Multifunctional Adjuvants Affect Sulfonylureas with Synthetic Auxin Mixture in Weed and Maize Grain Yield
Abstract
:1. Introduction
2. Results
2.1. Greenhouse Experiment
2.2. Field Experiment
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naeem, M.; Cheema, Z.A.; Ahmad, A.H.; Wahid, A.; Kamaran, M.; Arif, M. Weed dynamics in wheat canola intercropping systems. Chil. J. Agric. Res. 2012, 72, 434–439. [Google Scholar] [CrossRef]
- Landau, C.A.; Hager, A.G.; Williams, M.M., II. Diminishing weed control exacerbates maize yield loss to adverse weather. Glob. Chang. Biol. 2021, 27, 6156–6165. [Google Scholar] [CrossRef]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a Changing Climate: Vulnerabilities, Consequences, and Implications for Future Weed Management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. In Herbicides, Physiology of Action, and Safety; IntechOpen Limited: London, UK, 2015. [Google Scholar]
- Comont, D.; Lowe, C.; Hull, R.; Crook, L.; Hicks, H.L.; Onkokesung, N.; Beffa, R.; Childs, D.Z.; Edwards, R.; Freckleton, R.P.; et al. Evolution of generalist resistance to herbicide mixtures reveals trade-off in resistance management. Nat. Commun. 2020, 11, 3086. [Google Scholar] [CrossRef]
- Daramola, O.S.; Johnson, W.G.; Jordan, D.L.; Chahal, G.S.; Devkota, P. Spray water quality and herbicide performance: A review. Weed Technol. 2023, 36, 758–767. [Google Scholar] [CrossRef]
- Neto, R.C.A.; Ulguim, A.R.; Barbieri, G.F.; Thomasi, R.M.; Bortolin, E.; Leichtweis, E.M.; Melo, A.A. pH and water hardness on the efficiency of auxin mimics herbicides. Cienc. Rural 2024, 54, e20230005. [Google Scholar] [CrossRef]
- Grey, T.L.; McCullough, P.E. Sulfonylurea herbicides’ fate in soil: Dissipation, mobility, and other processes. Weed Technol. 2012, 26, 579–581. [Google Scholar] [CrossRef]
- Schilder, A. Effect of Water pH on the Stability of Pesticides; Michigan State University Extension, Department of Plant Pathology: East Lansing, MI, USA, 2008; Available online: https://www.canr.msu.edu/news/effect_of_water_ph_on_the_stability_of_pesticides (accessed on 19 April 2024).
- Zhong, J.; Wu, S.; Chen, W.J.; Huang, Y.; Lei, Q.; Mishra, S.; Bhatt, P.; Chen, S. Current insights into the microbial degradation of nicosulfuron: Strains, metabolic pathways, and molecular mechanisms. Chemosphere 2023, 326, 138390. [Google Scholar] [CrossRef]
- Rahman, A.; James, T.K.; Trolove, M.; Dowsett, C. Factors affecting the persistence of some residual herbicides in maize silage fields. N. Z. Plant Prot. 2011, 64, 125–132. [Google Scholar] [CrossRef]
- Piwowar, A. The use of pesticides in Polish agriculture after integrated pest management (IPM) implementation. Environ. Sci. Pollut. Res. 2021, 28, 26628–26642. [Google Scholar] [CrossRef]
- Akhter, M.J.; Abbas, R.N.; Waqas, M.A.; Noor, M.A.; Arshad, M.A.; Mahboob, W.; Nadeem, F.; Azam, M.; Gull, U. Adjuvants improves the efficacy of herbicide for weed management in maize sown under altered sowing methods. J. Exp. Biol. Agric. Sci. 2017, 5, 22–30. [Google Scholar] [CrossRef]
- Singh, S.; Rana, M. A review on adjuvants: A herbicide activator. Int. J. Res. Anal. Rev. 2019, 6, 60–63. [Google Scholar]
- Tu, M.; Randall, J.M. Adjuvants. In Weed Control Methods Handbook; The Nature Conservancy 21. Adjuvants; TNC: Davis, CA, USA; pp. 1–24.
- Palma-Bautista, C.; Vazquez-Garcia, J.G.; Travlos, I.; Tatarida, A.; Kanatas, P.; Dominguez-Valenzeula, J.A.; De Prado, R. Effect of adjuvants on glyphosate effectiveness, retention, absorption, adsorption and translocation in Lolium rigidum and Conyza canadensis. Plants 2020, 9, 297. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, H.; Ozkan, H.E.; Bagley, W.E.; Derksen, R.C.; Krause, C.R. Adjuvant effects on evaporation time and wetted areaof droplets on waxy leaves. ASABE 2010, 53, 13–20. [Google Scholar] [CrossRef]
- Kucharski, M.; Sadowski, J.; Domaradzki, K. Degradation rate of chloridazon in soil as influenced by adjuvants. J. Plant Prot. Res. 2012, 52, 114–117. [Google Scholar] [CrossRef]
- Pacanoski, Z. Herbicides and adjuvants. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; BoD—Books on Demand: Norderstedt, Germany, 2015; pp. 125–147. [Google Scholar]
- Mullin, C.A.; Fine, J.D.; Reynolds, R.D.; Frazier, M.T. Toxicological risks of agrochemical spray adjuvants: Organosilicone surfactants may not be safe. Front. Public Health 2016, 4, 92. [Google Scholar] [CrossRef]
- Surgan, M.; Condon, M.; Cox, C. Pesticide risk indicators: Unidentified inert ingredients compromise their integrity and utility. Environ. Manag. 2010, 45, 834–841. [Google Scholar] [CrossRef]
- Mesnage, R.; Antoniou, M.N. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front. Public Health 2018, 5, 361. [Google Scholar] [CrossRef]
- Wernecke, A.; Eckert, J.H.; Forster, R.; Kurlemann, N.; Odemer, R. Inert agricultural spray adjuvants may increase the adverse effects of selected insecticides on honey bees (Apis mellifera L.) under laboratory conditions. J. Plant Dis. Protect. 2022, 129, 93–105. [Google Scholar] [CrossRef]
- Bao, Z.; Wu, Y.; Liu, R.; Zhang, S.; Chen, Y.; Wu, T.; Gao, Y.; Zhang, C.; Du, F. Molecular selection and environmental evaluation of eco-friendly surfactants to efficiently reduce pesticide pollution. J. Clean. Prod. 2023, 416, 137954. [Google Scholar] [CrossRef]
- Lin, F.; Mao, Y.; Zhao, F.; Idris, A.L.; Liu, Q.; Zou, S.; Guan, X. Toward sustainable green adjuvants for microbial pesticides: Recent progress, upcoming challenges, and future perspectives. Microorganisms 2023, 11, 364. [Google Scholar] [CrossRef]
- Congreve, M.; Somervaille, A.; Betts, G.; Gordon, B.; Green, V.; Bugis, M. Adjuvants—Oils, Surfactants and Other Additives for Farm Chemicals Used in Grain Production, Revised 2019 ed.; Australian Government Grains Research & Development Corporation: Dulwich, Australia, 2019. [Google Scholar]
- Zabkiewicz, J. Adjuvants and herbicidal efficacy—Present status and future prospects. Weed Res. 2002, 40, 139–149. [Google Scholar] [CrossRef]
- Stock, D. Physicochemical properties of adjuvants: Values and applications. Weed Technol. 2009, 14, 798–806. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Q.; Huang, G.; Liu, M.; Cao, L.; Li, F.; Zhao, P.; Cao, C. The effects of adjuvants on the wetting and deposition of insecticide solutions on hydrophobic wheat leaves. Agronomy 2022, 12, 2148. [Google Scholar] [CrossRef]
- Bagula, E.M.; Majaliwa, J.G.M.; Basamba, T.A.; Mondo, J.G.M.; Vanlauwe, B.; Gabiri, G.; Tumuhairwe, J.; Mushagalusa, G.N.; Musinguzi, P.; Akello, S.; et al. Water use efficiency of maize (Zea mays L.) crop under selected soil and water conservation practices along the slope gradient in Ruzizi Watershed, Eastern D.R. Congo. Land 2022, 11, 1833. [Google Scholar] [CrossRef]
- Huang, C.; Duiker, S.W.; Deng, L.; Fang, C.; Zeng, W. Influence of precipitation on maize yield in the Eastern United States. Sustainability 2015, 7, 5996–6010. [Google Scholar] [CrossRef]
- Sobiech, Ł.; Idziak, R.; Skrzypczak, G.; Szulc, P.; Grzanka, M. Biodiversity of weed flora in maize on lessive soil. Prog. Plant Prot. 2018, 58, 282–287. [Google Scholar]
- Nguyen, H.T.X.; Liebman, M. Weed community composition in simple and more diverse cropping systems. Front. Agron. 2022, 4, 848548. [Google Scholar] [CrossRef]
- Chwastek, G.; Idziak, R.; Waligóra, H. Biodiversity of weed community in maize in the Cieszyńskie Foothills. Prog. Plant Prot. 2020, 60, 290–298. [Google Scholar]
- Green, J.M.; Beestman, G.B. Recently patented and commercialized formulation and adjuvant technology. Crop. Prot. 2007, 26, 320–327. [Google Scholar] [CrossRef]
- Chuah, T.S.; Kaben, A.M.; Thye-San, C. Proper adjuvant selection to enhance the activity of triclopyr combined with metsulfuron on the control of Hedyotis verticillate. Weed Biol. Manag. 2009, 9, 179–184. [Google Scholar]
- Imoloame, E.O.; Omolaiye, J.O. Weed infestation, growth and yield of maize (Zea mays L.) as influenced by periods of weed interference. Adv. Crop. Sci. Technol. 2017, 5, 267. [Google Scholar]
- Kumar, A.; Dhaka, A.K.; Kumar, S.; Singh, S.; Punia, S.S. Weed management indices as affected by different weed control treatments in pigeon pea [Cajanus cajan (L.) Millsp.]. J. Pharmacog. Phytochem. 2019, 8, 3490–3494. [Google Scholar]
- Hammond, M.E.; Pokorný, R. Diversity of Tree Species in Gap Regeneration under Tropical Moist Semi-Deciduous Forest: An Example from Bia Tano Forest Reserve. Diversity 2020, 12, 301. [Google Scholar] [CrossRef]
- Łukasiewicz, S. A modification suggestion of the method of drawing the wet humid period in the Walter’s climate diagram. Badania Fizjol. Nad Pol. Zachodnią 2006, A 57, 95–99. [Google Scholar]
| Herbicide | Rate | Adjuvant | ECHCG Control (%) | Average | ||
|---|---|---|---|---|---|---|
| pH | ||||||
| 4 | 7 | 9 | ||||
| N+R+D | FR | none | 48 ± 5.2 ef | 48 ± 5.1 ef | 56 ± 5.0 de | 51 ± 3.4 D |
| N+R+D | RR | none | 45 ± 8.3 ef | 37 ± 7.1 f | 42 ± 9.0 f | 42 ± 6.2 E |
| N+R+D | RR | +TEST-1 | 68 ± 6.4 bc | 83 ± 4.1 a | 76 ± 8.1 abc | 76 ± 5.3 AB |
| +TEST-2 | 81 ± 2.4 ab | 82 ± 2.4 a | 80 ± 4.1 ab | 81 ± 1.9 A | ||
| +TEST-3 | 80 ± 1.2 ab | 77 ± 11.1 ab | 80 ± 2.3 ab | 79 ± 4.8 A | ||
| +MSO | 73 ± 2.0 bc | 81 ± 1.1 ab | 79 ± 1.1 ab | 78 ± 2.1 AB | ||
| +S | 66 ± 7.3 bc | 71 ± 3.6 abc | 63 ± 5.0 cd | 67 ± 3.9 C | ||
| Average | 66 ± 14.4 A | 68 ± 18.2 A | 68 ± 14.7 A | - | ||
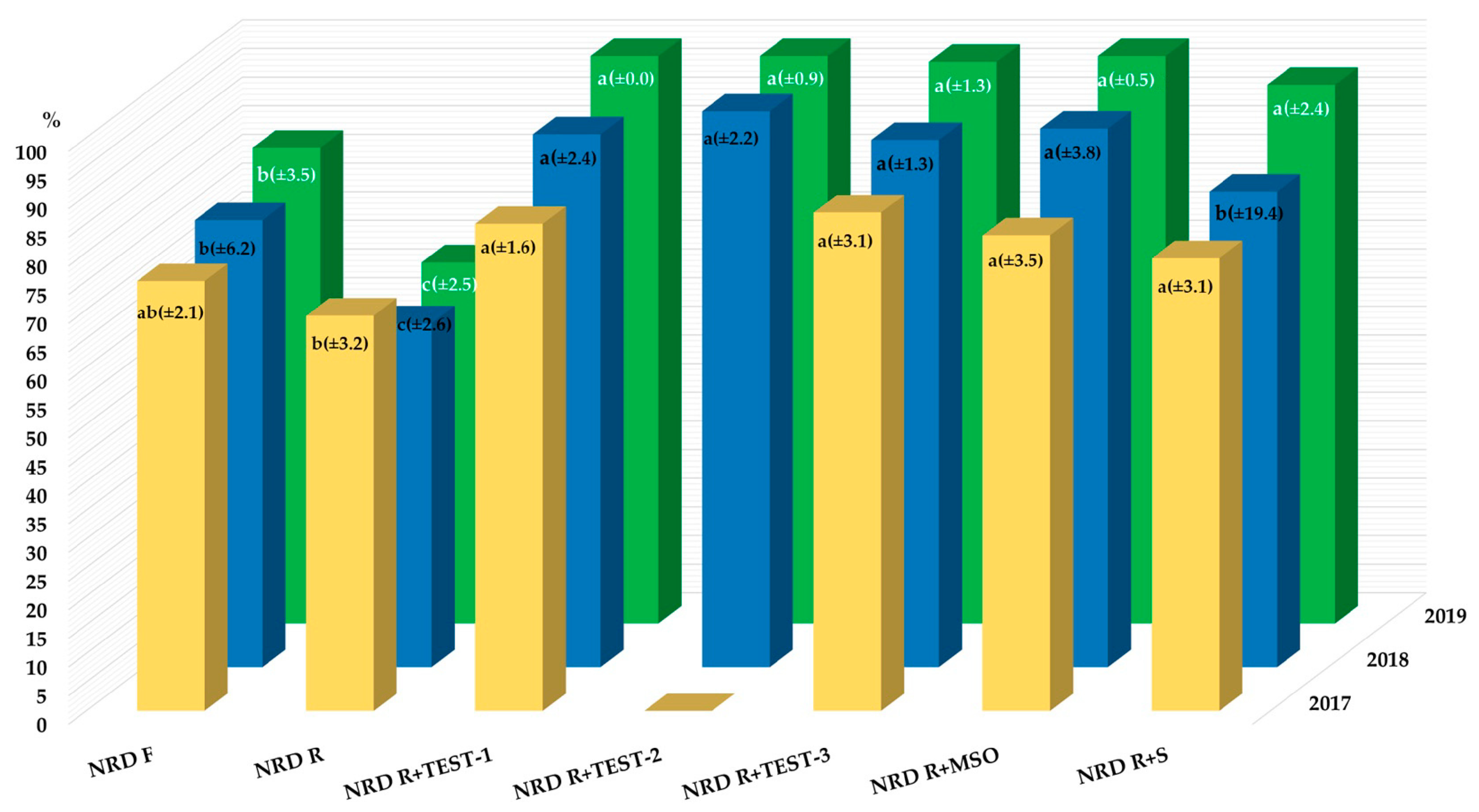
| Year | Herbicide | Rate | Adjuvant | CHEAL | ECHCG | GERPU | POLCO | VIOAR |
|---|---|---|---|---|---|---|---|---|
| WCE (%) | ||||||||
| 2017 | Untreated check (g m–2) | 3638 | 143 | 2258 | 572 | 87 | ||
| N+R+D 1 | FR | - | 84 ± 2.9 b | 100 ± 0.0 a | 84 ± 3.9 a | 89 ± 3.5 a | 74 ± 3.2 a | |
| N+R+D | RR | - | 65 ± 6.9 c | 95 ± 2.9 a | 64 ± 1.7 b | 83 ± 1.3 a | 67 ± 7.1 a | |
| N+R+D | RR | +TEST-1 | 92 ± 3.9 a | 100 ± 0.0 a | 74 ± 3.4 ab | 91 ± 1.8 a | 78 ± 2.4 a | |
| +TEST-2 2 | - | - | - | - | - | |||
| +TEST-3 | 95 ± 1.1 a | 100 ± 0.0 a | 73 ± 3.0 ab | 90 ± 0.8 a | 72 ± 4.6 a | |||
| +MSO | 95 ± 1.9 a | 100 ± 0.0 a | 71 ± 3.8 ab | 86 ± 1.4 a | 64 ± 2.5 a | |||
| +S | 82 ± 3.8 b | 100 ± 0.0 a | 68 ± 2.4 ab | 85 ± 3.3 a | 615.4 a | |||
| 2018 | Untreated check (g m–2) | 1066 | 253 | 11 | 31 | 28 | ||
| N+R+D 1 | FR | - | 81 ± 6.7 b | 81 ± 7.1 b | 100 ± 0.0 a | 86 ± 7.6 a | 100 ± 0.0 a | |
| N+R+D | RR | - | 66 ± 12.2 c | 61 ± 4.5 c | 100 ± 0.0 a | 80 ± 7.7 a | 97 ± 3.7 a | |
| N+R+D | RR | +TEST-1 | 93 ± 3.4 a | 99 ± 0.7 a | 97 ± 6.5 a | 81 ± 5.4 a | 98 ± 2.4 a | |
| +TEST-2 2 | 96 ± 2.2 a | 98 ± 1.0 a | 94 ± 6.9 a | 92 ± 5.6 a | 100 ± 0.0 a | |||
| +TEST-3 | 90 ± 1.8 ab | 100 ± 0.0 a | 100 ± 0.0 a | 86 ± 5.1 a | 100 ± 0.0 a | |||
| +MSO | 94 ± 3.4 a | 99 ± 1.1 a | 100 ± 0.0 a | 81 ± 2.1 a | 100 ± 0.0 a | |||
| +S | 79 ± 8.8 b | 93 ± 3.4 a | 97 ± 6.4 a | 77 ± 5.3 a | 91 ± 6.7 b | |||
| 2019 | Untreated check (g m–2) | 1746 | 171 | 12 | 37 | 10 | ||
| N+R+D 1 | FR | - | 86 ± 3.2 b | 85 ± 8.8 ab | 94 ± 6.6 ab | 100 ± 0.0 a | 100 ± 0.0 a | |
| N+R+D | RR | - | 60 ± 11.2 c | 77 ± 7.5 b | 67 ± 5.8 c | 85 ± 3.8 b | 97 ± 6.8 a | |
| N+R+D | RR | +TEST-1 | 99 ± 1.4 a | 96 ± 2.3 a | 87 ± 5.5 b | 96 ± 5.3 a | 98 ± 4.1 a | |
| +TEST-2 2 | 99 ± 0.9 a | 90 ± 8.1 ab | 100 ± 0.0 a | 94 ± 6.6 a | 100 ± 0.0 a | |||
| +TEST-3 | 99 ± 1.1 a | 90 ± 2.0 ab | 86 ± 4.2 b | 96 ± 8.1 a | 100 ± 0.0 a | |||
| +MSO | 99 ± 0.9 a | 94 ± 5.5 a | 90 ± 11.7 ab | 98 ± 2.1 a | 100 ± 0.0 a | |||
| +S | 96 ± 2.9 a | 88 ± 11.5 ab | 90 ± 3.7 ab | 89 ± 15.8 ab | 100 ± 0.0 a | |||
| Herbicide | Rate | Adjuvant | Grain Yield (t ha–1) | TKW (g) | ||||
|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2017 | 2018 | 2019 | |||
| Untreated check | - | - | 6.0 ± 1.58 c | 2.5 ± 0.86 b | 2.2 ± 1.05 c | 276 ± 20.5 ab | 238 ± 11.1 c | 248 ± 22.8 c |
| N+R+D | FR | none | 11.7 ± 0.45 a | 4.2 ± 0.16 b | 7.1 ± 1.13 b | 296 ± 14.6 ab | 247 ± 12.2 bc | 287 ± 18.7 b |
| N+R+D | RR | none | 9.3 ± 0.91 b | 3.9 ± 0.86 b | 7.1 ± 0.92 b | 261 ± 12.6 b | 245 ± 16.2 bc | 271 ± 17.5 b |
| N+R+D | RR | +TEST-1 | 12.5 ± 0.22 a | 8.3 ± 1.64 a | 10.7 ± 1.05 a | 306 ± 10.2 a | 270 ± 14.7 ab | 321 ± 6.1 a |
| +TEST-2 | - | 7.8 ± 2.00 a | 9.2 ± 0.71 ab | - | 289 ± 19.0 a | 324 ± 16.4 a | ||
| +TEST-3 | 12.4 ± 0.21 a | 7.4 ± 2.34 a | 10.4 ± 1.52 a | 308 ± 10.3 a | 276 ± 8.0 ab | 314 ± 7.3 a | ||
| +MSO | 11.7 ± 0.21 a | 7.6 ± 1.26 a | 10.9 ± 0.80 a | 290 ± 13.3 ab | 275 ± 14.3 ab | 325 ± 10.0 a | ||
| +S | 12.0 ± 0.39 a | 6.6 ± 1.25 a | 11.1 ± 0.92 a | 298 ± 14.7 a | 283 ± 10.8 a | 320 ± 5.9 a | ||
| Type of Test | TEST-2 | TEST-3 |
|---|---|---|
| IC50 (72 h)—OECD 201 1 | 3.157 mg L–1 | ˃100 mg L–1 |
| LOEC 1 (72 h) | 2.92 mg L–1 | - |
| NOEC 1 (72 h) | 1.47 mg L–1 | - |
| EC50 (48 h)—OECD 202 2 | ˃100 mg L–1 | ˃100 mg L–1 |
| LC50 (96 h)—OECD 203 3 | ˃100 mg L–1 | ˃100 mg L–1 |
| NOED—OECD 213 4 | ˃100 µg L–1 | ˃100 µg L–1 |
| NOED—OECD 214 4 | ˃100 µg L–1 | ˃100 µg L–1 |
| SB—OECD 301 | 72.9 ± 2.2 | 88.1 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idziak, R.; Waligóra, H.; Majchrzak, L.; Szulc, P. Multifunctional Adjuvants Affect Sulfonylureas with Synthetic Auxin Mixture in Weed and Maize Grain Yield. Plants 2024, 13, 1480. https://doi.org/10.3390/plants13111480
Idziak R, Waligóra H, Majchrzak L, Szulc P. Multifunctional Adjuvants Affect Sulfonylureas with Synthetic Auxin Mixture in Weed and Maize Grain Yield. Plants. 2024; 13(11):1480. https://doi.org/10.3390/plants13111480
Chicago/Turabian StyleIdziak, Robert, Hubert Waligóra, Leszek Majchrzak, and Piotr Szulc. 2024. "Multifunctional Adjuvants Affect Sulfonylureas with Synthetic Auxin Mixture in Weed and Maize Grain Yield" Plants 13, no. 11: 1480. https://doi.org/10.3390/plants13111480
APA StyleIdziak, R., Waligóra, H., Majchrzak, L., & Szulc, P. (2024). Multifunctional Adjuvants Affect Sulfonylureas with Synthetic Auxin Mixture in Weed and Maize Grain Yield. Plants, 13(11), 1480. https://doi.org/10.3390/plants13111480








