Culm Morphological Analysis in Moso Bamboo Reveals the Negative Regulation of Internode Diameter and Thickness by Monthly Precipitation
Abstract
:1. Introduction
2. Results
2.1. Culm Heights
2.2. Culm Heights under the First Branch
2.3. Ratios of Culm Heights under the First Branch to the Total Culm Height
2.4. Internode Number under the First Branch
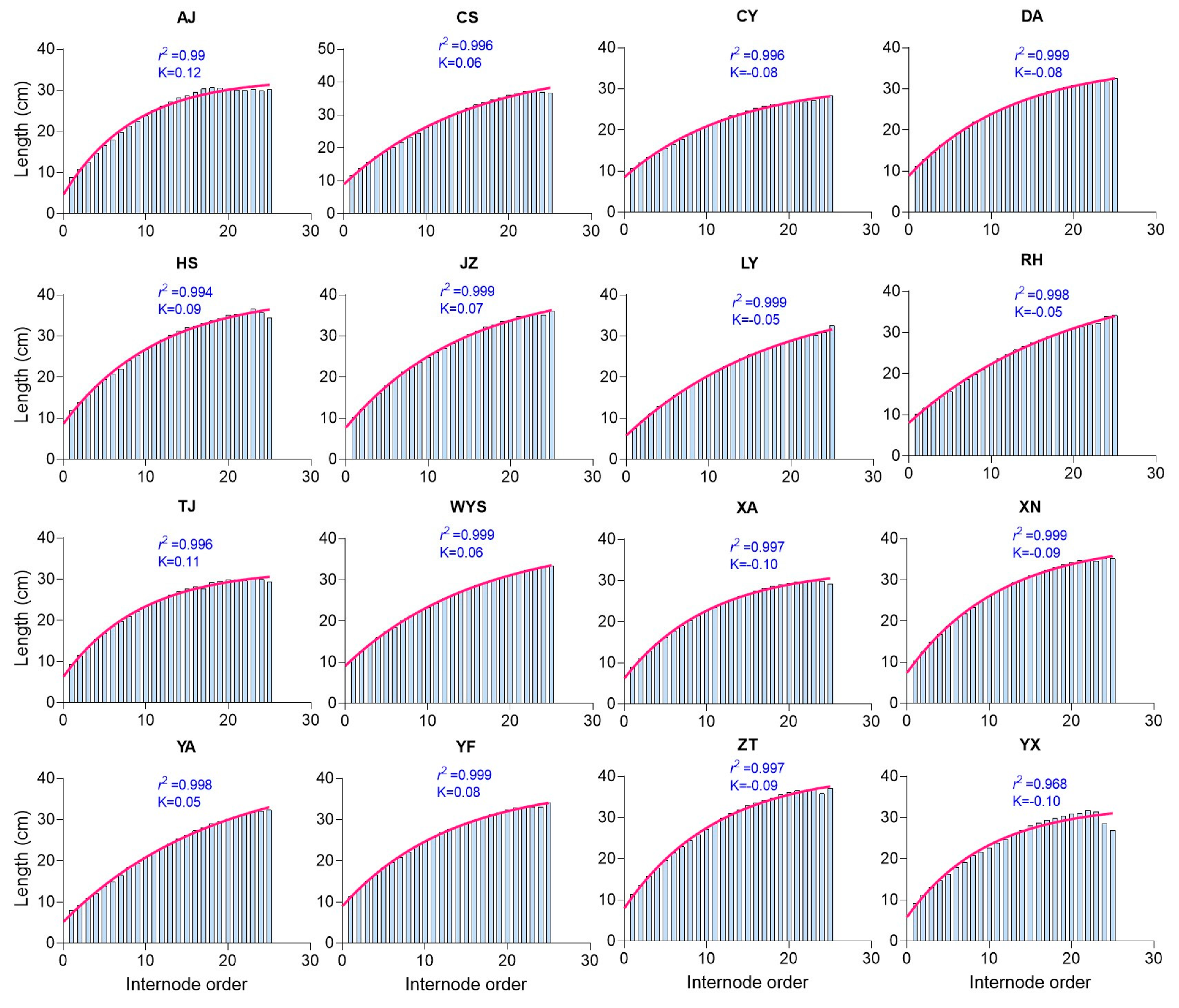
2.5. Internode Lengths
2.6. Internode Diameters
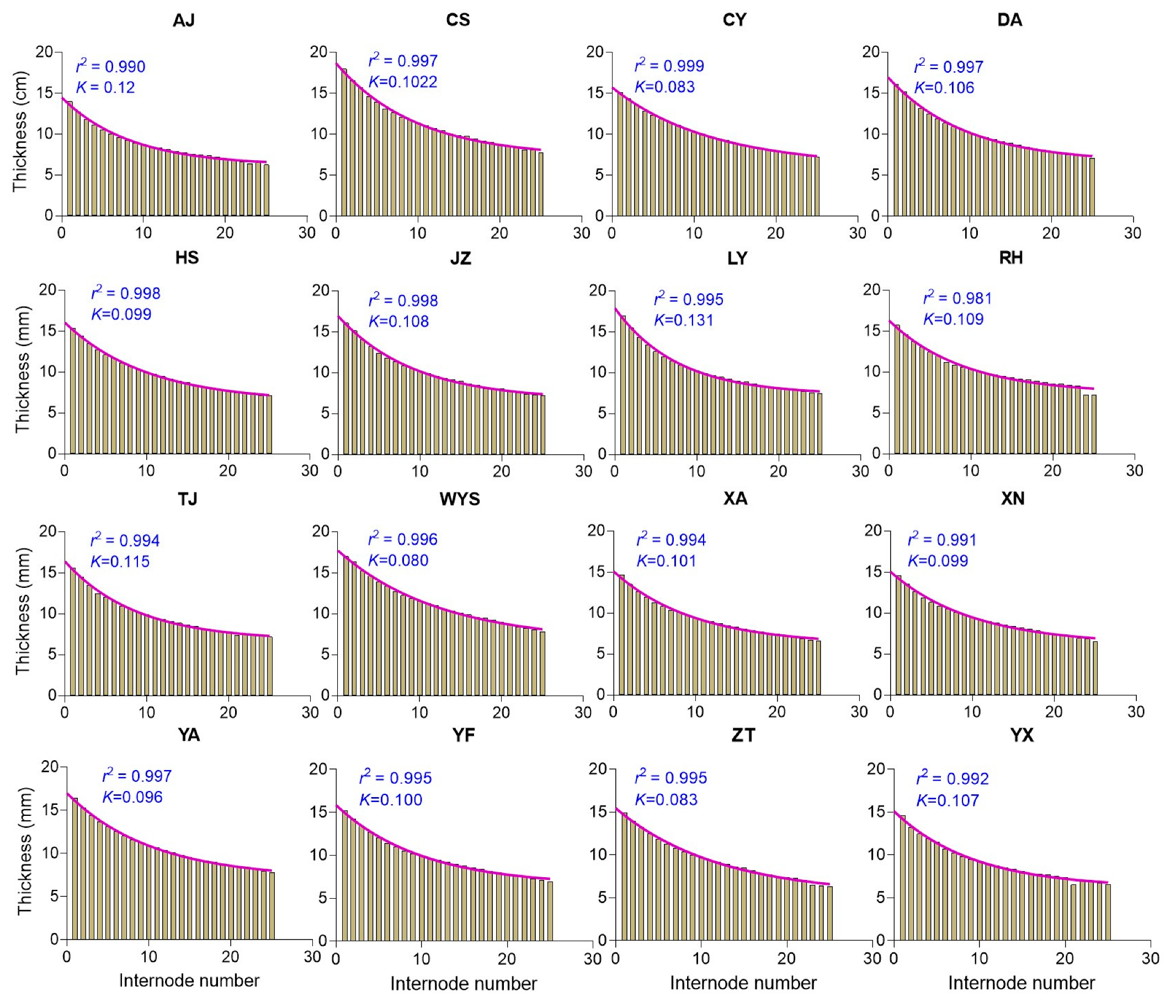
2.7. Internode Wall Thicknesses
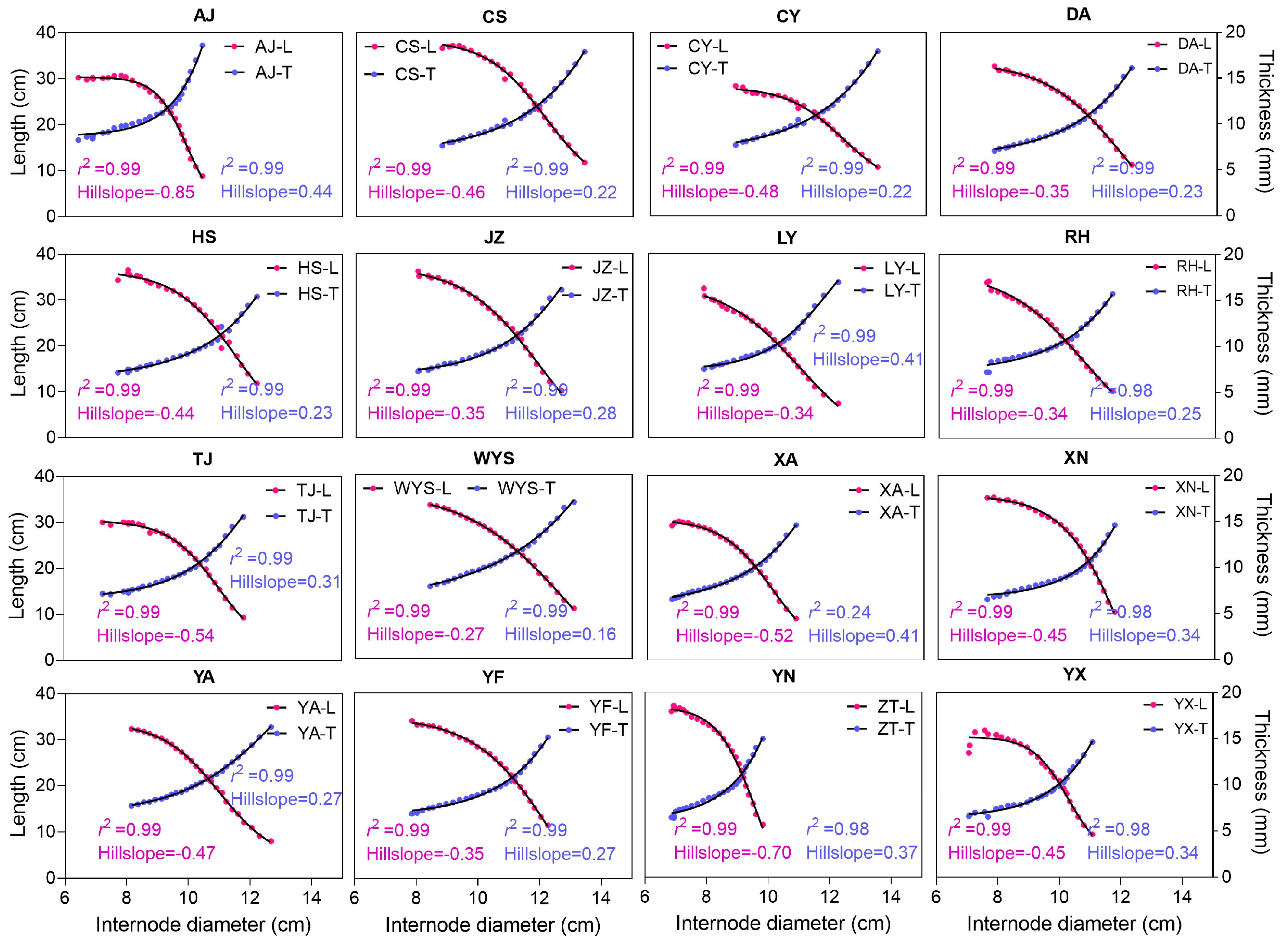
2.8. Correlation Analysis between Culm Parameters
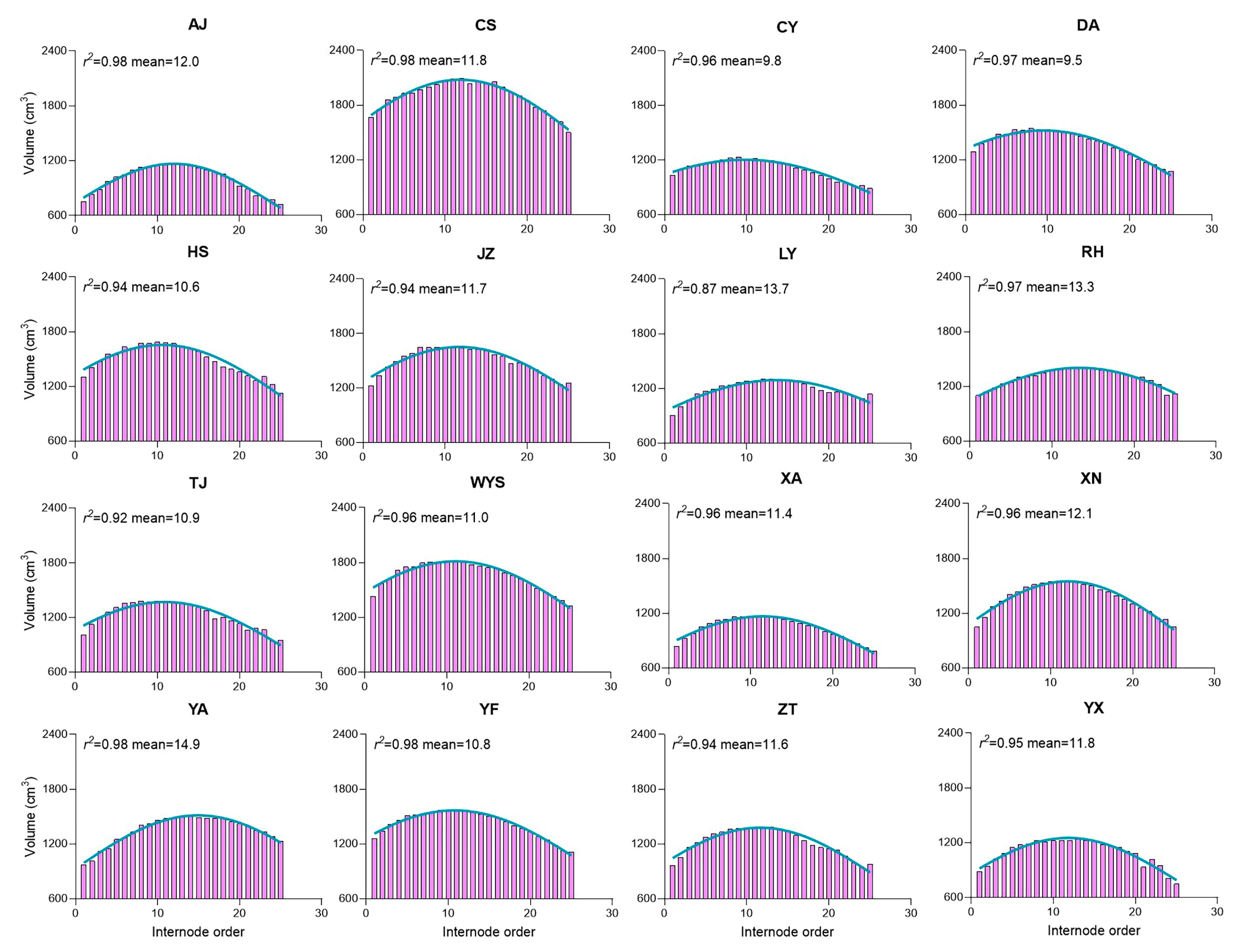
2.9. Culm Parameters Tend to Increase or Decrease Together to Influence Culm Height
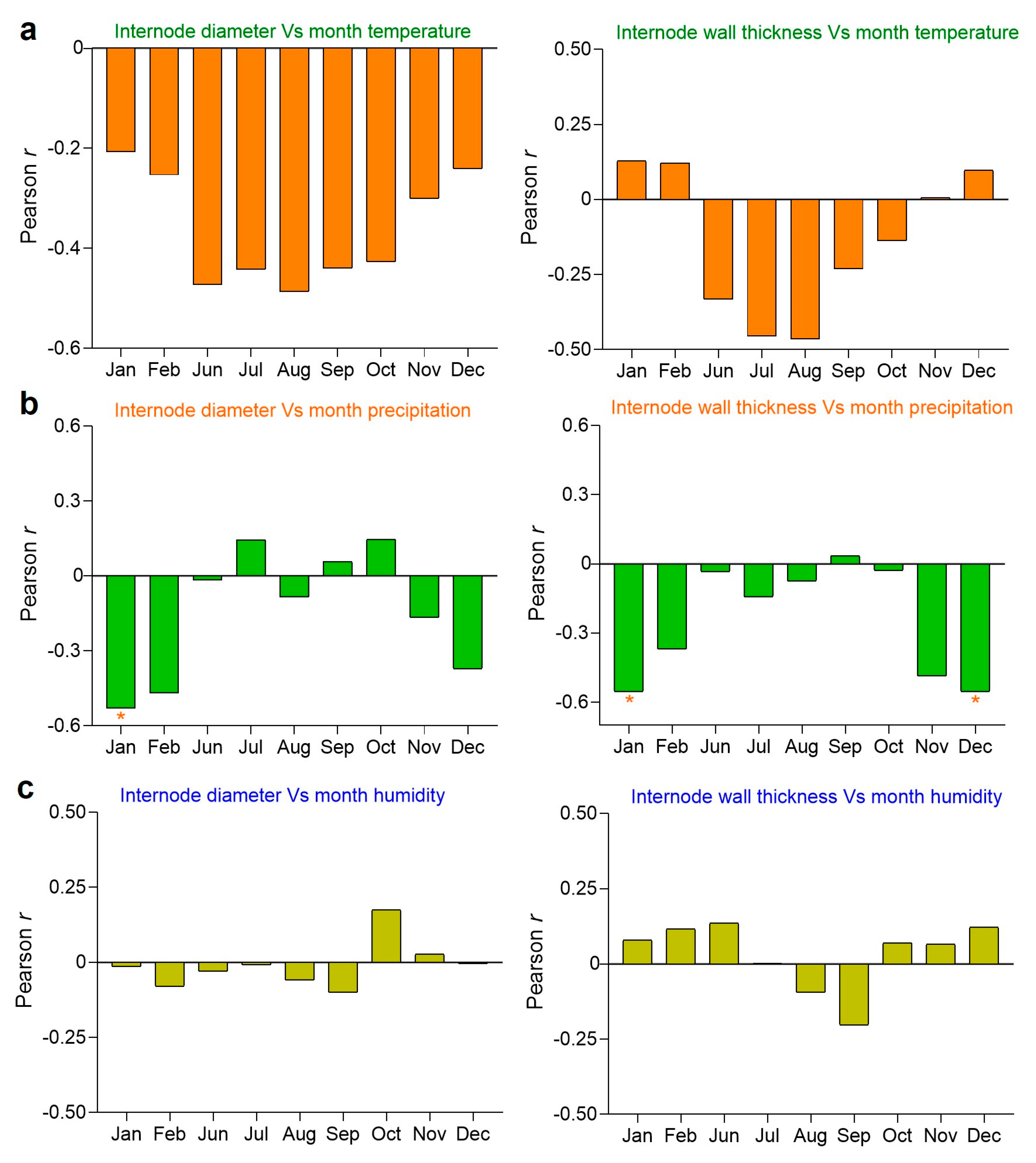
2.10. Influence of Environmental Factors on Internode Diameter and Thickness
3. Discussion
3.1. Bamboo Culm Material
3.2. Culm Morphological Variations among the Populations
3.3. Both Diameter and Thickness Significantly Contribute to Culm Development
3.4. Moso Bamboo Exhibits a Structural Resemblance to Building Construction Patterns
3.5. Monthly Precipitation Negatively Regulates Internode Diameter and Thickness
4. Materials and Methods
4.1. Plant Materials and Sampling Regions
4.2. Morphological Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, G.R.; Long, T.T.; Yanxia, L. A Review of International Bamboo and Timber Trade Regulations. A Multijurisdictional Study; International Bamboo and Rattan Organisation (INBAR): Beijing, China, 2021; pp. 1–70. [Google Scholar]
- Zhao, H.; Wang, J.; Meng, Y.; Li, Z.; Fei, B.; Das, M.; Jiang, Z. Bamboo and Rattan: Nature-based solutions for sustainable development. Innovation 2022, 3, 100337. [Google Scholar] [CrossRef]
- Razal, R.A. Enhancing sustainability and productivity of bamboo in the Philippines: Some initiatives. In World Forests XXV, Non-Wood Forest Products of Asia: Knowledge, Conservation and Lvelihood; Rashid, A.Z.M.M., Khan, N.A., Hossain, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 25, pp. 151–178. [Google Scholar]
- Marchi, M.; Marchettini, N.; Neri, E.; Esposito, G.; Niccolucci, V.; Pulselli, F.M.; Lajo, M.; Rissone, E.; Pulselli, R.M. Carbon footprint offset of a managed bamboo plantation in temperate regions. Sustain. Prod. Consum. 2023, 40, 220–235. [Google Scholar] [CrossRef]
- Liu, E.B.; Ren, Z.X.; Yao, H.W.; Zhou, G.M. Multi-scale estimation of carbon storage for Moso bamboo (Phyllostachys edulis) based on the mixed weibull density function. Glob. Ecol. Conserv. 2021, 31, e01856. [Google Scholar] [CrossRef]
- Xu, C.H.; Mao, F.J.; Du, H.Q.; Li, X.J.; Sun, J.Q.; Ye, F.F.; Zheng, Z.D.; Teng, X.F.; Yang, N.X. Full phenology cycle carbon flux dynamics and driving mechanism of Moso bamboo forest. Front. Plant Sci. 2024, 15, 1359265. [Google Scholar] [CrossRef]
- Chiti, T.; Blasi, E.; Chiriacò, M.V. Carbon sequestration in a bamboo plantation: A case study in a Mediterranean area. J. For. Res. 2024, 35, 51. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Yrjälä, K.; Vinod, K.K.; Sharma, A.; Cho, J.; Satheesh, V.; Zhou, M. Genetics and genomics of Moso bamboo (Phyllostachys edulis): Current status, future challenges, and biotechnological opportunities toward a sustainable bamboo industry. Food Energy Secur. 2020, 9, e229. [Google Scholar] [CrossRef]
- Dlamini, L.C.; Fakudze, S.; Makombe, G.G.; Muse, S.; Zhu, J. Bamboo as a valuable resource and its utilization in historical and modern-day China. BioResources 2022, 17, 1926–1938. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Z.; Yang, K.; Lou, Y.; Liu, Y.; Li, T.; Li, H.; Di, X.; Wang, J.; Sun, H.; et al. A bamboo ‘PeSAPK4-PeMYB99-PeTIP4-3’ regulatory model involved in water transport. New Phytol. 2024, 1–18. [Google Scholar] [CrossRef]
- Feng, P.; Li, Y. China’s bamboo resources in 2021. World Bamboo Ratt. 2023, 21, 100–103. [Google Scholar]
- Kim, Y.; Kim, B.; Park, H.; Choi, Y.-H.; Kim, K.-J.; Park, S.-Y. Improvement of Moso bamboo (Phyllostachys pubescens) properties using a heat treatment process for landscaping materials and evaluation of its durability against biotic/abiotic factors. Forests 2024, 15, 101. [Google Scholar] [CrossRef]
- Zhu, C.L.; Lin, Z.M.; Liu, Y.; Li, H.; Di, X.L.; Li, T.K.; Wang, J.F.; Gao, Z.M. A bamboo bHLH transcription factor PeRHL4 has dual functions in enhancing drought and phosphorus starvation tolerance. Plant Cell Environ. 2024, 1–15. [Google Scholar] [CrossRef]
- Ding, Y.; Zou, L.-H.; Ramakrishnan, M.; Chen, Y.; Zhu, B.; Yu, L.; Zhou, M. Abiotic stress-induced DNA methylation in transposable elements and their transcripts reveals a multi-layered response in Moso bamboo. Ind. Crops Prod. 2024, 210, 118108. [Google Scholar] [CrossRef]
- Zheng, H.F.; Xie, Y.L.; Mu, C.H.; Cheng, W.L.; Bai, Y.C.; Gao, J. Deciphering the regulatory role of PheSnRK genes in Moso bamboo: Insights into hormonal, energy, and stress responses. BMC Genom. 2024, 25, 252. [Google Scholar] [CrossRef]
- Guo, H.; Tan, J.Q.; Jiao, Y.; Huang, B.; Ma, R.F.; Ramakrishnan, M.; Qi, G.N.; Zhang, Z.J. Genome-wide identification and expression analysis of the HAK/KUP/KT gene family in Moso bamboo. Front. Plant Sci. 2024, 15, 1331710. [Google Scholar] [CrossRef]
- Jiao, Y.; Tan, J.Q.; Guo, H.; Huang, B.; Ying, Y.Q.; Ramakrishnan, M.; Zhang, Z.J. Genome-wide analysis of the KNOX gene family in Moso bamboo: Insights into their role in promoting the rapid shoot growth. BMC Plant Biol. 2024, 24, 213. [Google Scholar] [CrossRef]
- Dong, K.; Lan, L.Z.; Liu, M.Y.; Ge, B.H.; Bi, X.R.; Liu, Y.J.; Geng, X.; Chen, Y.Z.; Lu, C.F. Genome-wide identification, evolutionary and expression analysis of cyclin-dependent kinase gene family members in Moso bamboo (Phyllostachys edulis). J. Plant Growth Regul. 2024, 1–13. [Google Scholar] [CrossRef]
- Huang, F.Y.; Wang, J.X.; Tang, C. Genome-wide identification and analysis of ZF-HD gene family in Moso bamboo (Phyllostachys edulis). Plants 2023, 12, 4064. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.C.; Xie, Y.L.; Cai, M.M.; Jiang, J.T.; Wu, C.Y.; Zheng, H.F.; Gao, J. GA20ox family genes mediate gibberellin and auxin crosstalk in Moso bamboo (Phyllostachys edulis). Plants 2023, 12, 2842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Wang, H.H.; Wang, H.Y.; Zhou, R.F.; Wu, J.; Zhang, Z.K.; Jin, Y.D.; Li, T.; Kohnen, M.V.; Liu, X.Q.; et al. Multi-omics of circular RNAs and their responses to hormones in Moso bamboo (Phyllostachys edulis). Genom. Proteom. Bioinf. 2023, 21, 866–885. [Google Scholar] [CrossRef]
- Zhu, C.L.; Yuan, T.T.; Yang, K.B.; Liu, Y.; Li, Y.; Gao, Z.M. Identification and characterization of circRNA-associated CeRNA networks in Moso bamboo under nitrogen stress. BMC Plant Biol. 2023, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Liu, Z.H.; Yan, J.W.; Shi, W.H.; Ying, Y.Q. Genome-wide identification of SPX family genes and functional characterization of PeSPX6 and PeSPX-MFS2 in response to low phosphorus in Phyllostachys edulis. Plants 2023, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, D.Q. Comprehensive identification and expression analysis of CAMTA gene family in Phyllostachys edulis under abiotic stress. PeerJ 2023, 11, e15358. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Ge, B.H.; Liu, Y.J.; Wang, X.J.; Dong, K.; Zhang, Y.; Chen, Y.Z.; Lu, C.F. Genome-wide identification and functional analysis of silicon transporter family genes in Moso bamboo (Phyllostachys edulis). Int. J. Biol. Macromol. 2022, 223, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Yang, Y.; Kong, B.; Song, X.L.; Gao, Z.M.; Li, X.P. Identification and characterization of circRNAs under drought stress in Moso bamboo (Phyllostachys edulis). Forests 2022, 13, 426. [Google Scholar] [CrossRef]
- Xie, Y.L.; Zheng, H.F.; Bai, Y.C.; Xu, J.L.; Song, H.J.; Cao, Z.H.; Wu, C.Y.; Mu, C.H.; Jiang, J.T.; Gao, J. Photosynthesis in culms of non-foliar organs and effect of light on culm development of Moso bamboo (Phyllostachys edulis). Environ. Exp. Bot. 2024, 220, 105672. [Google Scholar] [CrossRef]
- Sun, H.Y.; Lou, Y.F.; Li, H.; Di, X.L.; Gao, Z.M. Unveiling the intrinsic mechanism of photoprotection in bamboo under high light. Ind. Crops Prod. 2024, 209, 118049. [Google Scholar] [CrossRef]
- Bai, Y.C.; Cai, M.M.; Mu, C.H.; Zheng, H.F.; Cheng, Z.C.; Xie, Y.L.; Gao, J. Integrative analysis of exogenous auxin mediated plant height regulation in Moso bamboo (Phyllostachys edulis). Ind. Crops Prod. 2023, 200, 116852. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sun, X.P.; Ding, Y.L.; Fei, Z.J.; Jiao, C.; Fan, M.Y.; Yao, B.F.; Xin, P.Y.; Chu, J.F.; Wei, Q. Cellular and molecular characterization of a thick-walled variant reveal a pivotal role of shoot apical meristem in transverse development of bamboo culm. J. Exp. Bot. 2019, 70, 3911–3926. [Google Scholar] [CrossRef]
- Wei, Q.; Jiao, C.; Guo, L.; Ding, Y.; Cao, J.; Feng, J.; Dong, X.; Mao, L.; Sun, H.; Yu, F.; et al. Exploring key cellular processes and candidate genes regulating the primary thickening growth of Moso underground shoots. New Phytol. 2017, 214, 81–96. [Google Scholar] [CrossRef]
- Cui, K.; He, C.-Y.; Zhang, J.-G.; Duan, A.-G.; Zeng, Y.-F. Temporal and spatial profiling of internode elongation-associated protein expression in rapidly growing culms of bamboo. J. Proteome Res. 2012, 11, 2492–2507. [Google Scholar] [CrossRef]
- Wei, Q.; Guo, L.; Jiao, C.; Fei, Z.J.; Chen, M.; Cao, J.J.; Ding, Y.L.; Yuan, Q.S. Characterization of the developmental dynamics of the elongation of a bamboo internode during the fast growth stage. Tree Physiol. 2019, 39, 1201–1214. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Z.; Ma, Y.; Bai, Q.; Li, X.; Cao, Z.; Wu, Z.; Gao, J. The association of hormone signalling genes, transcription and changes in shoot anatomy during Moso bamboo growth. Plant Biotechnol. J. 2018, 16, 72–85. [Google Scholar] [CrossRef]
- Li, L.; Zhou, B.A.; Liu, D.; Wu, H.Y.; Shi, Q.Q.; Lin, S.Y.; Yao, W.J. Transcriptomic complexity of culm growth and development in different types of Moso bamboo. Int. J. Mol. Sci. 2023, 24, 7425. [Google Scholar] [CrossRef]
- Li, Y.; Vasupalli, N.; Cai, O.; Lin, X.F.; Wu, H.Y. Network of miR396-mRNA in tissue differentiation in Moso bamboo (Phyllostachys edulis). Plants 2023, 12, 1103. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Zhang, Y.; Hu, T.; Mu, S.; Li, X.; Gao, J. Transcriptome sequencing and analysis of the fast growing shoots of Moso bamboo (Phyllostachys edulis). PLoS ONE 2013, 8, e78944. [Google Scholar] [CrossRef]
- Gamuyao, R.; Nagai, K.; Ayano, M.; Mori, Y.; Minami, A.; Kojima, M.; Suzuki, T.; Sakakibara, H.; Higashiyama, T.; Ashikari, M. Hormone distribution and transcriptome profiles in bamboo shoots provide insights on bamboo stem emergence and growth. Plant Cell Physiol. 2017, 58, 702–716. [Google Scholar] [CrossRef]
- Tao, G.-Y.; Ramakrishnan, M.; Vinod, K.K.; Yrjälä, K.; Satheesh, V.; Cho, J.; Fu, Y.; Zhou, M. Multi-omics analysis of cellular pathways involved in different rapid growth stages of Moso bamboo. Tree Physiol. 2020, 40, 1487–1508. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Zhang, Y.; Zhang, H.M.; Lin, X.C.; Xia, R.; Song, L.; Wu, A.M. MicroRNAs play important roles in regulating the rapid growth of the Phyllostachys edulis culm internode. New Phytol. 2021, 231, 2215–2230. [Google Scholar] [CrossRef]
- Bai, Y.C.; Cai, M.M.; Mu, C.H.; Cheng, W.L.; Zheng, H.F.; Cheng, Z.C.; Li, J.; Mu, S.H.; Gao, J. New insights into the local auxin biosynthesis and its effects on the rapid growth of Moso bamboo (Phyllostachys edulis). Front. Plant Sci. 2022, 13, 858686. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Zhang, Y.; Wang, H.; Zhang, Z.; Liu, X.; Zhang, Z.; Liu, K.; Yang, D.; Zhang, H.; et al. Comprehensive profiling of epigenetic modifications in fast-growing Moso bamboo shoots. Plant Physiol. 2022, 191, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, S.; Hu, J.; Zou, X.; Tie, L.; Li, Y.; Cui, X.; Huang, C.; Sardans, J.; Peñuelas, J. Variations and trade-offs in leaf and culm functional traits among 77 woody bamboo species. BMC Plant Biol. 2024, 24, 387. [Google Scholar] [CrossRef] [PubMed]
- Zha, R.; Chen, T.; Liu, Q.; Wei, Q.; Que, F. Morphological and anatomical analysis of the internodes of a new dwarf variant of Moso bamboo, Phyllostachys edulis f. exaurita. Plants 2023, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, G.; Xu, J.; Jin, K.; Fan, M.; Ding, Y.; Wei, Q.; Zhuo, R. Anatomical characteristics and variation mechanisms on the thick-walled and dwarfed culm of Shidu bamboo (Phyllostachys nidularia f. farcta). Front. Plant Sci. 2022, 13, 876658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, T.; Chang, Y.; Fei, B.; Ma, Y.; Deng, Y.; Xia, M.; Fan, K.; Zhang, X.; Jiang, Z. Correlation between genetic characteristics, cell structure and material properties of Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) in different areas of China. Forests 2022, 13, 107. [Google Scholar] [CrossRef]
- Huanying, F.; Wenhui, S.; Manyi, D.; Guanglu, L.; Shaohui, F. An index to assess the health of Moso bamboo (Phyllostachys edulis) forest. J. For. Res. 2023, 28, 453–462. [Google Scholar] [CrossRef]
- Zhao, H.S.; Sun, S.; Ding, Y.L.; Wang, Y.; Yue, X.H.; Du, X.; Wei, Q.; Fan, G.Y.; Sun, H.Y.; Lou, Y.F.; et al. Analysis of 427 genomes reveals Moso bamboo population structure and genetic basis of property traits. Nat. Commun. 2021, 12, 5466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, C.; Yue, X.; Lin, Z.; Li, H.; Di, X.; Wang, J.; Gao, Z. Evolutionary relationship of Moso bamboo forms and a multihormone regulatory cascade involving culm shape variation. Plant Biotechnol. J. 2024, 1–15. [Google Scholar] [CrossRef]
- Guo, L.; Chen, T.; Chu, X.; Sun, K.; Yu, F.; Que, F.; Ahmad, Z.; Wei, Q.; Ramakrishnan, M. Anatomical and transcriptome analyses of Moso bamboo culm neck growth: Unveiling key insights. Plants 2023, 12, 3478. [Google Scholar] [CrossRef]
- Ma, P.F.; Liu, Y.L.; Guo, C.; Jin, G.H.; Guo, Z.H.; Mao, L.; Yang, Y.Z.; Niu, L.Z.; Wang, Y.J.; Clark, L.G.; et al. Genome assemblies of 11 bamboo species highlight diversification induced by dynamic subgenome dominance. Nat. Genet. 2024, 56, 710–720. [Google Scholar] [CrossRef]
- Ouyang, M.; Yang, C.; Tian, D.; Pan, J.; Chen, G.; Su, H.; Yan, Z.; Ji, C.; Tang, Z.; Fang, J. A field-based estimation of Moso bamboo forest biomass in China. For. Ecol. Manag. 2022, 505, 119885. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, S.; Wei, Q.; Yao, W.; Que, F.; Li, L. Advances in developmental biology of bamboos. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 44, 23–40. [Google Scholar]
- Zhang, Z.; Rao, F.; Wang, Y. Morphological, chemical, and physical-mechanical properties of a clumping bamboo (Thyrsostachys oliveri) for construction applications. Polymers 2022, 14, 3681. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics (Statistical Package for the Social Sciences) for Windows, Version 28.0, Released 2021; IBM Corp.: Armonk, NY, USA, 2021.


| D | T | D Slope | T Slope | DT Slope | DL Slope | TL Slope | L | H | HUFB | INUFB | IV | CVHUFB | Ratio FBRS | Vmax IS | Vmax in ratio | Vmax H ratio | |
| D | 1 | ||||||||||||||||
| T | 0.69 * | 1 | |||||||||||||||
| D Slope | −0.85 * | −0.37 | 1 | ||||||||||||||
| T Slope | 0.04 | −0.23 | 0.47 | 1 | |||||||||||||
| DT Slope | −0.62 * | −0.67 * | −0.61 * | 0.54 * | 1 | ||||||||||||
| DL Slope | 0.62 * | 0.54 * | −0.38 | −0.07 | −0.62 * | 1 | |||||||||||
| TL Slope | 0.52 * | 0.64 * | −0.23 | −0.11 | −0.42 | 0.67 * | 1 | ||||||||||
| L | 0.48 | 0.27 | −0.43 | −0.38 | −0.39 | 0.10 | 0.07 | 1 | |||||||||
| H | 0.71 * | 0.66 * | −0.45 | −0.41 | −0.74 | 0.65 * | 0.50 * | 0.66 * | 1 | ||||||||
| HUFB | 0.63 * | 0.47 | −0.39 | −0.35 | −0.66 * | 0.42 | 0.25 | 0.69 * | 0.87 * | 1 | |||||||
| INUFB | 0.44 | 0.40 | −0.60 | −0.25 | −0.56 * | 0.52 * | 0.35 | 0.18 | 0.68 * | 0.81 * | 1 | ||||||
| IV | 0.89 * | 0.75 * | −0.62 * | −0.23 | −0.66 * | 0.46 | 0.43 | 0.77 * | 0.82 * | 0.74 * | 0.39 | 1 | |||||
| CVHUFB | 0.86 * | 0.75 * | −0.04 | −0.26 | −0.75 * | 0.54 * | 0.43 | 0.64 * | 0.89 * | 0.90 * | 0.70 * | 0.92 * | 1 | ||||
| Ratio FBRS | −0.12 | −0.19 | 0.06 | 0.14 | 0.004 | −0.27 | −0.57 * | 0.14 | −0.11 | 0.32 | 0.28 | −0.05 | 0.11 | 1 | |||
| Vmax IS | −0.04 | −0.01 | 0.40 | 0.34 | 0.33 | −0.02 | −0.11 | −0.08 | 0.07 | 0.23 | 0.41 | −0.06 | 0.11 | 0.38 | 1 | ||
| Vmax in ratio | −0.41 | −0.34 | 0.44 | 0.54 * | 0.81 * | −0.48 | −0.41 | −0.23 | −0.52 * | −0.47 | −0.45 | −0.39 | −0.49 | 0.15 | 0.63 * | 1 | |
| Vmax H ratio | −0.41 | −0.33 | 0.47 | 0.48 | 0.80 * | −0.49 | −0.30 | −0.17 | −0.53 * | −0.53 * | −0.54 * | −0.36 | −0.52 * | 0.001 | 0.50 * | 0.96 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Chu, X.; Gao, Z.; Ding, Y.; Que, F.; Ahmad, Z.; Yu, F.; Ramakrishnan, M.; Wei, Q. Culm Morphological Analysis in Moso Bamboo Reveals the Negative Regulation of Internode Diameter and Thickness by Monthly Precipitation. Plants 2024, 13, 1484. https://doi.org/10.3390/plants13111484
Zhang Q, Chu X, Gao Z, Ding Y, Que F, Ahmad Z, Yu F, Ramakrishnan M, Wei Q. Culm Morphological Analysis in Moso Bamboo Reveals the Negative Regulation of Internode Diameter and Thickness by Monthly Precipitation. Plants. 2024; 13(11):1484. https://doi.org/10.3390/plants13111484
Chicago/Turabian StyleZhang, Qianwen, Xue Chu, Zhipeng Gao, Yulong Ding, Feng Que, Zishan Ahmad, Fen Yu, Muthusamy Ramakrishnan, and Qiang Wei. 2024. "Culm Morphological Analysis in Moso Bamboo Reveals the Negative Regulation of Internode Diameter and Thickness by Monthly Precipitation" Plants 13, no. 11: 1484. https://doi.org/10.3390/plants13111484
APA StyleZhang, Q., Chu, X., Gao, Z., Ding, Y., Que, F., Ahmad, Z., Yu, F., Ramakrishnan, M., & Wei, Q. (2024). Culm Morphological Analysis in Moso Bamboo Reveals the Negative Regulation of Internode Diameter and Thickness by Monthly Precipitation. Plants, 13(11), 1484. https://doi.org/10.3390/plants13111484






