The Influence of Piriformospora indica Colonization on the Root Development and Growth of Cerasus humilis Cuttings
Abstract
:1. Introduction
2. Results
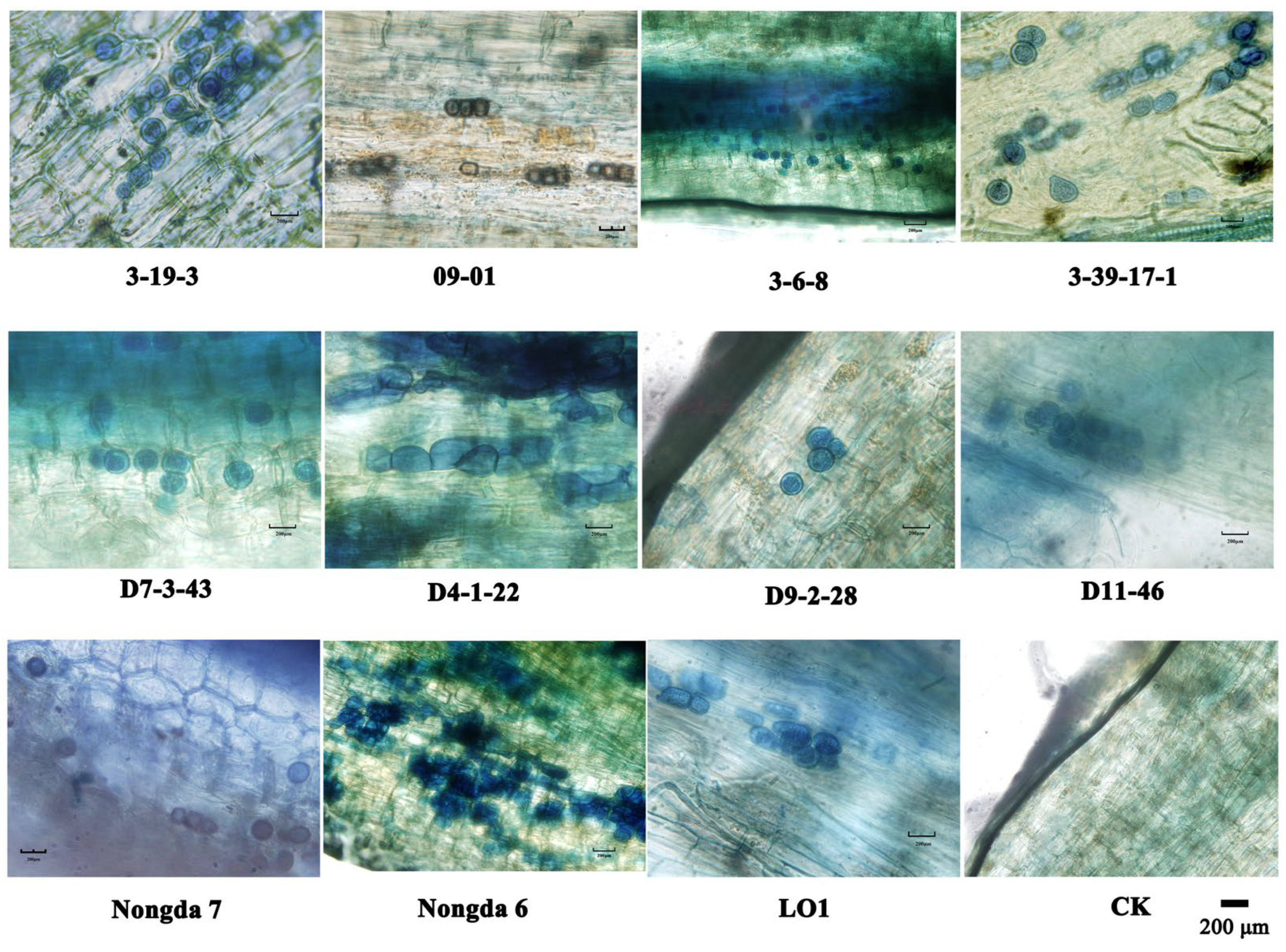
2.1. P. indica Colonization Detection Results in Roots of Cerasus humilis Cuttings
2.2. Influence of P. indica Colonization on the Growth and Development of Cerasus humilis
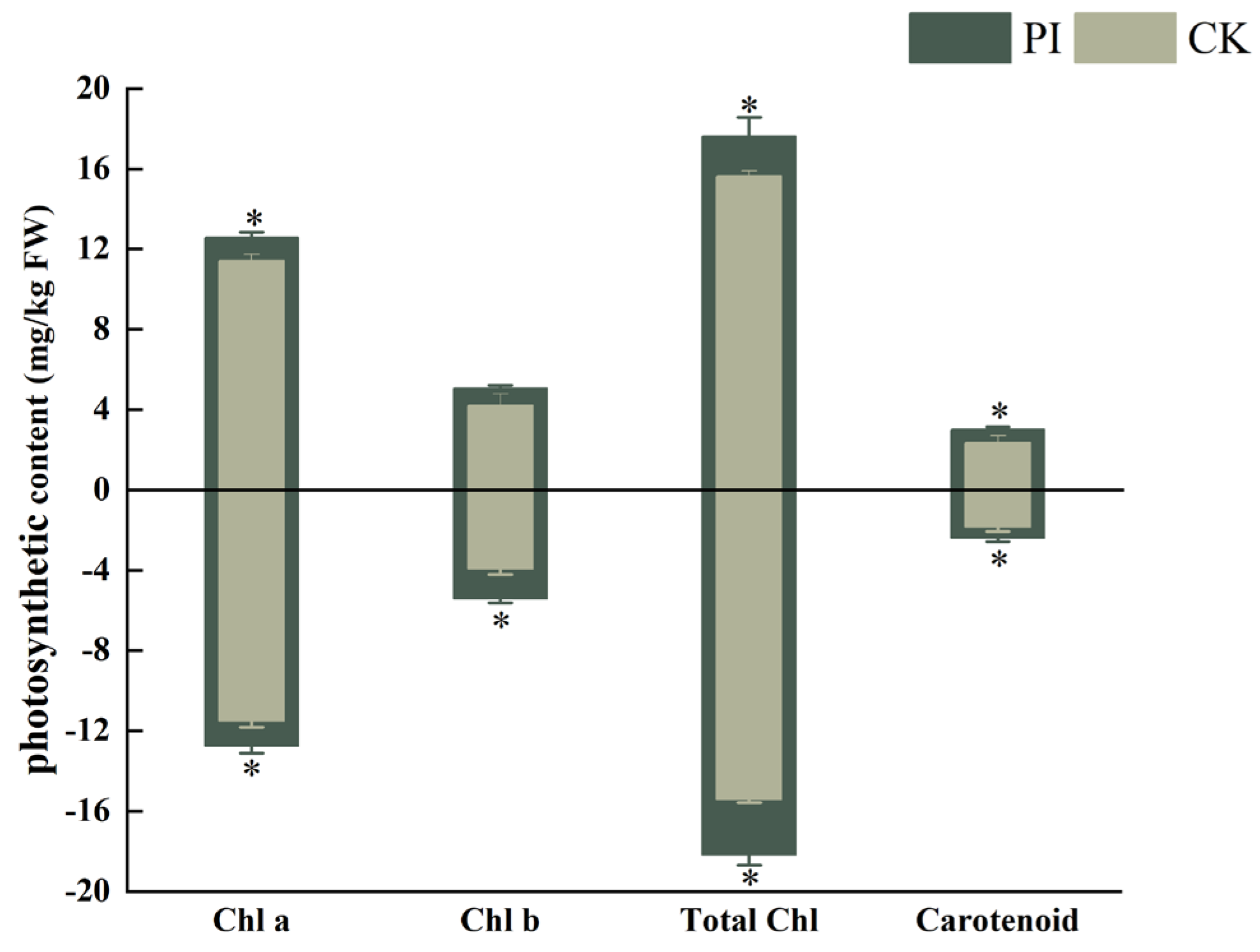
2.3. Effect of P. indica Colonization on Photosynthetic Pigments in Cerasus humilis Leaves
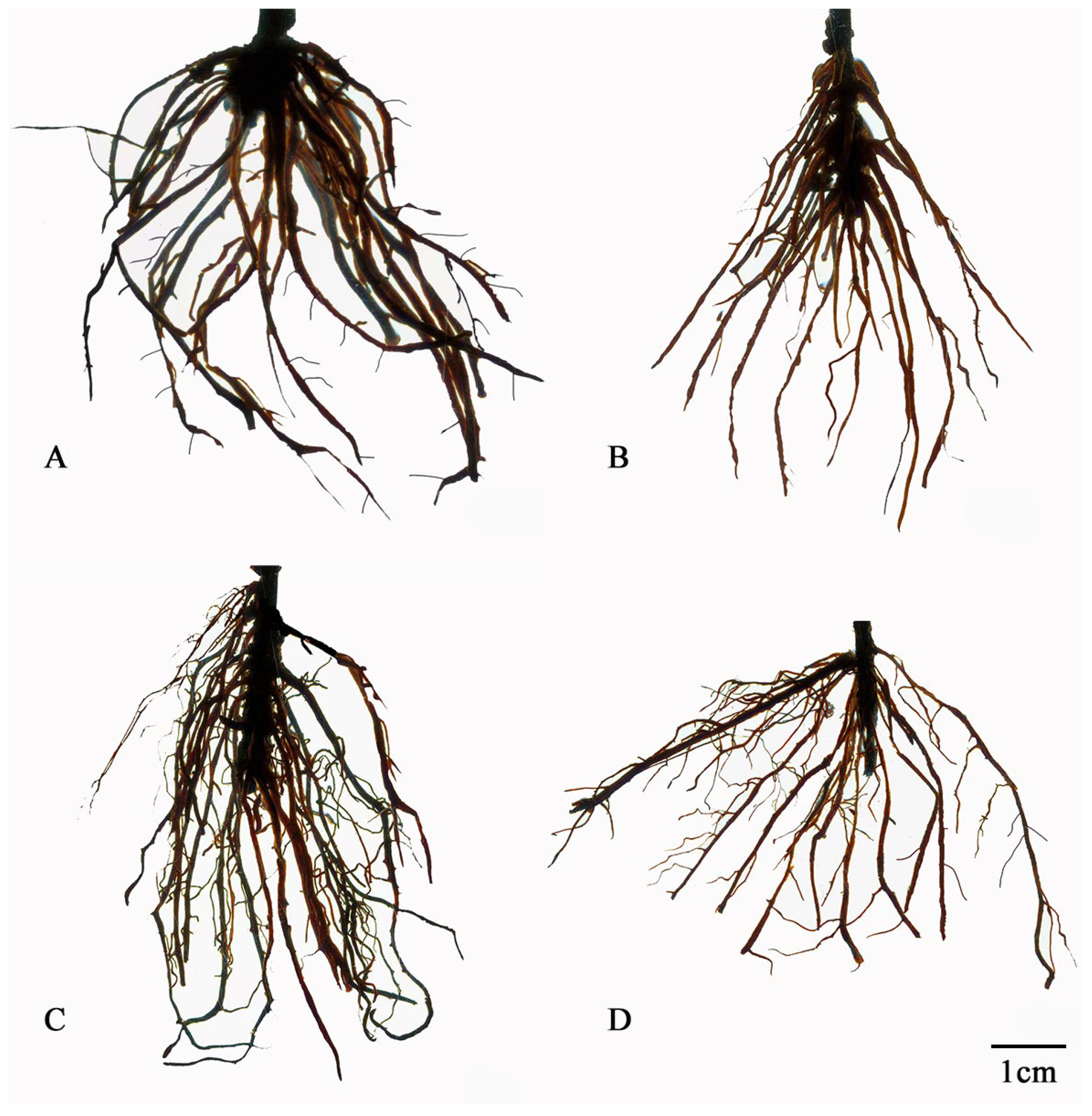
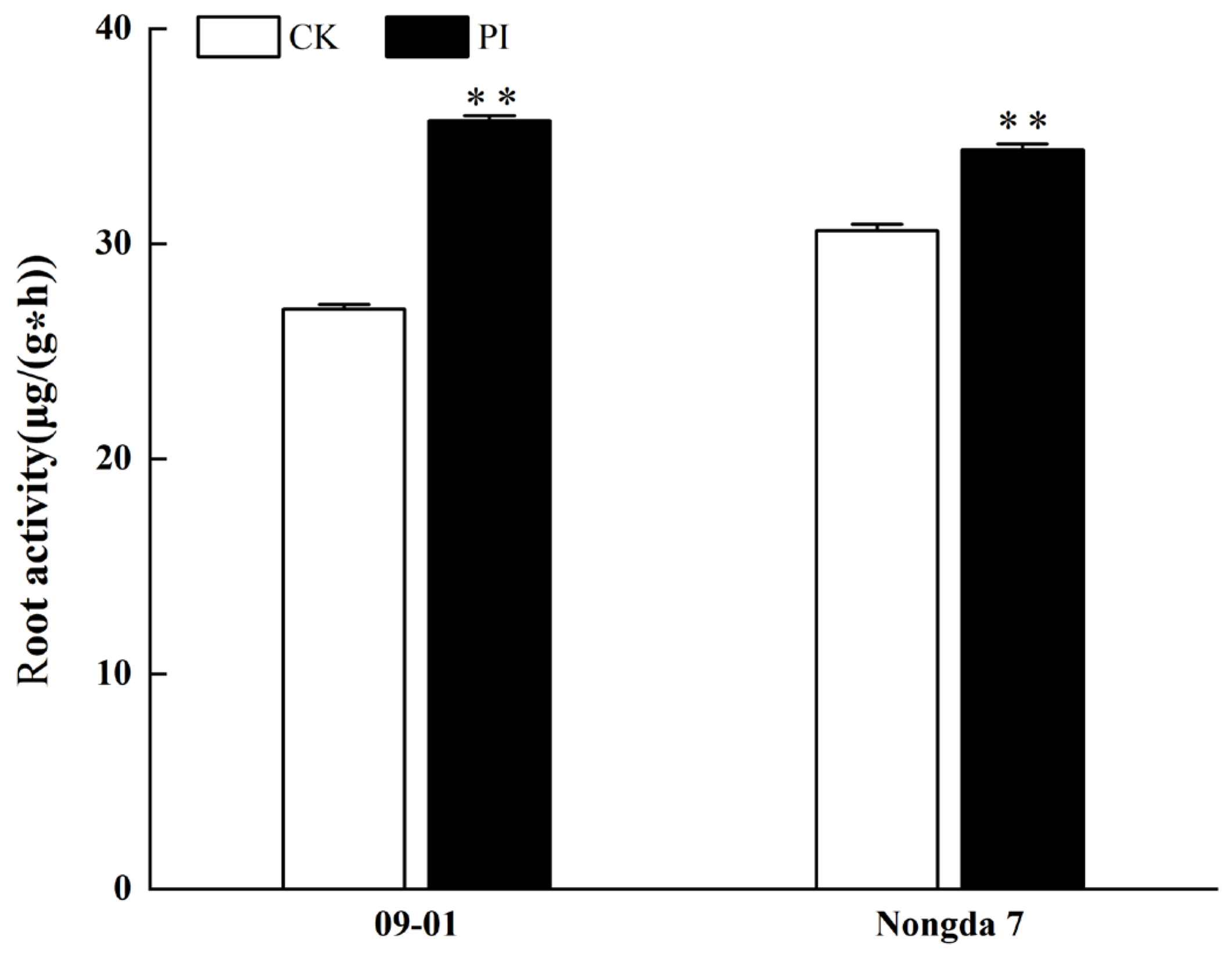
2.4. Effect of P. indica Colonization on Root Development and Root Activities in Cerasus humilis
2.5. Effect of P. indica Colonization on POD and Hormone Levels
3. Discussion
3.1. P. indica Establishes Symbiotic Relationship with Cerasus humilis
3.2. P. indica Colonization Promotes the Growth and Root Development of Cerasus humilis Cuttings
3.3. P. indica Colonization Greatly Induces the Accumulation of Chlorophyll in Cerasus humilis Leaves
3.4. P. indica Colonization Enhances Root POD Activity Beneficial for Root Growth and Development
3.5. P. indica Promotes Cerasus humilis Root Growth by Inducing IAA Hormone Synthesis, Inhibiting JA and ACC Accumulation
4. Materials and Methods
4.1. Plant Materials and Fungal Preparation
4.2. Piriformospora indica Colonization Detection
4.3. Determination of Plant Growth Parameters
4.4. Determination of Photosynthetic Pigments in Cerasus humilis Leaves
Total chlorophyll = Ca + Cb
4.5. Determination of Root Development and Activities in Cerasus humilis
4.6. Determination of Root Antioxidant Enzyme Activities and Hormone Levels in Cerasus humilis
4.7. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Varma, A.; Verma, S.; Sudha; Sahay, N.; Butehom, B.; Franken, P. Pinrmospora indica, a cultivable plant-growth promoting root endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef]
- Li, L.; Feng, Y.; Qi, F.; Hao, R. Research progress of Piriformospora indicain improving plant growth and stress resistance to plant. J. Fungi 2023, 9, 965. [Google Scholar] [CrossRef]
- Li, Q.; Kuo, Y.W.; Lin, K.H.; Huang, W.; Deng, C.; Yeh, K.W.; Chen, S.P. Piriformospora indica colonization increases the growth, development, and herbivory resistance of sweet potato (Ipomoea batatas L.). Plant Cell Rep. 2021, 40, 339–350. [Google Scholar] [CrossRef]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Tuteja, N. Piriformospora indica: Potential and significance in plant stress tolerance. Front. Microbiol. 2016, 7, 332. [Google Scholar] [CrossRef]
- Liu, B.H.; Jing, D.W.; Liu, F.C.; Ma, H.; Liu, X.; Peng, L. Serendipita indica alleviates drought stress responses in walnut (Juglans regia L.) cuttings by stimulating osmotic adjustment and antioxidant defense system. Appl. Microbiol. Biotechnol. 2021, 105, 8951–8968. [Google Scholar] [CrossRef]
- Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Sun, X.; Hao, X.; Cheng, C.Z. Versatile Piriformospora indica and its potential applications in horticultural crops. Hortic. Plant J. 2020, 6, 111–121. [Google Scholar] [CrossRef]
- Liu, W.; Tan, M.; Qu, P.Y.; Huo, C.; Liang, W.; Li, R.; Cheng, C.Z. Use of Piriformospora indica to promote growth of strawberry daughter plants. Horticulturae 2022, 8, 370. [Google Scholar] [CrossRef]
- Wang, H.L.; Zheng, X.D. Effects of Piriformospora indica on yield, quality and postharvest resistance of cherry tomato. J. Nucl. Agric. Sci. 2022, 36, 466–472. [Google Scholar]
- Su, Z.Z.; Wang, T.; Shrivastava, N.; Chen, Y.Y.; Liu, X.; Sun, C.; Lou, B.G. Piriformospora indica promotes growth, seed yield and quality of Brassica napus L. Microbiol. Res. 2017, 199, 29–39. [Google Scholar] [CrossRef]
- Cheng, C.Z.; Li, D.; Wang, B.; Liao, B.; Qu, P.Y.; Liu, W.; Lu, P. Piriformospora indica colonization promotes the root growth of Dimocarpus longan cuttings. Sci. Hortic. 2022, 301, 111137. [Google Scholar] [CrossRef]
- Wu, J.D.; Chen, Q.; Liu, X.X.; Ling, F.C.; Gao, Q.K.; Long, B.G. A preliminary study on the growth-promoting effect of Piriformospora indica on rice and its mechanism. Chin. J. Rice Sci. 2015, 29, 200–207. [Google Scholar]
- Liu, M.; Du, J.J. Distribution of germplasm resources and performance of varietal traits in Cerasus humilis. Deciduous Fruit 2010, 42, 18–21. [Google Scholar]
- Li, H.; Yue, J.H.; Xia, W.X.; Li, T.; Huang, X.; Zhang, Y.; Fu, X. Exploring the beneficial effects and mechanisms of Cerasus humilis (Bge) Sok fruit for calcium supplementation and promotion. Food Biosci. 2023, 54, 102846. [Google Scholar] [CrossRef]
- Wu, C.Y.; Han, X.Y.; Wu, J.Y.; Wang, W.; Xie, C.; Xiao, B.Y.; Zhou, Z.H.; Dong, X.J. Progress of nutritional value and development and utilisation of Cerasus humilis. China Fruit 2024, 2, 1–5+25. [Google Scholar]
- Zhang, J.; Yan, F.J. A review of the research on seed selection and propagation of Cerasus humilis. J. Northeast Agric. Sci. 2007, 6, 55–57. [Google Scholar]
- Hou, G.H.; Ma, J.J.; Wu, S.G.; Lv, D.; Sheng, B.W.; Wang, L.B. A preliminary study on the propagation techniques of Cerasus humilis. For. Sci. Technol. 2015, 3, 32–33. [Google Scholar]
- Xu, M.L.; Qiao, C.X.; Liu, Y.J.; Yu, R.; Xie, J.; Ma, L. Effects of different rootstocks on grafting survival and fruit quality of Cerasus humilis. J. Nucl. Agric. Sci. 2023, 37, 2503–2509. [Google Scholar]
- Huang, L.H.; Long, Z.W.; Zhang, P. Research on tissue culture technology of Cerasus humilis. China New Technol. New Prod. 2009, 11, 227. [Google Scholar]
- Cheng, M.Y.; Geng, L.; Luo, J.; Wen, H.; Liu, C.S.; Li, W.D.; Liu, Y.; Gao, Y. Research on quality grading standards of Cerasus humilis cuttings. Mod. Chin. Med. 2016, 18, 326–328. [Google Scholar]
- Du, J.J.; Li, J.; Li, Z.J.; Wen, P.F.; Tian, C.F. Research on the method of seedling preparation and cutting of Cerasus humilis. Shanxi Agric. Sci. 1998, 3, 62–66. [Google Scholar]
- Jiang, R.D. Research on the Key Technology of Tissue Culture and Cuttings Breeding of Cerasus humilis. Master’s Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- He, Q.Q.; Zhang, J.; Mu, X.P.; Zhang, J.C.; Du, J.J.; Wang, P.F. Effects of foliar spraying of plant growth regulators on rooting and physiological changes in shoot cuttings of Cerasus humilis. Plant Physiol. J. 2024, 60, 108–116. [Google Scholar]
- Scheer, H. Chlorophylls: A personal snapshot. Molecules 2022, 27, 1093. [Google Scholar] [CrossRef]
- Gong, M.; Lu, N.P. In vivo determination of root avtivity in crops. J. Nucl. Agric. Sci. 1982, 4, 54. [Google Scholar]
- Ben, K.L.; Gilabert, P.M.; Dreyer, B.; Oihabi, A.; Honrubia, M.; Morte, A. Peroxidase changes in Phoenix dactylifera palms inoculated with mycorrhizal and biocontrol fungi. Agron. Sustain. Dev. 2008, 28, 411–418. [Google Scholar]
- Wang, X.L.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, E. Mycorrhizal Symbiosis Modulates the Rhizosphere Microbiota to Promote Rhizobia-Legume Symbiosis. Mol. Plant 2020, 14, 503–516. [Google Scholar] [CrossRef]
- De La Fuente Canto, C.; Simonin, M.; King, E.; Moulin, L.; Bennett, M.J.; Castrillo, G.; Laplaze, L. An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Cell Mol. Biol. 2020, 103, 951–964. [Google Scholar] [CrossRef]
- Achatz, B.; Kogel, K.H.; Franken, P.; Waller, F. Piriformospora indica mycorrhization increases grain yield by accelerating early development of barley plants. Plant Signal. Behav. 2010, 5, 1685–1687. [Google Scholar] [CrossRef]
- Zhang, K.L.; Zhang, H.X.; Xie, C.; Zhu, Z.; Lin, L.; An, Q.; Li, D. Piriformospora indica colonization enhances remediation of cadmium and chromium co-contaminated soils by king grass through plant growth promotion and rhizosphere microecological regulation. J. Hazard. Mater. 2023, 462, 132728. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Liu, F.; Ma, H.; Ma, B.; Zhang, W.; Peng, L. Growth improvement of Lolium multiforum Lam. induced by seed inoculation with fungus suspension of Xerocomus badius and Serendipita Indica. AMB Express 2019, 9, 145. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.; Zhu, J.W.; Gao, W.; Chen, L.; Li, X.; Liu, Y. Nitric oxide generated by Piriformospora indica-induced nitrate reductase promotes tobacco growth by regulating root architecture and ammonium and nitrate transporter gene expression. J. Plant Interact. 2022, 17, 861–872. [Google Scholar] [CrossRef]
- Chen, W.; Lin, F.; Lin, K.H.; Chen, C.; Xia, C.; Liao, Q.; Kuo, Y.W. Growth Promotion and Salt-Tolerance Improvement of Gerbera jamesonii by Root Colonization of Piriformospora indica. J. Plant Growth Regul. 2021, 41, 1219–1228. [Google Scholar] [CrossRef]
- Narayan, O.P.; Verma, N.; Jogawat, A.; Dua, M.; Johri, A.K. Sulfur transfer from the endophytic fungus Serendipita indica improves maize growth and requires the sulfate transporter SiSulT. Plant Cell 2021, 33, 1268–1285. [Google Scholar] [CrossRef]
- Shikha, S.; Samta, G.; Rupam, K. Chemically Synthesized AgNPs and Piriformospora indica Synergistically Augment Nutritional Quality in Black Rice. Fungi 2023, 9, 611. [Google Scholar] [CrossRef]
- Kundu, A.; Mishra, S.; Kundu, P.; Jogawat, A.; Vadassery, J. Piriformospora indica Recruits Host-Derived Putrescine for Growth Promotion in Plants. Plant Physiol. 2022, 188, 2289–2307. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wang, L.; Bao, F.H.; Chen, J.X.; Lin, Y.; Bao, W.Q.; Ao, D. Genetic diversity of leaf phenotypic traits in good single plants of Cerasus humilis. Non-Wood For. Res. 2023, 41, 183–190. [Google Scholar]
- Wu, M.Y.; Liu, F.C.; Liu, B.H.; Liu, X.H.; Si, D.X.; Ma, H.L. Effects of Piriformospora indica on the growth and phosphorus absorption of peonies under cultivation. North. Hortic. 2023, 9, 46–53. [Google Scholar]
- Jogawat, A.; Meena, M.K.; Kundu, A.; Varma, M.; Vadassery, J. Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J. Exp. Bot. 2020, 71, 2752–2768. [Google Scholar] [CrossRef]
- Zheng, M.J.; Zhong, S.P.; Wang, W.J.; Tang, Z.; Bu, T.; Li, Q. Serendipita indica Promotes the Growth of Tartary Buckwheat by Stimulating Hormone Synthesis, Metabolite Production, and Increasing Systemic Resistance. Fungi 2023, 9, 1114. [Google Scholar] [CrossRef]
- Johnson, J.M.; Alex, T.; Oelmüller, R. Piriformospora indica–The versatile and multifunctional root endophytic fungus for enhanced yield and tolerance to biotic and abiotic stress in crop plants. J. Trop. Agric. 2014, 52, 103–122. [Google Scholar]
- Lin, H.F.; Xiong, J.; Zhou, H.M.; Chen, C.M.; Lin, F.Z.; Xu, X.N.; Yeh, K.W. Growth promotion and disease resistance induced in Anthurium colonized by the beneficial root endophyte Piriformospora indica. BMC Plant Biol. 2019, 19, 40. [Google Scholar] [CrossRef]
- Zhang, D.P.; Wang, X.S.; Zhang, Z.Y.; Li, C.; Xing, Y.; Luo, Y.; Cai, H. Symbiotic System Establishment between Piriformospora indica and Glycine max and Its Effects on the Antioxidant Activity and Ion-Transporter-Related Gene Expression in Soybean under Salt Stress. Int. J. Mol. Sci. 2022, 23, 14961. [Google Scholar] [CrossRef]
- Li, A.H.; Xu, X.H.; Li, J.Z.; Pu, Y.; Wu, C. Effects of selenium inoculation with Piriformospora indica on root development, gas exchange and leaf selenium accumulation of Cephalotaxus sinensis cuttings under selenium fertiliser. J. Anhui Agric. Univ. 2022, 49, 735–740. [Google Scholar]
- Saxena, C.; Samantaray, S.; Rout, G.R.; Das, P. Effect of Auxins on in vitro Rooting of Plumbago Zeylanica: Peroxidase Activity as a Marker for Root Induction. Biol. Plant. 2000, 43, 121–124. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Hausman, J.F.; Berthon, J.Y.; Ripetti, V. Practical uses of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomie 1992, 12, 757–765. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Hausman, J.F.; Ripetti, V. Peroxidase activity and endogenous free auxin during adventitious root formation. In Growth and Development of Plants in Culture; Springer: Dordrecht, The Netherlands, 1994; pp. 289–298. [Google Scholar]
- Rahou, Y.A.; Boutaj, H.; Boutasknit, A.; Douira, A.; Benkirane, R.; Modafar, C.; Meddich, A. Colonization of tomato roots with arbuscular mycorrhizal fungi changes of antioxidative activity and improves tolerance to Verticillium dahliae. Plany Cell Biotechnol. Mol. Biol. 2021, 22, 65–81. [Google Scholar]
- Ur Rahman, S.; Khalid, M.; Hui, N.; Rehman, A.; Kayani, S.L.; Fu, X. Piriformospora indica alter root-associated microbiome structure to enhance Artemisia annua L. tolerance to arsenic. J. Hazard. Mater. 2023, 457, 131752. [Google Scholar] [CrossRef]
- Santos, B.A.; Maia, L.C.; Cavalcante, U.M.T.; Correia, M.T.S.; Coelho, L. Effect of arbuscular mycorrhizal fungi and soil phosphorus level on expression of protein and activity of peroxidase on passion fruit roots. Braz. J. Biol. 2001, 61, 693–700. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Fu, Y.; Shao, J.; Liu, Y.; Xuan, W.; Zhang, R. Signal communication during microbial modulation of root system architecture. J. Exp. Bot. 2024, 75, 526–537. [Google Scholar] [CrossRef]
- Lin, P.C.; Lilananda, I.; Shao, K.H.; Wu, H.Y.; Wang, S.J. Role of auxin in the symbiotic relationship between Piriformospora indica and rice plants. Rhizosphere 2023, 25, 100632. [Google Scholar] [CrossRef]
- Inaji, A.; Okazawa, A.; Taguchi, T.; Nakamoto, M.; Katsuyama, N.; Yoshikawa, R.; Ohta, D. Rhizotaxis modulation in arabidopsis is induced by diffusible compounds produced during the cocultivation of arabidopsis and the endophytic fungus Serendipita indica. Plant Cell Physiol. 2020, 61, 838–850. [Google Scholar] [CrossRef]
- Vahabi, K.; Sherameti, I.; Bakshi, M.; Mrozinska, A.; Ludwig, A.; Reichelt, M.; Oelmuller, R. The interaction of Arabidopsis with Piriformospora indica shifts from initial transient stress induced by fungus-released chemical mediators to a mutualistic interaction after physical contact of the two symbionts. BMC Plant Biol. 2015, 15, 58. [Google Scholar] [CrossRef]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronien, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef]
- Huang, Y.P.; Wang, S.L.; Shi, L.; Xu, F.S. JASMONATE RESISTANT 1 negatively regulates root growth under boron deficiency in Arabidopsis. J. Exp. Bot. 2021, 72, 3108–3121. [Google Scholar] [CrossRef]
- Yoshida, Y.; Sano, R.; Wada, T.; Takabayashi, J.; Okada, K. Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 2009, 136, 1039–1048. [Google Scholar] [CrossRef]
- Xie, Y.; Li, A.N.; Ding, Z.J.; Zhen, S.J. Progress of ethylene-regulated root hair development in plants. Plant Physiol. J. 2020, 56, 2517–2525. [Google Scholar]
- Negi, S.; Sukumar, P.; Liu, X.; Cohen, J.D.; Muday, G.K. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant 2010, 61, 3–15. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef]
- Cheng, C.Z.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Lai, Z. The root endophytic fungus Serendipita indica improves resistance of Banana to Fusarium oxysporum f. sp. cubense tropical race 4. Plant Pathol. 2020, 156, 87–100. [Google Scholar]
- Chen, J.X.; Wang, X.F. Experimental Guide to Plant Physiology; South China University of Technology Press: Guangzhou, China, 2002; pp. 119–124. [Google Scholar]


| 2W | 3W | 4W | 5W | |||||
|---|---|---|---|---|---|---|---|---|
| CK | PI | CK | PI | CK | PI | CK | PI | |
| Rootlet length (cm) | 4.09 ± 0.10 | 6.49 ± 0.05 | 6.12 ± 0.12 | 7.08 ± 0.11 | 7.79 ± 0.10 | 8.28 ± 0.09 ** | 9.40 ± 0.10 | 10.03 ± 0.21 ** |
| Lateral root number | 3.00 ± 0.58 | 4.00 ± 1.00 | 5.00 ± 0.96 | 7.00 ± 0.82 | 7.00 ± 1.50 | 10.25 ± 0.96 * | 10.67 ± 1.15 | 12.33 ± 0.58 * |
| Plant height (cm) | 5.66 ± 0.66 | 6.63 ± 0.55 | 7.36 ± 0.32 | 8.88 ± 0.44 ** | 8.32 ± 0.16 | 13.03 ± 0.49 ** | 16.57 ± 1.06 | 20.20 ± 0.69 ** |
| Stem thickness | 1.13 ± 0.14 | 1.12 ± 0.15 | 1.44 ± 0.12 | 1.61 ± 0.04 | 1.56 ± 0.06 | 1.78 ± 0.04 | 1.95 ± 0.04 | 2.12 ± 0.01 |
| Blade number | 4.67 ± 0.58 | 4.33 ± 0.58 | 6.25 ± 0.50 | 8.75 ± 0.96 * | 12.00 ± 0.82 | 13.75 ± 0.96 * | 16.00 ± 0.66 | 17.67 ± 0.58 * |
| Aboveground part fresh | 0.29 ± 0.03 | 0.50 ± 0.04 | 0.39 ± 0.04 | 0.53 ± 0.05 | 0.42 ± 0.03 | 0.84 ± 0.04 ** | 1.15 ± 0.06 | 1.33 ± 0.03 ** |
| Root fresh weight (g) | 0.16 ± 0.02 | 0.42 ± 0.03 | 0.31 ± 0.03 | 0.45 ± 0.03 | 0.34 ± 0.04 | 0.60 ± 0.02 ** | 0.53 ± 0.03 | 1.23 ± 0.03 ** |
| Plant fresh weight (g) | 0.45 ± 0.05 | 0.94 ± 0.05 | 0.70 ± 0.07 | 0.98 ± 0.06 | 0.76 ± 0.07 | 1.44 ± 0.05 ** | 1.68 ± 0.08 | 2.56 ± 0.05 ** |
| Aboveground part dry | 0.14 ± 0.03 | 0.18 ± 0.03 | 0.16 ± 0.05 | 0.27 ± 0.04 | 0.19 ± 0.02 | 0.35 ± 0.02 ** | 0.39 ± 0.04 | 0.59 ± 0.04 ** |
| Root dry weight (g) | 0.10 ± 0.02 | 0.13 ± 0.03 | 0.12 ± 0.03 | 0.17 ± 0.04 | 0.16 ± 0.02 | 0.25 ± 0.03 ** | 0.26 ± 0.05 | 0.40 ± 0.04 ** |
| Plant dry weight (g) | 0.24 ± 0.05 | 0.31 ± 0.06 | 0.27 ± 0.05 | 0.44 ± 0.04 | 0.35 ± 0.03 | 0.60 ± 0.06 ** | 0.64 ± 0.02 | 0.98 ± 0.04 ** |
| 2W | 3W | 4W | 5W | |||||
|---|---|---|---|---|---|---|---|---|
| CK | PI | CK | PI | CK | PI | CK | PI | |
| Rootlet length (cm) | 7.41 ± 0.10 | 7.59 ± 0.26 | 8.18 ± 0.14 | 9.82 ± 0.10 * | 9.07 ± 0.15 | 10.26 ± 0.28 ** | 9.27 ± 0.32 | 10.93 ± 0.15 ** |
| Lateral root number | 3.67 ± 0.58 | 5.00 ± 1.00 | 4.33 ± 0.58 | 5.67 ± 0.95 | 5.00 ± 1.00 | 6.00 ± 1.00 | 5.67 ± 0.58 | 7.00 ± 0.58 * |
| Plant height (cm) | 7.43 ± 0.41 | 8.13 ± 0.44 | 8.35 ± 0.19 | 9.43 ± 0.55 | 10.76 ± 0.46 | 14.37 ± 0.12 ** | 13.63 ± 0.32 | 16.77 ± 0.50 ** |
| Stem thickness | 1.58 ± 0.04 | 1.47 ± 0.08 | 1.62 ± 0.03 | 1.64 ± 0.03 | 1.67 ± 0.05 | 1.68 ± 0.05 | 1.68 ± 0.03 | 1.70 ± 0.02 |
| Blade number | 5.55 ± 0.47 | 5.67 ± 0.58 | 6.33 ± 0.58 | 6.67 ± 0.58 | 10.33 ± 0.65 | 12.67 ± 0.58 | 11.33 ± 0.96 | 14.00 ± 0.85 * |
| Aboveground part fresh | 0.33 ± 0.02 | 0.45 ± 0.03 | 0.41 ± 0.03 | 0.50 ± 0.02 | 0.53 ± 0.04 | 0.73 ± 0.05 * | 0.56 ± 0.05 | 0.81 ± 0.03 * |
| Root fresh weight (g) | 0.30 ± 0.01 | 0.36 ± 0.02 | 0.32 ± 0.02 | 0.37 ± 0.02 | 0.30 ± 0.03 | 0.33 ± 0.01 | 0.40 ± 0.03 | 0.39 ± 0.03 |
| Plant fresh weight (g) | 0.63 ± 0.04 | 0.81 ± 0.04 | 0.73 ± 0.01 | 0.87 ± 0.01 | 0.83 ± 0.05 | 1.06 ± 0.03 * | 0.96 ± 0.06 | 1.20 ± 0.04 * |
| Aboveground part dry | 0.18 ± 0.03 | 0.21 ± 0.02 | 0.23 ± 0.03 | 0.26 ± 0.03 | 0.26 ± 0.02 | 0.35 ± 0.05 | 0.28 ± 0.04 | 0.47 ± 0.03 ** |
| Root dry weight (g) | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.17 ± 0.02 | 0.18 ± 0.02 | 0.16 ± 0.01 | 0.19 ± 0.01 | 0.22 ± 0.03 | 0.24 ± 0.02 ** |
| Plant dry weight (g) | 0.30 ± 0.03 | 0.34 ± 0.04 | 0.40 ± 0.05 | 0.45 ± 0.03 | 0.42 ± 0.02 | 0.55 ± 0.09 | 0.50 ± 0.02 | 0.70 ± 0.04 ** |
| 09-01 | Nongda 7 | |||
|---|---|---|---|---|
| CK | PI | CK | PI | |
| Length (m) | 0.80 ± 0.01 | 0.94 ± 0.03 ** | 0.74 ± 0.02 | 1.32 ± 0.02 ** |
| Average diameter (mm) | 0.86 ± 0.03 | 0.94 ± 0.05 * | 0.57 ± 0.06 | 1.06 ± 0.04 ** |
| Volume (mm3) | 551.36 ± 13.18 | 1043.56 ± 17.91 ** | 924.50 ± 28.77 | 870.68 ± 32.78 |
| Surface area (mm2) | 1440.02 ± 23.73 | 2594.65 ± 37.31 ** | 2204.5 ± 81.58 | 1990.56 ± 91.23 * |
| Projected area (mm2) | 458.37 ± 7.56 | 825.90 ± 9.40 ** | 791.16 ± 7.54 | 612.39 ± 5.53 * |
| Number of root tips | 61.33 ± 4.04 | 111.67 ± 7.02 ** | 93.00 ± 2.65 | 144.00 ± 2.00 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Qu, P.; Wang, D.; Yan, S.; Gong, Q.; Yang, R.; Hu, Y.; Liu, N.; Cheng, C.; Wang, P.; et al. The Influence of Piriformospora indica Colonization on the Root Development and Growth of Cerasus humilis Cuttings. Plants 2024, 13, 1482. https://doi.org/10.3390/plants13111482
Yin L, Qu P, Wang D, Yan S, Gong Q, Yang R, Hu Y, Liu N, Cheng C, Wang P, et al. The Influence of Piriformospora indica Colonization on the Root Development and Growth of Cerasus humilis Cuttings. Plants. 2024; 13(11):1482. https://doi.org/10.3390/plants13111482
Chicago/Turabian StyleYin, Lu, Pengyan Qu, Dongmei Wang, Songtao Yan, Qinghua Gong, Rui Yang, Yang Hu, Niru Liu, Chunzhen Cheng, Pengfei Wang, and et al. 2024. "The Influence of Piriformospora indica Colonization on the Root Development and Growth of Cerasus humilis Cuttings" Plants 13, no. 11: 1482. https://doi.org/10.3390/plants13111482





