraction Conditions for Recovering Carotenoids and Antioxidant Capacity from Gac Peel Using Response Surface Methodology. Int. J. Food Sci. Technol. 2017, 52, 972–980. [Google Scholar] [CrossRef]
- Rahimi, S.; Mikani, M. Lycopene Green Ultrasound-Assisted Extraction Using Edible Oil Accompany with Response Surface Methodology (RSM) Optimization Performance: Application in Tomato Processing Wastes. Microchem. J. 2019, 146, 1033–1042. [Google Scholar] [CrossRef]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication Enhances Polyphenolic Compounds, Sugars, Carotenoids and Mineral Elements of Apple Juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Tsiaka, T.; Zoumpoulakis, P.; Sinanoglou, V.J.; Makris, C.; Heropoulos, G.A.; Calokerinos, A.C. Response Surface Methodology toward the Optimization of High-Energy Carotenoid Extraction from Aristeus antennatus Shrimp. Anal. Chim. Acta 2015, 877, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, D.P.; Rech, R.; Marczak, L.D.F.; Mercali, G.D. Ultrasound as an Alternative Technology to Extract Carotenoids and Lipids from Heterochlorella luteoviridis. Bioresour. Technol. 2017, 224, 753–757. [Google Scholar] [CrossRef]
- Izadi-NajafAbadi, P.; Ahmadi-Dastgerdi, A. Optimization of Emulsification and Microencapsulation of Balangu (Lallemantia royleana) Seed Oil by Surface Response Methodology. J. Food Qual. 2022, 2022, e5898937. [Google Scholar] [CrossRef]
- Yan, F.; Fan, K.; He, J.; Gao, M. Ultrasonic-Assisted Solvent Extraction of Carotenoids From Rapeseed Meal: Optimization Using Response Surface Methodology. J. Food Qual. 2015, 38, 377–386. [Google Scholar] [CrossRef]
- Provesi, J.G.; Amante, E.R. Carotenoids in Pumpkin and Impact of Processing Treatments and Storage. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015; pp. 71–80. [Google Scholar] [CrossRef]
- Dianursanti, A.R.; Siregar, Y.; Maeda, T.; Yoshino, T.; Tanaka, T. The Effects of Solvents and Solid-to-Solvent Ratios on Ultrasound-Assisted Extraction of Carotenoids from Chlorella vulgaris. Int. J. Technol. 2020, 11, 941–950. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Technological Aspects of β-Carotene Production. Food Bioprocess Technol. 2011, 4, 693–701. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Pinzón-Zarate, L.X.; González-Salcedo, L.O. Optimization of Ultrasonic-Assisted Extraction of Total Carotenoids from Peach Palm Fruit (Bactris gasipaes) by-Products with Sunflower Oil Using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 560–566. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, G.; Chen, F.; Wang, Z.; Wu, J.; Hu, X. Different Effects of Microwave and Ultrasound on the Stability of (All-E)-Astaxanthin. J. Agric. Food Chem. 2006, 54, 8346–8351. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, G.; Ye, X.; Kakuda, Y.; Meng, R. Stability of All-Trans-β-Carotene under Ultrasound Treatment in a Model System: Effects of Different Factors, Kinetics and Newly Formed Compounds. Ultrason. Sonochem. 2010, 17, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (αTEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Teng, H.; Choi, Y.H. Optimization of Ultrasonic-Assisted Extraction of Bioactive Alkaloid Compounds from Rhizoma Coptidis (Coptis chinensis Franch.) Using Response Surface Methodology. Food Chem. 2014, 142, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, A.; Jamil, M.A.; Noreen, S.; Iqbal, M.A. Antioxidant and Antimicrobial Properties of Pumpkin (Cucurbita maxima) Peel, Flesh and Seeds Powders. J. Biol. Agric. Healthc. 2021, 11, 42. [Google Scholar]
- De, B.; Bhandari, K.; Katakam, P.; Goswami, T.K. Development of a Standardized Combined Plant Extract Containing Nutraceutical Formulation Ameliorating Metabolic Syndrome Components. SN Appl. Sci. 2019, 1, 1484. [Google Scholar] [CrossRef]
- Nistor, O.V.; Mocanu, G.D.; Andronoiu, D.G.; Barbu, V.V.; Ceclu, L. A Complex Characterization of Pumpkin and Quince Purees Obtained by a Combination of Freezing and Conventional Cooking. Foods 2022, 11, 2038. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Gavril, R.N.; Carlescu, P.M.; Veleșcu, I.D.; Arsenoaia, V.N.; Stoica, F.; Stanciuc, N.; Aprodu, I.; Constantin, O.E.; Rapeanu, G. The development of value-added yogurt based on pumpkin peel powder as a bioactive powder. JAFR 2024, 16, 101098. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Ferreira, S.R.S.; Parada-Alfonso, F. Extraction of Phenolic Fraction from Guava Seeds (Psidium guajava L.) Using Supercritical Carbon Dioxide and Co-Solvents. J. Supercrit. Fluids 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of Thermal Treatment on Phenolic Compounds from Plum (Prunus domestica) Extracts—A Kinetic Study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]

| Run | Factor 1 A: Temperature (°C) | Factor 2 B: Timp (min) | Factor 3 C: Solvent Ratio (ml) | Response 1 TCs (mg/g DW) | Response 2 AA (µM/g DW) |
|---|---|---|---|---|---|
| 1 | 80 | 15 | 10 | 0.97 | 6.03 |
| 2 | 98.75 | 57.5 | 25 | 0.96 | 5.12 |
| 3 | 52.5 | 57.5 | 25 | 0.91 | 1.26 |
| 4 | 80 | 100 | 10 | 0.97 | 7.28 |
| 5 | 25 | 15 | 10 | 0.96 | 2.48 |
| 6 | 52.5 | 57.5 | 50.23 | 0.53 | 0.87 |
| 7 | 52.5 | 57.5 | 25 | 0.92 | 1.40 |
| 8 | 52.5 | 57.5 | 0.27 | 1.02 | 6.70 |
| 9 | 25 | 100 | 40 | 0.65 | 1.01 |
| 10 | 25 | 100 | 10 | 1.06 | 3.50 |
| 11 | 52.5 | 128.98 | 25 | 0.93 | 3.57 |
| 12 | 80 | 100 | 40 | 0.82 | 2.75 |
| 13 | 52.5 | 57.5 | 25 | 0.92 | 1.24 |
| 14 | 52.5 | 57.5 | 25 | 0.90 | 1.41 |
| 15 | 52.5 | 13.98 | 25 | 0.87 | 1.43 |
| 16 | 80 | 15 | 40 | 0.78 | 2.36 |
| 17 | 52.5 | 57.5 | 25 | 0.91 | 1.31 |
| 18 | 25 | 15 | 40 | 0.56 | 0.86 |
| 19 | 52.5 | 57.5 | 25 | 0.92 | 1.72 |
| 20 | 6.25 | 57.5 | 25 | 0.81 | 0.34 |
| TC | AA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F-Value | p-Value | Source | SS | df | MS | F-Value | p-Value |
| Model | 0.3841 | 9 | 0.0427 | 705.14 | <0.0001 a | Model | 82.17 | 9 | 9.13 | 233.19 | <0.0001 a |
| A-Temperature | 0.0236 | 1 | 0.0236 | 390.02 | <0.0001 | A-Temperature | 25.33 | 1 | 25.33 | 647.00 | <0.0001 |
| B-Time | 0.0083 | 1 | 0.0083 | 137.29 | <0.0001 | B-Time | 1.52 | 1 | 1.52 | 38.82 | <0.0001 |
| C-Solvent ratio | 0.2822 | 1 | 0.2822 | 4662.55 | <0.0001 | C-Solvent ratio | 36.26 | 1 | 36.26 | 926.08 | <0.0001 |
| AB | 0.0031 | 1 | 0.0031 | 51.17 | <0.0001 | AB | 0.0296 | 1 | 0.0296 | 0.7554 | 0.4052 |
| AC | 0.0262 | 1 | 0.0262 | 432.85 | <0.0001 | AC | 2.08 | 1 | 2.08 | 53.22 | <0.0001 |
| BC | 0.0003 | 1 | 0.0003 | 4.41 | 0.0621 | BC | 0.3734 | 1 | 0.3734 | 9.54 | 0.0115 |
| A² | 0.0016 | 1 | 0.0016 | 25.88 | 0.0005 | A² | 3.37 | 1 | 3.37 | 86.08 | <0.0001 |
| B² | 0.0006 | 1 | 0.0006 | 10.68 | 0.0085 | B² | 2.67 | 1 | 2.67 | 68.32 | <0.0001 |
| C² | 0.0351 | 1 | 0.0351 | 579.13 | <0.0001 | C² | 10.84 | 1 | 10.84 | 276.95 | <0.0001 |
| Residual | 0.0006 | 10 | 0.0001 | Residual | 0.3915 | 10 | 0.0392 | ||||
| Lack of Fit | 0.0004 | 5 | 0.0001 | 1.93 | 0.2445 b | Lack of Fit | 0.2383 | 5 | 0.0477 | 1.56 | 0.3197 b |
| Pure Error | 0.0002 | 5 | 0.0000 | Pure Error | 0.1532 | 5 | 0.0306 | ||||
| Cor Total | 0.3847 | 19 | Cor Total | 82.56 | 19 | ||||||
| Dependent Variable | Predicted Value | 95% Confidence Intervals | Experimental Value |
|---|---|---|---|
| TCs (mg/g DW) | 0.979 | 0.96–1.01 | 0.97 |
| AA (µM TE/g DW) | 7.281 | 6.72–7.84 | 7.25 |
| Code | Independent Variables | Units | Minimum | Maximum | Coded Low | Coded High |
|---|---|---|---|---|---|---|
| A | Temperature | °C | 6.2507 | 98.7493 | −1 = 0.10 | +1 = 2.00 |
| B | Time | min | 13.9762 | 128.976 | −1 = 20.00 | +1 = 60.00 |
| C | Solvent ratio | mL | 0.2268 | 50.2269 | −1 = 25.00 | +1 = 50.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavril, R.N.; Constantin, O.E.; Enachi, E.; Stoica, F.; Lipșa, F.D.; Stănciuc, N.; Aprodu, I.; Râpeanu, G. Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Carotenoids Extracted from Pumpkin Peel Using a Central Composite Design. Plants 2024, 13, 1447. https://doi.org/10.3390/plants13111447
Gavril RN, Constantin OE, Enachi E, Stoica F, Lipșa FD, Stănciuc N, Aprodu I, Râpeanu G. Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Carotenoids Extracted from Pumpkin Peel Using a Central Composite Design. Plants. 2024; 13(11):1447. https://doi.org/10.3390/plants13111447
Chicago/Turabian StyleGavril (Rațu), Roxana Nicoleta, Oana Emilia Constantin, Elena Enachi, Florina Stoica, Florin Daniel Lipșa, Nicoleta Stănciuc, Iuliana Aprodu, and Gabriela Râpeanu. 2024. "Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Carotenoids Extracted from Pumpkin Peel Using a Central Composite Design" Plants 13, no. 11: 1447. https://doi.org/10.3390/plants13111447








