Histological and Physiological Study of the Effects of Biostimulants and Plant Growth Stimulants in Viburnum opulus ‘Roseum’
Abstract
1. Introduction
2. Results
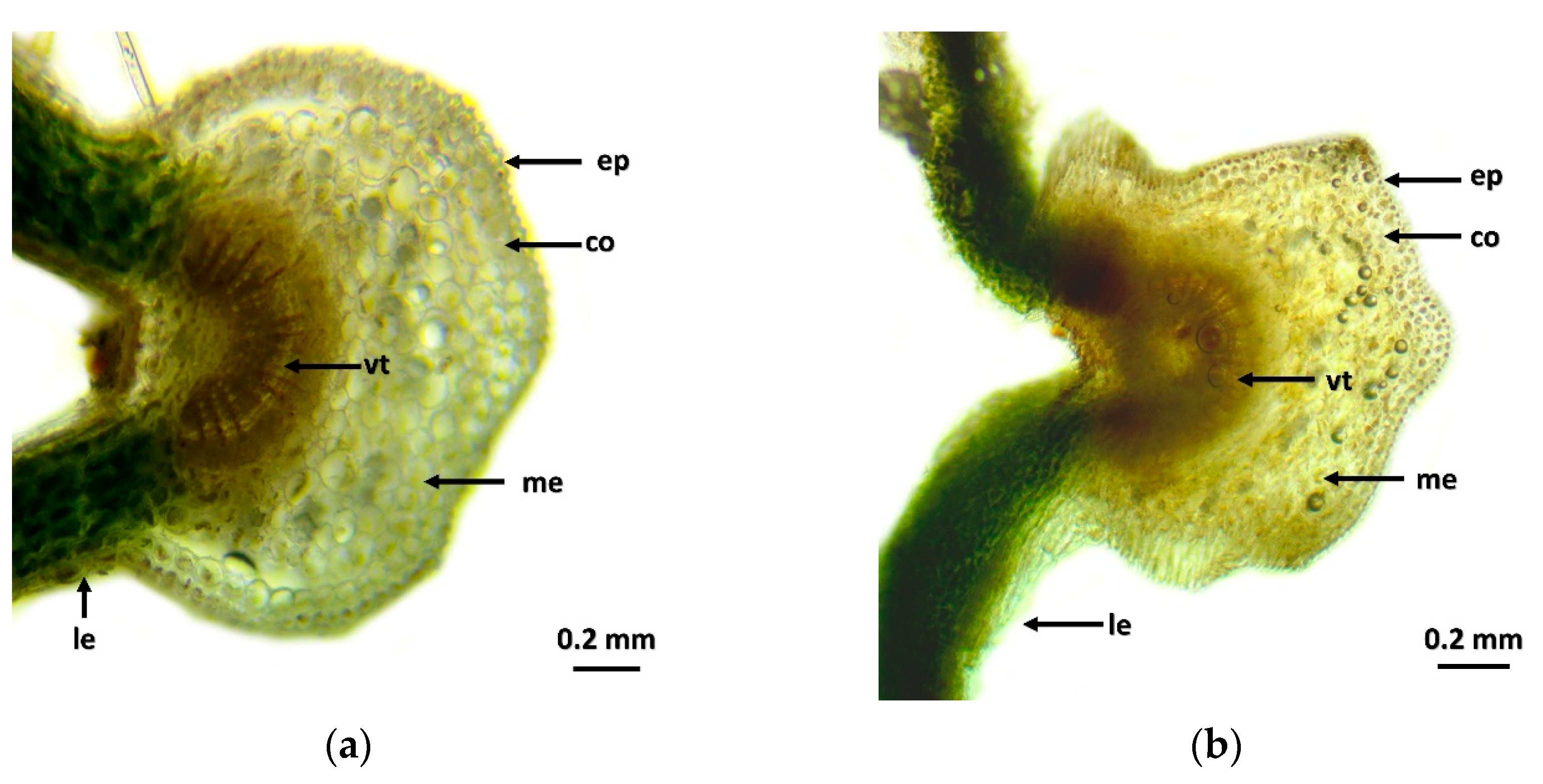
2.1. Histological Results
2.2. Physiological Results
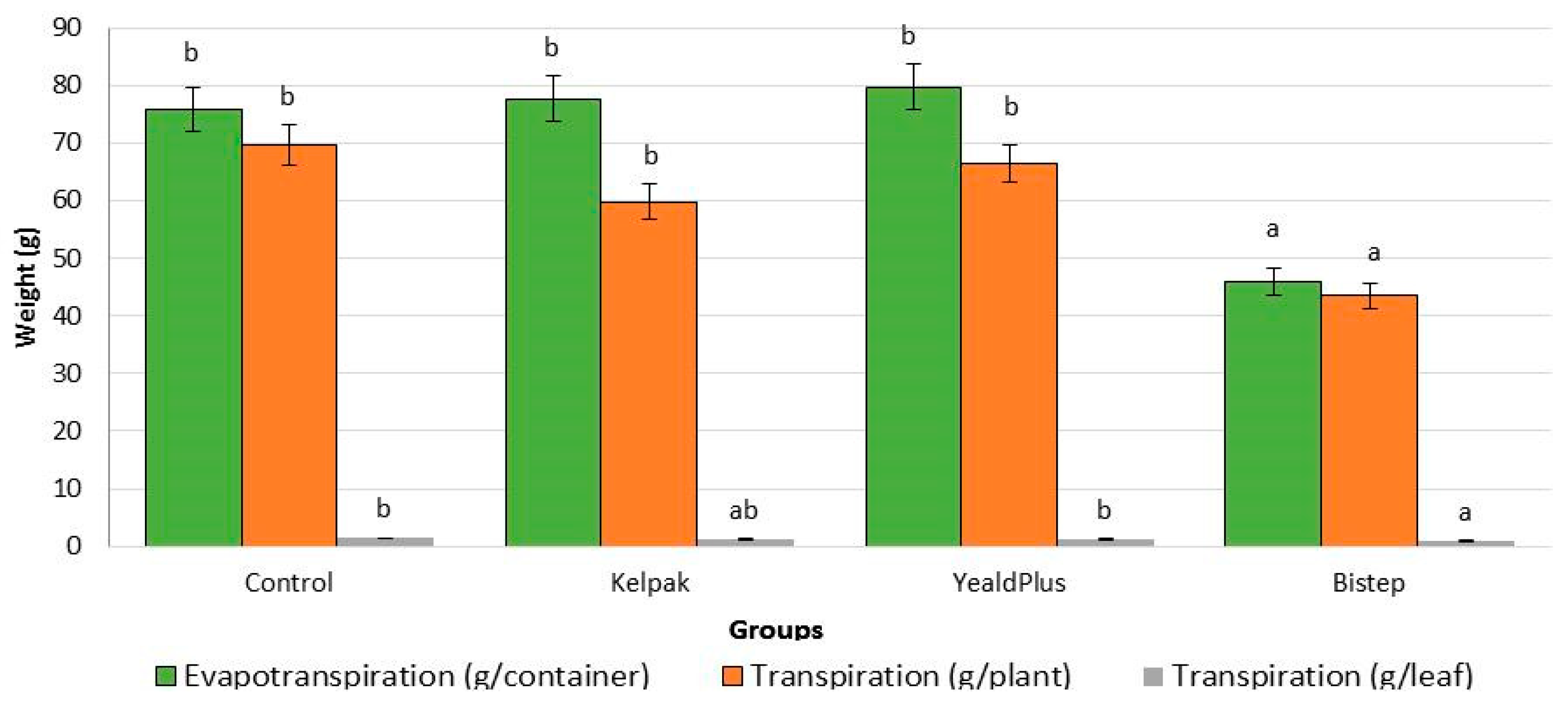
2.2.1. Transpiration and Evapotranspiration
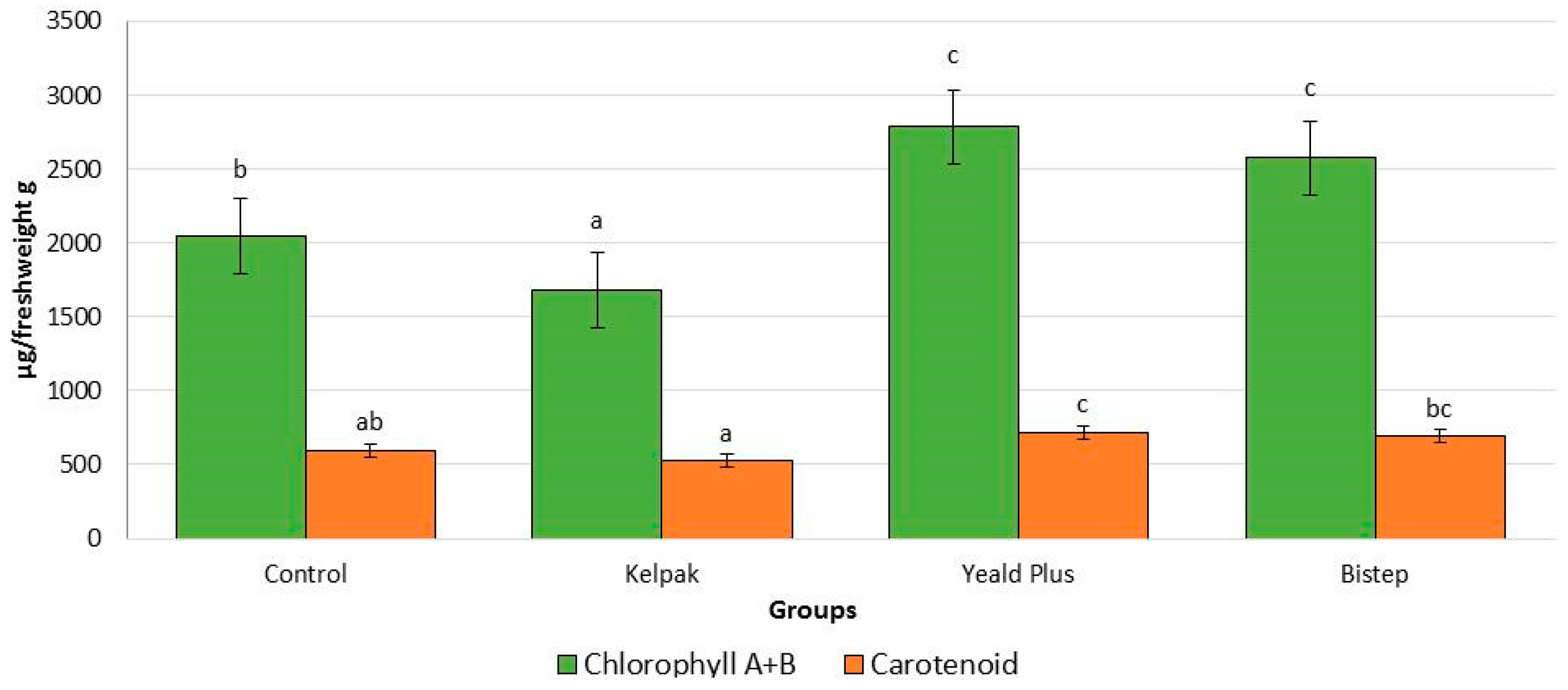
2.2.2. Chlorophyll and Carotenoid Content
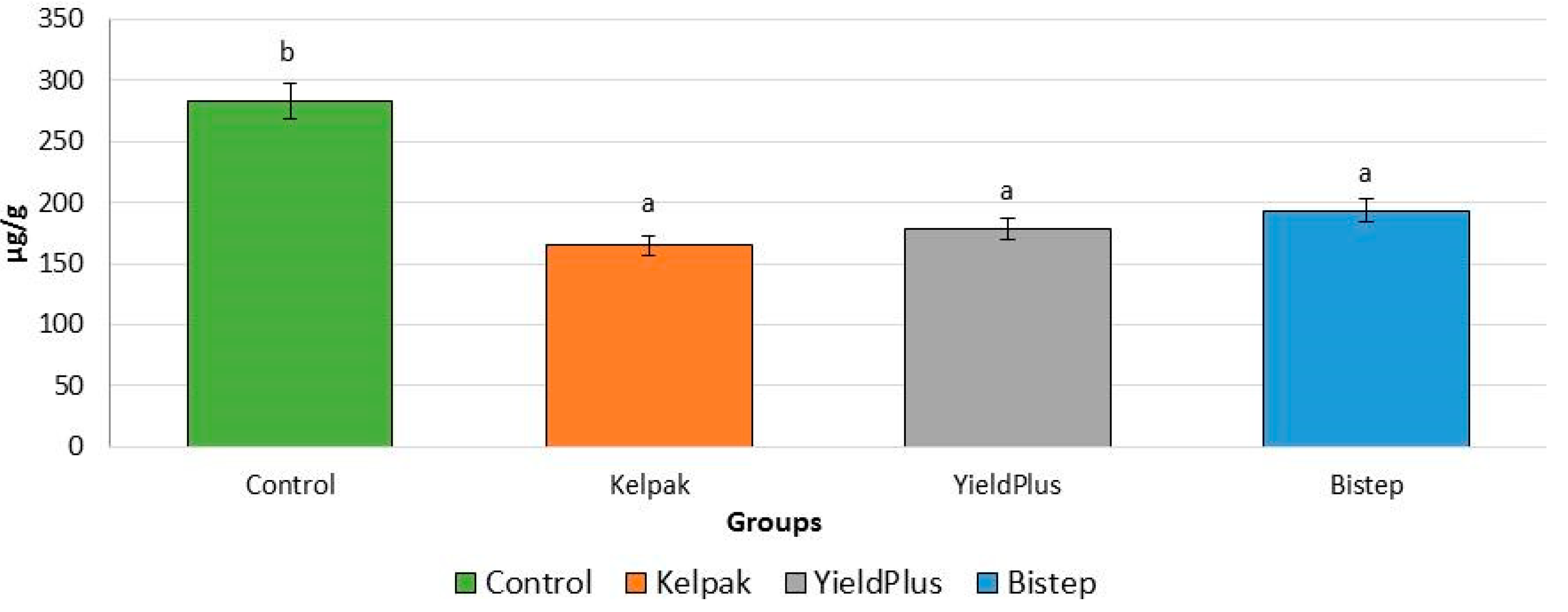
2.2.3. Proline Level
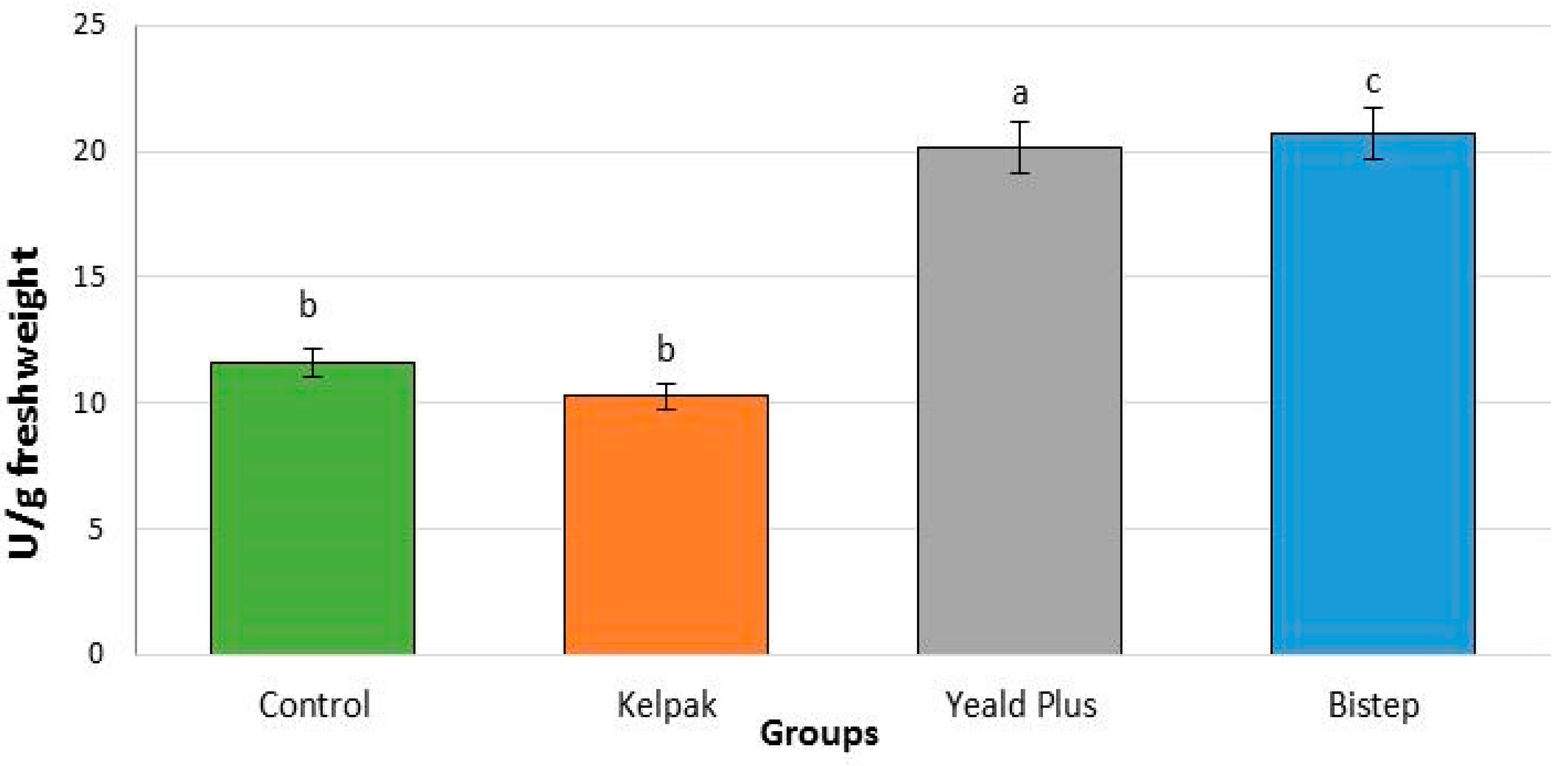
2.2.4. Peroxidase Enzyme
2.2.5. Rhizosphere Enzyme Activities
3. Discussion
4. Materials and Methods
4.1. Biostimulants Used for Treatment
4.1.1. Bistep
4.1.2. Kelpak®
4.1.3. Yeald Plus
4.2. Plant Material Used in the Experiment
4.3. Planting Parameters
- -
- Kelpak® 0.4% solution;
- -
- Yeald Plus 0.3% solution;
- -
- Bistep 0,5% solution;
- -
- Control group received only water at the same time as the treatments.
4.4. Histological Measurements
4.5. Physiological Measurements
4.6. Chlorophyll and Carotenoid Content Measurement
- -
- Ax—absorbance at a given wavelength;
- -
- V—final volume of the sample;
- -
- w—the sampled and homogenised plant fresh weight.
4.7. Peroxidase Enzyme Activity Measurement
4.8. Proline Level Measurement
4.9. Rhizosphere Measurements
4.10. Statistical Evaulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucini, L.; Colla, G.; Moreno, M.B.M.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphael, Y. Inoculation of Rhizoglomus irregulare or Trichoderma atroviride differentially modulates metabolite profiling of wheat root exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review. Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Rajabi Hamedani, S.; Rouphael, Y.; Colla, G.; Colantoni, A.; Cardarelli, M. Biostimulants as a tool for improving environmental sustainability of greenhouse vegetable crops. Sustainability 2020, 12, 5101. [Google Scholar] [CrossRef]
- Kisvarga, S.; Farkas, D.; Boronkay, G.; Neményi, A.; Orlóci, L. Effects of biostimulants in horticulture, with emphasis on ornamental plant production. Agronomy 2022, 12, 1043. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Bistimulant of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- Padmaja, V.V.; Pavani, K.; Srilatha, P.; Lalitha, K.; Sarada, G.; Naik, M.R.; Kiran, Y.D.; Gopal, K. Role of Biostimulants in Horticulture: A Review. Int. J. Environ. Clim. Change 2023, 13, 1146–1157. [Google Scholar] [CrossRef]
- Bell, J.C.; Bound, S.A.; Buntain, M. Biostimulants in agricultural and horticultural production. Hortic. Rev. 2022, 49, 35–95. [Google Scholar]
- Joly, P.; Calteau, A.; Wauquier, A.; Dumas, R.; Beuvin, M.; Vallenet, D.; Crovadore, J.; Cochard, B.; Lefort, F.; Berthon, J.Y. From strain characterization to field authorization: Highlights on Bacillus velezensis strain B25 beneficial properties for plants and its activities on phytopathogenic fungi. Microorganisms 2021, 9, 1924. [Google Scholar] [CrossRef]
- Research and Markets. Global Biostimulants Market Report 2022–2030—Compound Annual Growth of 10.4% with Market Set to Reach $6.79 Billion by 2030. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/06/17/2464668/28124/en/Global-Biostimulants-Market-Report-2022-2030-Compound-Annual-Growth-of-10-4-with-Market-Set-to-Reach-6-79-Billion-by-2030.html (accessed on 6 March 2024).
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 155882. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Giannattasio, M.; Vendramin, E.; Fornasier, F.; Alberghini, S.; Zanardo, M.; Stellin, F.; Squartini, A. Microbiological features and bioactivity of a fermented manure product (preparation 500) used in biodynamic agriculture. J. Microbiol. Biotechnol. 2013, 23, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kałużewicz, A.; Krzesiński, W.; Spiżewski, T.; Zaworska, A. Effect of biostimulants on several physiological characteristics and chlorophyll content in broccoli under drought stress and re-watering. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 197–202. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Testolin, R.; Zanotelli, D.; Andreotti, C. Effect of biostimulants on apple quality at harvest and after storage. Agronomy 2020, 10, 1214. [Google Scholar] [CrossRef]
- Conselvan, G.B.; Fuentes, D.; Merchant, A.; Peggion, C.; Francioso, O.; Carletti, P. Effects of humic substances and indole-3-acetic acid on Arabidopsis sugar and amino acid metabolic profile. Plant Soil 2018, 426, 17–32. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Bio/Technol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wadas, W.; Dziugieł, T. Changes in assimilation area and chlorophyll content of very early potato (Solanum tuberosum L.) cultivars as influenced by biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Ashraf, N.; Ashraf, M. Effect of plant biostimulants on growth, chlorophyll content, flower drop and fruit set of pomegranate cv. Kandhari Kabuli. Int. J. Agric. Environ. Biotechnology 2013, 6, 305–309. [Google Scholar]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Gupta, S.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Effect of organic biostimulants on the growth and biochemical composition of Amaranthus hybridus L. South Afr. J. Bot. 2019, 124, 87–93. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Peripolli, M.; Dornelles, S.H.; Lopes, S.J.; Tabaldi, L.A.; Trivisiol, V.S.; Rubert, J. Application of biostimulants in tomato subjected to water deficit: Physiological, enzymatic and production responses. Rev. Bras. De Eng. Agrícola E Ambient. 2021, 25, 90–95. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Biostimulant priming in Oryza sativa: A novel approach to reprogram the functional biology under nutrient-deficient soil. Cereal Reasearch Commun. 2022, 50, 45–52. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Yang, E.Y.; Cho, M.C.; Chae, S.Y.; Jeong, H.B.; Chae, W.B. Heat-tolerant hot pepper exhibits constant photosynthesis via increased transpiration rate, high proline content and fast recovery in heat stress condition. Sci. Rep. 2021, 11, 14328. [Google Scholar] [CrossRef]
- Surendar, K.K.; Devi, D.D.; Ravi, I.; Jeyakumar, P.; Velayudham, K. Effect of water stress on leaf temperature, transpiration rate, stomatal diffusive resistance and yield of banana. Plant Gene Trait. 2013, 4, 43–47. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Gaur, J.P. Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 2004, 219, 397–404. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Akensous, F.Z.; Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Ait-Rahou, Y.; Ahmed, H.B.; Nasri, N.; Hafidi, M.; Meddich, A. Boosting date palm (Phoenix dactylifera L.) growth under drought stress: Effects of innovative biostimulants. Gesunde Pflanz. 2022, 74, 961–982. [Google Scholar] [CrossRef]
- Santacruz-García, A.C.; Senilliani, M.G.; Gómez, A.T.; Ewens, M.; Yonny, M.E.; Villalba, G.F.; Nazareno, M.A. Biostimulants as forest protection agents: Do these products have an effect against abiotic stress on a forest native species? Aspects to elucidate their action mechanisms. For. Ecol. Manag. 2022, 522, 120446. [Google Scholar] [CrossRef]
- Miler, N.; Tymoszuk, A.; Woźny, A.; Michalik, T.; Wiśniewska, J.; Kulus, D. Microbiological Biostimulants in the Improvement of Extended Storage Quality of In Vitro-Derived Plants of Popular Ornamental Perennials. Agronomy 2024, 14, 289. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Huang, B. Lipidomic reprogramming associated with drought stress priming-enhanced heat tolerance in tall fescue (Festuca arundinacea). Plant Cell Environ. 2019, 42, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.; Li, C.; Vollbracht, K.; Hooper, D.G.; Wills, S.A.; Margenot, A.J. Prescribed pH for soil β-glucosidase and phosphomonoesterase do not reflect pH optima. Geoderma 2021, 401, 115161. [Google Scholar] [CrossRef]
- Parham, J.A.; Deng, S.P. Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol. Biochem. 2000, 32, 1183–1190. [Google Scholar] [CrossRef]
- Cristiano, G.; De Lucia, B. Petunia performance under application of animal-based protein hydrolysates: Effects on visual quality, biomass, nutrient content, root morphology, and gas exchange. Front. Plant Sci. 2021, 12, 640608. [Google Scholar] [CrossRef]
- Kumar, S.B.; Prasanth, P.; Sreenivas, M.; Gouthami, P.; Sathish, G. Effect of NPK, biofertilizers and biostimulants on flowering, flower quality and yield of China aster cv.‘Arka Kamini’. Pharma Innov. J. 2022, 11, 1239–1243. [Google Scholar]
- El-Baset, A.; Kasem, M.M. Improving growth characteristics and vase life of Dendranthema grandiflorum ‘Flyer’using humic and fulvic acids as biostimulants substances. Sci. J. Flowers Ornam. Plants 2022, 9, 87–102. [Google Scholar] [CrossRef]
- Suchitha, N.; Babu, K.K.; Lakshminarayana, D.; Kumar, S.P. Studies on the Effect of Biostimulants on Quality of Cut Flower of Chrysanthemum (Dendranthema grandiflora) cv. Denjigar Whitec. Int. J. Environ. Clim. Change 2023, 13, 687–692. [Google Scholar] [CrossRef]
- Smolin, N.V.; Ivoilov, A.V.; Volgin, V.V.; Potapova, N.V.; Nikolskiy, A.N.; Nedayborshch, J.N.; Potapov, I.V. Growth regulators and mineral fertilizers effect on morphometric indicators and decorative qualities of Zinnia elegans varieties. Ornam. Hortic. 2023, 29, 163–170. [Google Scholar] [CrossRef]
- Lin, Y.; Jones, M.L. Evaluating the growth-promoting effects of microbial biostimulants on greenhouse floriculture crops. Hortic. Sci. 2022, 57, 97–109. [Google Scholar] [CrossRef]
- Loconsole, D.; Sdao, A.E.; Cristiano, G.; De Lucia, B. Different responses to adventitious rhizogenesis under indole-3-butyric acid and seaweed extracts in ornamental’s cuttings: First Results in Photinia x fraseri ‘Red Robin’. Agriculture 2023, 13, 513. [Google Scholar] [CrossRef]
- Maciejewska, I. Pollen morphology of the Polish species of the family Caprifoliaceae. Part 1. Acta Soc. Bot. Pol. 1997, 66, 133–142. [Google Scholar] [CrossRef]
- Mazur, M.; Szperlik, J.; Salejda, A.M.; Krasnowska, G.; Kolniak-Ostek, J.; Bąbelewski, P. Description of the guelder rose fruit in terms of chemical composition, antioxidant capacity and phenolic compounds. Appl. Sci. 2021, 11, 9221. [Google Scholar] [CrossRef]
- Rop, O.; Reznicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant properties of European cranberrybush fruit (Viburnum opulus var. edule). Molecules 2010, 15, 4467–4477. [Google Scholar] [CrossRef]
- Kajszczak, D.; Zakłos-Szyda, M.; Podsędek, A. Viburnum opulus L.—A review of phytochemistry and biological effects. Nutrients 2020, 12, 3398. [Google Scholar] [CrossRef] [PubMed]
- Bubulica, V.M.; Anghel, I.; Grumezescu, A.M.; Saviuc, C.; Anghel, G.A.; Chifriuc, M.C.; Gheorghe, I.; Lazar, V.; Popescu, A. In vitro evaluation of bactericidal and antibiofilm activity of Lonicera tatarica and Viburnum opulus plant extracts on Staphylococcus strains. Farmacia 2012, 60, 80–91. [Google Scholar]
- Kajszczak, D.; Kowalska-Baron, A.; Sosnowska, D.; Podsędek, A. In vitro inhibitory effects of Viburnum opulus bark and flower extracts on digestion of potato starch and carbohydrate hydrolases activity. Molecules 2022, 27, 3118. [Google Scholar] [CrossRef]
- Levent Altun, M.; Saltan Çitoğlu, G.; Sever Yilmaz, B.; Çoban, T. Antioxidant properties of Viburnum opulus and Viburnum lantana growing in Turkey. Int. J. Food Sci. Nutr. 2008, 59, 175–180. [Google Scholar] [CrossRef]
- Akbulut, M.; Calisir, S.; Marakoglu, T.; Coklar, H. Chemical and technological properties of European cranberrybush (Viburnum opulus L.) fruits. Asian J. Chem. 2008, 20, 1875. [Google Scholar]
- Jouili, H.; Bouazizi, H.; El Ferjani, E. Plant peroxidases: Biomarkers of metallic stress. Acta Physiol. Plant. 2011, 33, 2075–2082. [Google Scholar] [CrossRef]
- Vujinović, T.; Zanin, L.; Venuti, S.; Tomasi, N.; Pinton, R.; Cesco, S. Biostimulant action of dissolved humic substances from a conventionally and an organically managed soil on nitrate acquisition in maize plants. Front. Plant Sci. 2020, 10, 502598. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kleber, M. A talaj szerves anyagainak vitás természete. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kwizda. 2008. Available online: https://kwizda.hu/yeald-plus~p36277 (accessed on 16 March 2024).
- Masny, A.; Basak, A.; Żurawicz, E. Effects of foliar applications of Kelpak SL and Goëmar BM 86® preparations on yield and fruit quality in two strawberry cultivars. J. Fruit Ornam. Plant Res. 2004, 12, 23–27. [Google Scholar]
- Kumar, R.; Trivedi, K.; Anand, K.V.; Ghosh, A. Science behind biostimulant action of seaweed extract on growth and crop yield: Insights into transcriptional changes in roots of maize treated with Kappaphycus alvarezii seaweed extract under soil moisture stressed conditions. J. Appl. Phycol. 2020, 32, 599–613. [Google Scholar] [CrossRef]
- Kularathne, M.N.; Srikrishnah, S.; Sutharsan, S. Effect of Seaweed Extracts on Ornamental Plants: Article Review. Curr. Agric. Res. J. 2021, 9, 149–160. [Google Scholar] [CrossRef]
- Davet, P. Microbial Ecology of Soil and Plant Growth; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Li, J.; Xie, T.; Zhu, H.; Zhou, J.; Li, C.; Xiong, W.; Xu, L.; Wu, Y.; He, Z.; Li, X. Alkaline phosphatase activity mediates soil organic phosphorus mineralization in a subalpine forest ecosystem. Geoderma 2021, 404, 115376. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, K.; Luo, Y.; Du, L.; Tian, R.; Wang, S.; Shen, Y.; Zhang, J.; Li, N.; Shao, W.; et al. Responses of soil enzyme activity to long-term nitrogen enrichment and water addition in a typical steppe. Agronomy 2023, 13, 1920. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant substances for sustainable agriculture: Origin, operating mechanisms and effects on cucurbits, leafy greens, and nightshade vegetables species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Kumar Sootahar, M.; Zeng, X.; Wang, Y.; Su, S.; Soothar, P.; Bai, L.; Kumar, M.; Zhang, Y.; Mustafa, A.; Ye, N. The short-term effects of mineral-and plant-derived fulvic acids on some selected soil properties: Improvement in the growth, yield, and mineral nutritional status of wheat (Triticum aestivum L.) under soils of contrasting textures. Plants 2020, 9, 205. [Google Scholar] [CrossRef]
- Kumar, H.D.; Aloke, P. Role of biostimulant formulations in crop production: An overview. Int. J. Appl. Res. Vet. M 2020, 8, 38–46. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Zodape, S.T. Seaweeds as a biofertilizer. J. Sci. Ind. Reaserch 2001, 60, 378–382. [Google Scholar]
- Basak, A. Effect of preharvest treatment with seaweed products, Kelpak® and Goëmar BM 86®, on fruit quality in apple. Int. J. Fruit Sci. 2008, 8, 1–14. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 462648. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.Q.; Critchley, A.T. A review of multiple biostimulant and bioeffector benefits of AMPEP, an extract of the brown alga Ascophyllum nodosum, as applied to the enhanced cultivation and micropropagation of the commercially important red algal carrageenophyte Kappaphycus alvarezii and its selected cultivars. J. Appl. Phycol. 2018, 30, 2859–2873. [Google Scholar]
- Szabó, V.; Magyar, L.; Hrotkó, K. Effect of leaf spray treatments on rooting and quality of Prunus mahaleb (L.) cuttings. Acta Sci. Polonorum. Hortorum Cultus 2016, 15, 77–87. [Google Scholar]
- Kovács, D.; Magyar, L.; Diószegi, M.; Hrotkó, K. Biostimulátorok hatása Forsythia x intermedia Zabel.” Beatrix Farrand” konténeres díszcserjék növekedésére = Treatments Affecting the Growth of Forsythia x Intermedia Zabel.’Beatrix Farrand’Container Grown Shrubs. GRADUS 2017, 4, 284–289. [Google Scholar]
- Pearcy, R.W.; Schulze, E.D.; Zimmermann, R. Measurement of transpiration and leaf conductance. In Plant Physiological Ecology: Field Methods and Instrumentation; Springer Netherlands: Dordrecht, The Netherlands, 2000; pp. 137–160. [Google Scholar]
- Van Leperen, W.; Madery, H. A new method to measure plant water uptake and transpiration simultaneously. J. Exp. Bot. 1994, 45, 51–60. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Shannon, L.M.; Kay, E.; Lew, J.Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J. Biol. Chem. 1966, 241, 2166–2172. [Google Scholar] [CrossRef]
- Chinard, F.P. Photometric estimation of proline and ornithine. J. Biol. Chem. 1952, 199, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance: Methods and Protocols; Humana: Totowa, NJ, USA, 2010; pp. 317–331. [Google Scholar]
- Tong, A.Z.; Liu, W.; Liu, Q.; Xia, G.Q.; Zhu, J.Y. Diversity and composition of the Panax ginseng rhizosphere microbiome in various cultivation modesand ages. BMC Microbiol. 2021, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]



| Key Characteristics | Bistep (syn. Ferbanat L) | Kelpak® | Yeald Plus |
|---|---|---|---|
| Manufacturer | UAB ALJARA LT-11219 Vilnius, Geniu str. 16-38. Lithuania | KELP PRODUCTS (PTY) LTD 7975 Simon’s Town, Blue Water Close South Africa | De Sangosse Ltd. Hillside Mill Quarry Lane, Swaffham Bulbeck, Cambridge CB5 0LU, UK |
| Base elements | Microhumates and coupled microelements | Aqueous extracts of Ecklonia maxima | 6% nitrogen, 5% zinc, 1% potassium peroxide, 0.03% boron, 0.25% copper, 0.25% iron, 0.25% manganese, 0.001% molybdenum |
| Effects | Increase soil humus volume, support root development, increase yield and green weight | Support shoot and bud development, support root development | Leaf fertilisation, irrigation, watering |
| Economic benefit | 50% reduction in fertiliser use | It also has a beneficial effect on seed development | Increases root mass by 30–35% in two weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, D.; Horotán, K.; Orlóci, L.; Makádi, M.; Mosonyi, I.; Sütöri-Diószegi, M.; Kisvarga, S. Histological and Physiological Study of the Effects of Biostimulants and Plant Growth Stimulants in Viburnum opulus ‘Roseum’. Plants 2024, 13, 1446. https://doi.org/10.3390/plants13111446
Kovács D, Horotán K, Orlóci L, Makádi M, Mosonyi I, Sütöri-Diószegi M, Kisvarga S. Histological and Physiological Study of the Effects of Biostimulants and Plant Growth Stimulants in Viburnum opulus ‘Roseum’. Plants. 2024; 13(11):1446. https://doi.org/10.3390/plants13111446
Chicago/Turabian StyleKovács, Dezső, Katalin Horotán, László Orlóci, Marianna Makádi, István Mosonyi, Magdolna Sütöri-Diószegi, and Szilvia Kisvarga. 2024. "Histological and Physiological Study of the Effects of Biostimulants and Plant Growth Stimulants in Viburnum opulus ‘Roseum’" Plants 13, no. 11: 1446. https://doi.org/10.3390/plants13111446
APA StyleKovács, D., Horotán, K., Orlóci, L., Makádi, M., Mosonyi, I., Sütöri-Diószegi, M., & Kisvarga, S. (2024). Histological and Physiological Study of the Effects of Biostimulants and Plant Growth Stimulants in Viburnum opulus ‘Roseum’. Plants, 13(11), 1446. https://doi.org/10.3390/plants13111446







