Abstract
Orobanche aegyptiaca Pers. is a holoparasitic plant that severely reduces tomato (Solanum lycopersicum L.) production in China. However, there is a lack of effective control methods and few known sources of genetic resistance. In this study, we focused on key genes in the JAZ family, comparing the JAZ family in Arabidopsis thaliana (L. Heynh.) to the tomato genome. After identifying the JAZ family members in S. lycopersicum, we performed chromosomal localization and linear analysis with phylogenetic relationship analysis of the JAZ family. We also analyzed the gene structure of the JAZ gene family members in tomato and the homology of the JAZ genes among the different species to study their relatedness. The key genes for O. aegyptiaca resistance were identified using VIGS (virus-induced gene silencing), and the parasitization rate of silenced tomato plants against O. aegyptiaca increased by 47.23–91.13%. The genes were localized in the nucleus by subcellular localization. Heterologous overexpression in A. thaliana showed that the key gene had a strong effect on the parasitization process of O. aegyptiaca, and the overexpression of the key gene reduced the parasitization rate of O. aegyptiaca 1.69-fold. Finally, it was found that the SLJAZ15 gene can positively regulate the hormone content in tomato plants and affect plant growth and development, further elucidating the function of this gene.
1. Introduction
Tomato (Solanum lycopersicum L.) is a widely grown vegetable crop around the world with many uses and high nutritional value. Broomrapes (Phelipanche spp. and Orobanche spp.) are parasitic plants that affect the growth of cash crops throughout the world, causing major parasitism of tomato, oilseed rape (Brassica napus L.), legumes (Fabaceae spp.), and sunflower (Helianthus annuus L.). They parasitize on plant roots, which can lead to devastating yield losses and is an increasing threat to tomato cultivation. A wide range of control methods are available for parasitic weeds, but no effective control strategy has been developed to date [1]. Traditional control methods include seed quarantine, trapping, manual removal, induced eradication, and chemical control [2]. Current research suggests that breeding resistant species is also an effective strategy and much work has been conducted to identify resistance (R) genes; however, the function of the R genes may be overcome by new strains of parasitic plants, as most R genes mediate race-specific resistance through a gene-for-gene mechanism [3,4,5].
Currently, modified plant viral vectors are used to express RNAi inducers, which is known as virus-induced gene silencing (VIGS). VIGS has many advantages. First, it can be applied to a wide range of host plant types. Some virus vectors can infect monocotyledons, legumes, and even perennial woody plants, for which Agrobacterium tumefaciens-mediated transformation is difficult. Second, viruses can achieve systemic infection of plants [6] The selection of ideal target genes, especially those involved in the parasitization process, is crucial for VIGS to control parasitized plants. Agrobacterium-mediated VIGS was first used in the Nicotiana benthamiana experiment, and researchers have successfully used it in Solanaceae, Lactuca sativa, Arabidopsis thaliana, and S. lycopersicum by optimizing it and obtained stable silencing results [7,8]. Because VIGS can specifically induce the silencing of genes in various parts of the plant, it is often used as an efficient reverse genetic technique in the study of functional genes in the plant body, especially in the study of the function of certain disease resistance genes and disease resistance signaling pathways, which plays an increasingly important role. Tobacco rattle virus (TRV) was originally discovered in tobacco and was shown to be able to replicate, translocate, and efficiently transmit silencing signals to plant tissues in host plants, opening up the possibility of studying gene function in primary and secondary plant metabolism [9]. In recent years, the use of TRV as a vector to induce transient silencing in plants has been rapidly developed, not only in tomato and tobacco but also in other lycopene plants. Ghamsari successfully induced silencing of the PDS gene in Datura stramonium using the TRV vector system [10], and Zhang successfully induced silencing of the PDS, MPE2, and MPF3 genes in Clinopodium polycephalum with high efficacy [11]. In addition, TRV vectors can often be used to introduce the host’s PDS gene into the vector along with it so that it can be used as a reporter gene for silencing through the phenomenon of photobleaching. Phytoene desaturase (PDS) is a key enzyme in the plant carotenoid synthesis pathway and is located upstream of the carotenoid synthesis pathway, which catalyzes the conversion of colorless octahydro lycopene into colored carotenoids [12]. Carotenoids are important pigments that constitute the color of different haustorium in plants and are mainly distributed in the pigment cells of plant haustorium, which make plant haustorium, such as flowers and leaves, show different colors [13]. When the PDS gene is silenced, the plant carotenoid synthesis pathway is blocked, which leads to the breakdown of chlorophyll and causes albinism and dwarfism in plants [14]. Recently, it has been demonstrated that jasmonic acid (JA), salicylic acid (SA), and ethylene glycol all play important roles in host resistance to parasitic plants [15]. In the study of JA, we found that it plays a crucial role in host resistance re-response to parasitic plants. For example, JA and phytochemical synthesis-related genes are highly expressed in Lotus japonicus (Regel) K. Larsen when it is parasitized by O. aegyptiaca and S. hermonthica [16]. The knockdown of a JA synthesis gene in S. hermonthica-resistant rice resulted in a substantial increase in susceptibility, while the application of exogenous JA restored the resistant phenotype [17]. In addition, JA promotes the thickening of plant cell walls, thereby directly or indirectly resisting pathogen invasion [18].
The JA signaling pathway is a more complex process, which includes a large number of related synthetic genes and proteins. Through searching the literature, we found that the JA signaling pathway mainly includes downstream gene response, JA signaling, and the creation and metabolism of signal transduction molecules [19]. In response to external biotic or abiotic stresses, plants produce substantial amounts of jasmonic acid, which is subsequently converted into the downstream compound JA-Ile by the adenylate-forming enzyme JAR1 [20]. Subsequently, JA-Ile further interacts with the jasmonate receptor F-box protein COI1 (Coronatine Insensitive 1) to generate the JAS structural domain. Additionally, JAZ is believed to serve an inhibitory function in the JA pathway [21]. When JA-Ile is absent from the pathway, JAZ proteins collaborate with NINJA proteins [22] to integrate the co-inhibitory factor, TPL (topless). This interaction allows JAZ proteins to synergize with downstream transcription factors, such as MYC2, preventing MYC2 from transcriptionally activating JA-responsive genes. When JA-Ile is present, JA-Ile binds to COI1 to form the COI1-JAZ complex. Upon formation of these complexes, JAZ proteins are ubiquitinated by the E3 ubiquitin ligase SCFCOI1 (Skp/Cullin/F-box) complex and used to interpret JAZ repressors [23]. Among these, MYC2 can work in concert with MTB (MYC2-targeted HLH) to modulate jasmonic acid signaling [24]. Numerous members of the JAZ gene family, each with a distinct biological role, are involved in the control of plant development, responses to abiotic stressors, and abiotic stress tolerance. For example, the overexpression of the OsJAZ9 gene improves rice (Oryza sativa) tolerance to potassium deficiency by changing the JA level and JA signal transduction pathway [25]. The overexpression of the GsJAZ2 gene in soybean (Glycine max) significantly enhanced the resistance of transgenic lines to saline stress [26] The overexpression of AtJAZ1 in Arabidopsis can enhance host resistance to Spodoptera exigua [27] The overexpression of OsJAZs in rice can lead to malformations in haustorium development [28,29]. Previous studies showed that most SLJAZs are induced by JA and are able to respond to a wide range of biotic and abiotic stresses, which provides clues to analyze the function of JAZ genes in biotic and abiotic responses in tomato [30,31].
In previous studies of the JAZ family of genes in tomato, we have identified many genes that are resistant to biotic stresses. For example, SLJAZ2 (Solyc12g009220), SLJAZ6 (Solyc01g005440), and SLJAZ7 (Solyc11g011030) silenced tomato plants and showed enhanced disease-associated cell death for Pst DC3000 (Pesudomonas syringae pv. Tomato DC3000) [31]. In addition, SLJAZ25 (Solyc12g009220) interacted with SLJAR1 (Solyc07g042170), SLCOI1, SLMYC2, and other resistance-related genes to form a regulatory network, and these genes played an important role in the regulation of tomato gray leaf spots [32]. Moreover, it has been reported that SLJAZs are involved in tomato plant development, and SLJAZ2 (Solyc12g009220) is involved in mediating the transition from early asexual to reproductive growth in plants [33]. From the above studies, it was found that despite the different nomenclature, the Solyc12g009220 gene has a pivotal position in the JAZ family. It plays a role in the development of plants towards sexual regeneration, as well as in the development of resistance to biotic stresses.
By screening the whole genome of tomato, we hoped to obtain all the genes of the JAZ family in tomato. The screened genes were also structurally analyzed. Finally, we aimed to achieve effective silencing of the tomato host plant using TRV (tobacco rattle virus) and identify the level of resistance of the target gene to O. aegyptiaca using VIGS technology. Then, we accurately located the action position of the resistance gene using subcellular localization technology to lay the foundation for further resolving its function. Finally, the mechanism of action was further elucidated through the determination of heterologous overexpression in A. thaliana and the change in enzyme activity after parasitism by O. aegyptiaca.
2. Results
2.1. Identification and Characterization of JAZ Genes in Tomato
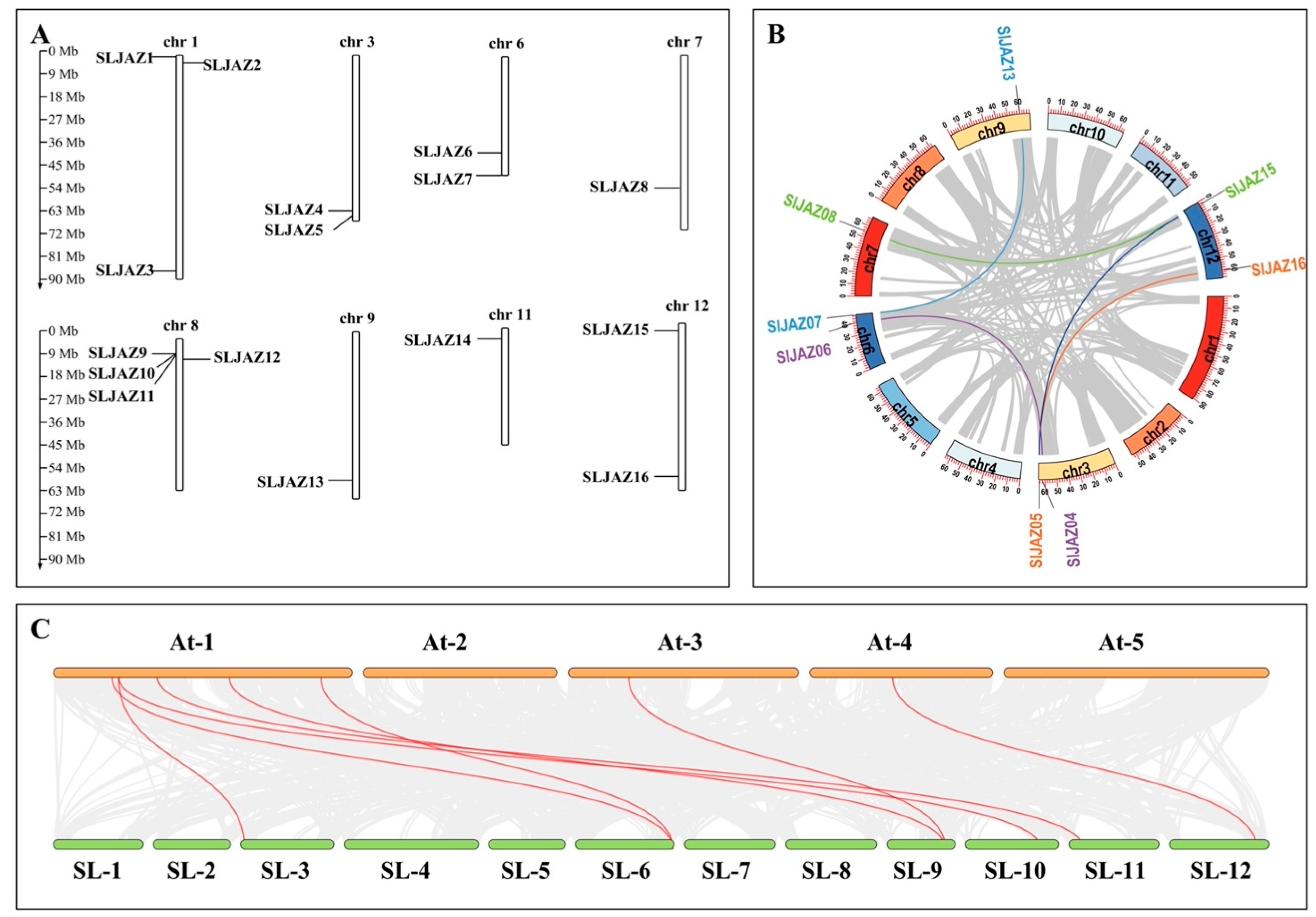
After visualizing the data using several pieces of software, such as HMMER (hidden Markov model of contours), the Simple Module Architecture Research Tool (SMART), the Conserved Structural Domains Database (CDD), and TBtools, the results revealed that 16 SLJAZ genes were localized on 12 chromosomes of tomato, and SLJAZ genes were present on chromosomes 1, 3, 6, 7, 8, 9, 11, and 12. One to three SLJAZ genes were present on the above chromosomes, with only one SLJAZ gene on chromosomes 7, 9, and 11; two on chromosomes 3, 6, and 12; and three on chromosome 1. These genes were named SLJAZ1 to SLJAZ16 for the convenience of subsequent studies, and their locations on the chromosomes are shown in Figure 1A. Gene duplication is an important factor in gene functional differentiation and gene amplification [34]. The results show that we detected five pairs of segmental duplications in SLJAZ genes, such as SLJAZ4 and SLJAZ6, SLJAZ5 and SLJAZ16, SLJAZ7 and SLJAZ13, and SLJAZ8 and SLJAZ15 (Figure 1B).
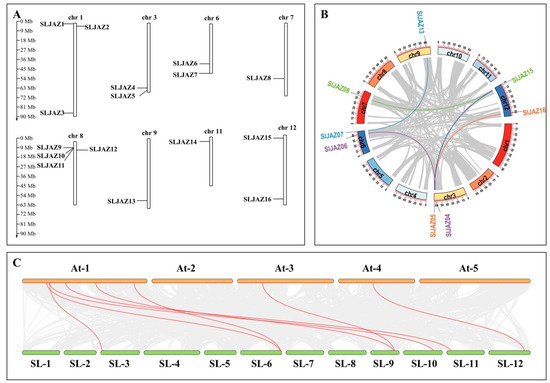
Figure 1.
Chromosomal distribution of tomato JAZ genes and gene duplications of JAZs. (A) Chromosomal distribution of SLJAZ genes. Chromosome length can be judged from the left scale. (B) Gene duplication in JAZs. The inner circle shows the chromosome position where the gene is located. The same color indicates gene duplication. (C) Status of homologous SLJAZ genes in tomato and A. thaliana. Red line connections represent homology between the different species. The first line is the A. thaliana chromosome, and the second line is the S. lycopersicum chromosome. SLJAZ1, Solyc01g005440; SLJAZ2, Solyc01g009740; SLJAZ3, Solyc01g103595; SLJAZ4, Solyc03g118540; SLJAZ5, Solyc03g122190; SLJAZ6, Solyc06g068930; SLJAZ7, Solyc06g084120; SLJAZ8, Solyc07g042170; SLJAZ9, Solyc08g036660; SLJAZ10, Solyc08g036640; SLJAZ11, Solyc08g036620; SLJAZ12, Solyc08g036505; SLJAZ13, Solyc09g065630; SLJAZ14, Solyc11g011030; SLJAZ15, Solyc12g009220; SLJAZ16, Solyc12g049400.
2.2. Phylogenetic Relationships and Gene Structure Analysis of SLJAZ Genes
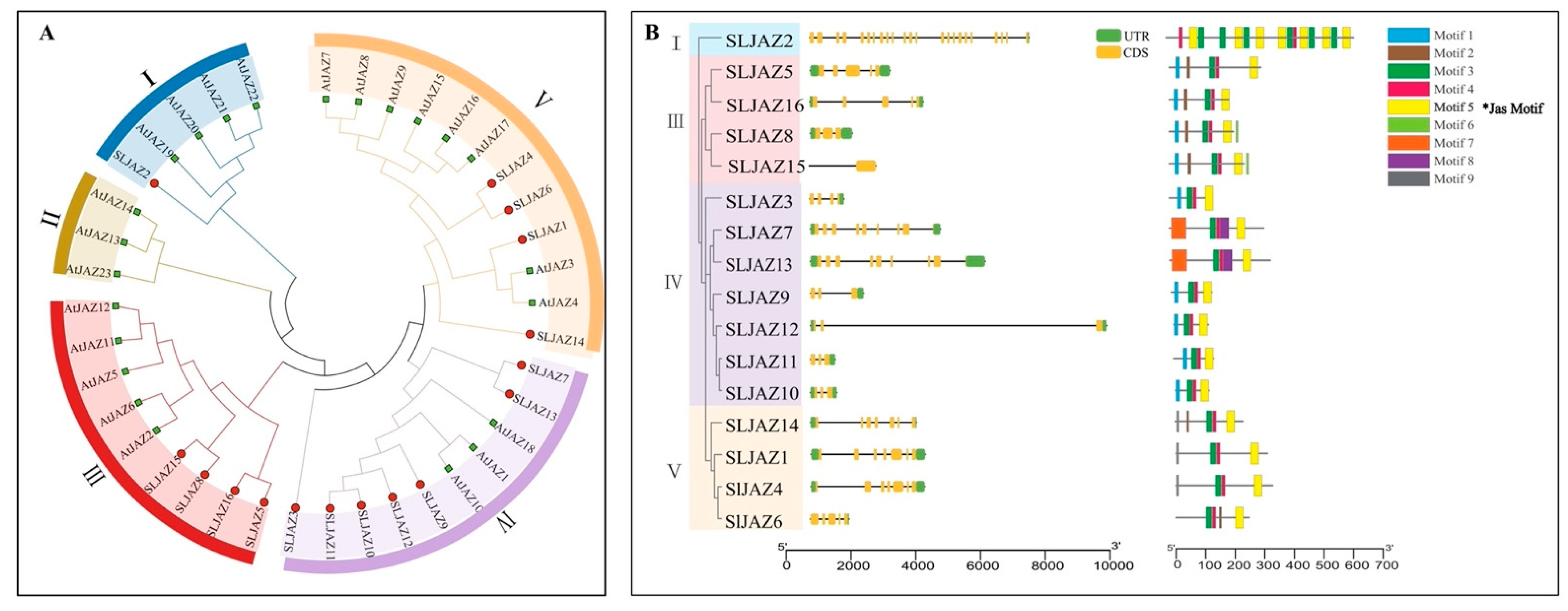
To further clarify the phylogeny of JAZs in tomato, a phylogenetic tree of 16 SLJAZ proteins and 23 AtJAZ proteins expressed in A. thaliana was constructed using molecular evolutionary genetics analysis (MEGA X) (Figure 2A). As shown in the figure, the thirty-nine JAZ genes were categorized into five subfamilies, of which only four branches contained both screened JAZ genes from tomato and A. thaliana. One of our most interesting genes, SLJAZ15, was located in subfamily III, with the strongest homology to SLJAZ8 (Figure 2A,B).
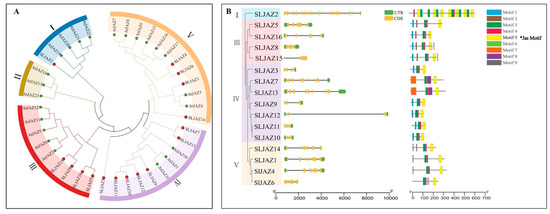
Figure 2.
Phylogenetic tree of JAZ genes in tomato and Arabidopsis thaliana and gene structures of SLJAZ genes’ family members. (A) Phylogenetic tree of SLJAZ and AtJAZ genes. (B) Gene structure of JAZ gene family members in tomato. Branches of the same color represent the same subgroup. Asterisks indicate the motifs shared by SLJAZ genes.
Based on the above results, we divided the phylogenetic tree into four branches (Figure 2B). After analyzing the genetic structure and visualizing the data, we found that most of the SLJAZ genes consisted of four exons and three introns (Figure 2), and the rest of the SLJAZ genes contained more than five exons, among which SLJAZ2 had the highest number of exons (i.e., 15), and SLJAZ15 and SLJAZ8 had six exons. The above conditions indicate that the two genes have a high probability of homology. To further explore the possible functions of SLJAZ genes, we analyzed the conserved motifs of the nine proteins and found that all members contained the Jas motif (Motif 5) (Figure 2). In the phylogenetic tree, five other motifs were specific to different branches. The functional differences in the tomato SLJAZ genes may be due to the clade-specific distribution of conserved motifs.
2.3. Resistant Plants Showed Increased Parasitism of O. aegyptiaca after Silencing SLJAZ15
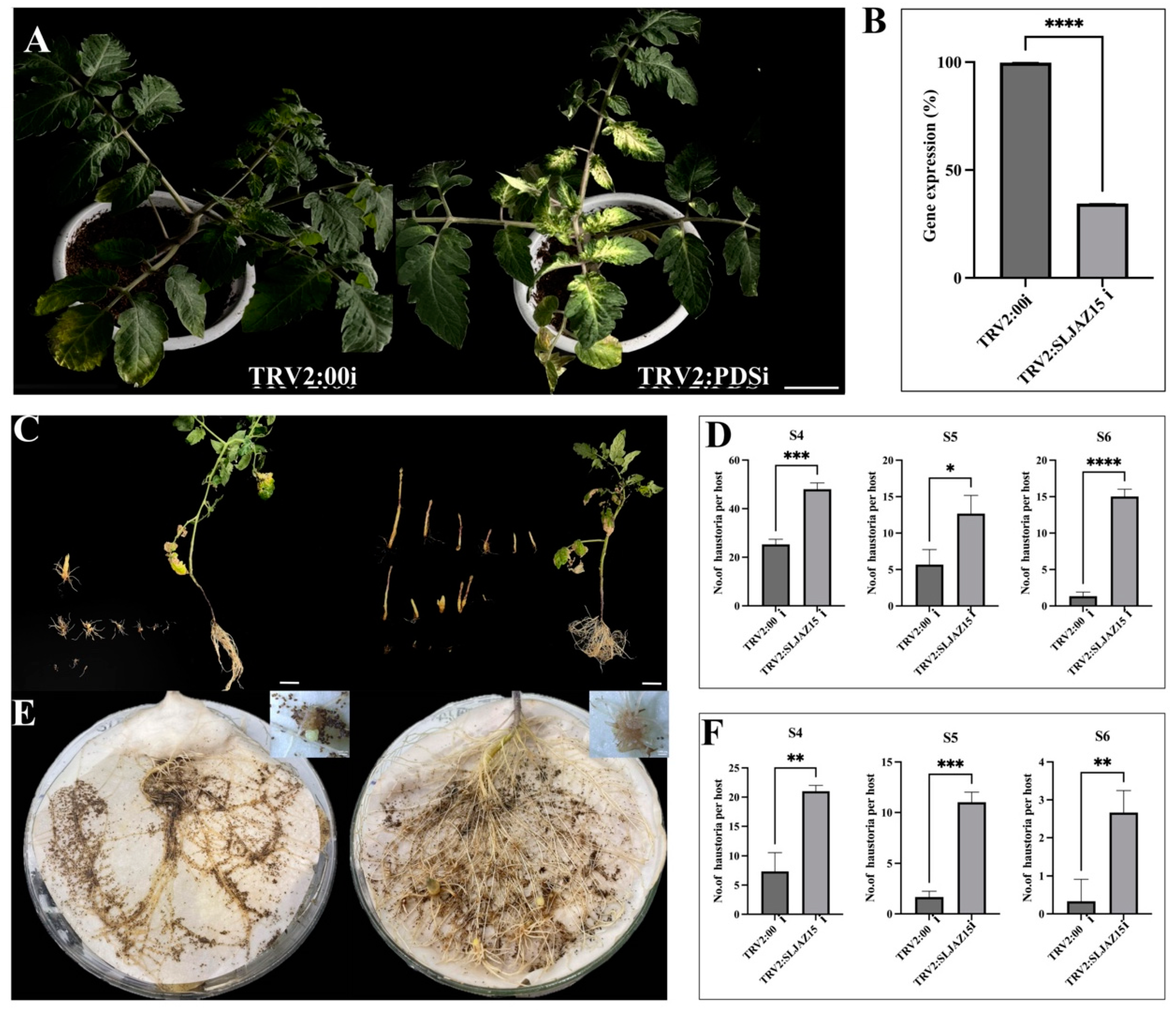
In tomato plants infected with Agrobacterium tumefaciens carrying the TRV-PDSi construct, significant bleaching was observed in the leaves after 15–20 d of incubation, and subsequent experiments were performed after stabilization of the bleaching phenomenon (Figure 3A). To further determine the efficiency of VIGS, quantitative real-time polymerase chain reaction (qRT-PCR) assays were performed on leaves collected from plants containing the TRV2:00i empty vector and the TRV2:SLJAZ15i ced plants compared with that in the TRV2:00i empty vector-infected plants (Figure 3B). These results collectively suggest that the target gene was successfully knocked down in resistant plants. A comparison of the root chamber method with the potting method revealed that SLJAZ15-silenced plants showed symptoms of susceptibility, i.e., an increase in the rate of O. aegyptiaca parasitism by 6.32 and 4.84 flod, respectively, of the mean original, whereas the untreated control plants showed better resistance characteristics to O. aegyptiaca. As shown in Figure 3C,D, after 20 days of co-cultivation of different treated plants with O. aegyptiaca, the average parasitization rate of a single plant was significantly increased in plants silenced with SlJAZ15 compared to plants carrying the TRV2 vector alone. Growth was increased by 47.23%, 51.39%, and 91.13% at different periods of S4, S5, and S6, respectively. Based on the potting method of co-cultivation (Figure 3E,F), after silencing the resistance genes, the plants produced obvious deformities and dwarfing, accompanied by the problem of impeded growth of leaves. Additionally, using the root chamber method for secondary validation, we found that co-cultivation produced the same pattern in the growth of O. aegyptiaca. As shown in Figure 3, the root growth of the plants was enhanced after co-cultivation, and the parasitism of O. aegyptiaca was more significant. An analysis showed that the three phases had increased 1.86-, 5.59-, and 7.09-fold, and in the co-cultivation phase of the root chamber, the developmental attitude was best in the period of S5, which was significantly different from that in the TRV2:00i plants. According to the longitudinal comparison experiments, O. aegyptiaca-parasitized TRV2:00i plants were more prone to necrosis and less likely to be parasitized by O. aegyptiaca, while O. aegyptiaca-parasitized TRV2:SLJAZ15i plants had increased root growth, grew more vigorously, and had a good developmental status.
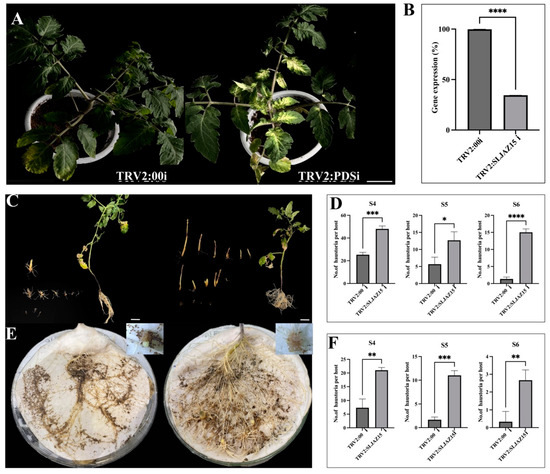
Figure 3.
Efficiency of gene silencing and parasitic results of O. aegyptiaca. (A,B) Bleaching phenomenon in tomato plants and qRT-PCR results. (C,D) Co-cultivation of O. aegyptiaca with tomato for 20 days with the potting method. (E,F) Co-cultivation of O. aegyptiaca with tomato for 20 days with the root chamber method. (A) Bleaching of tomato plants after inoculation with the TRV2:00i (left) and TRV2:PDSi vector (right). (B) qRT-PCR results after SLJAZ15 gene silencing. (C) O. aegyptiaca co-cultivated with TRV2:00i (left) and TRV2:SLJAZ15i (right) tomato for 20 days with the potting method. (D) Parasite differences between TRV2:00i and TRV2:SLJAZ15i co-cultured with O. aegyptiaca during S4–S6 for 20 days with the potting method. (E) O. aegyptiaca co-cultivated with TRV2:00i (left) and TRV2:SLJAZ15i (right) tomato for 20 days with the root chamber method. (F) Parasite differences between TRV2:00i and TRV2:SLJAZ15i co-cultured with O. aegyptiaca during S4–S6 for 20 days with the root chamber method. S4: O. aegyptiaca produces spider web-like structures when the aspirator expands. S5: Pre-emergence shoot structure of O. aegyptiaca. S6: Post-emergence shoots of O. aegyptiaca. * Represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001, and **** represents p < 0.0001.
2.4. Subcellular Localization of SLJAZ15 in Nicotiana tabacum
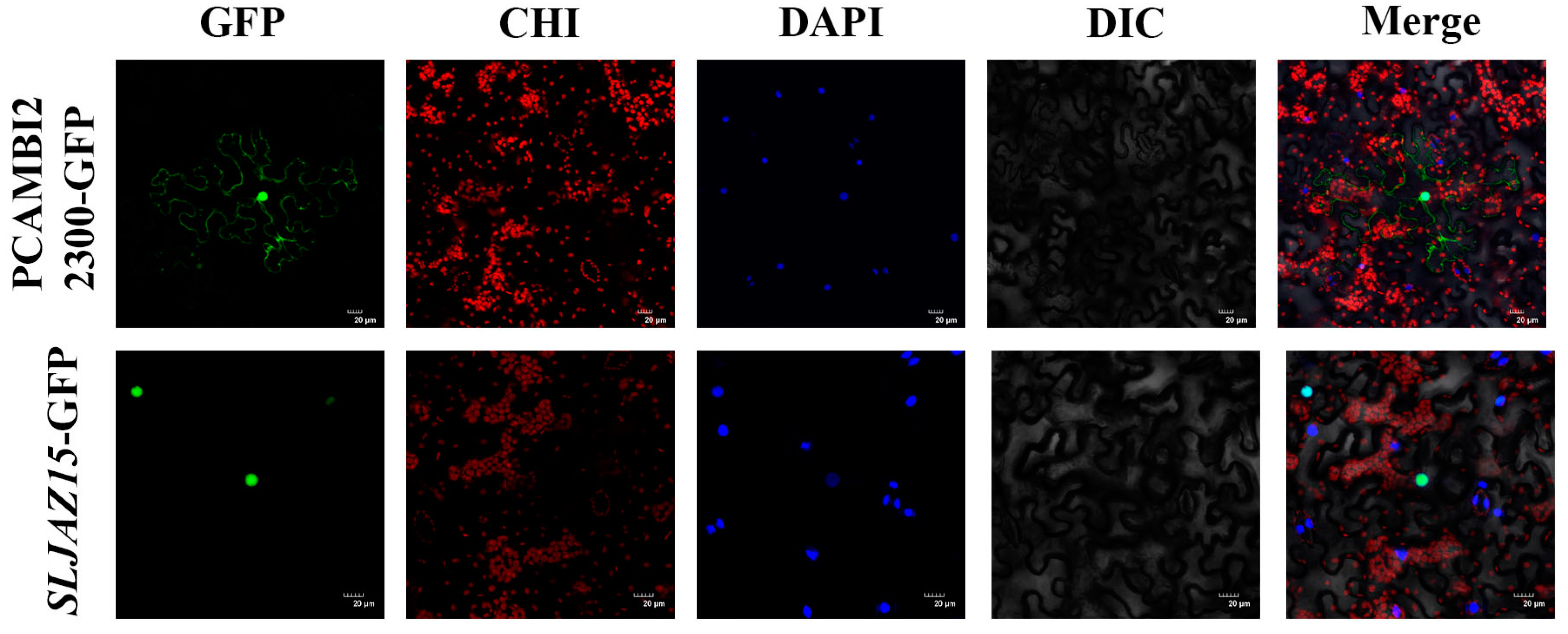
To determine where SLJAZ15 exerts resistance in plants, we utilized a GFP fusion protein expression assay to determine where the SLJAZ15 gene is expressed in cells. As shown in Figure 4, pCAMBIA2300-GFP can be clearly seen under the microscope with obvious fluorescence signals in the cell membrane and nucleus of the lower epidermal cells, but SLJAZ15-GFP only has a fluorescence response in the nucleus, which confirms that SLJAZ15 is localized in the nucleus.
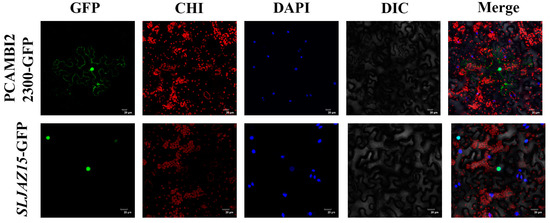
Figure 4.
Subcellular localization of SLJAZ15 in Nicotiana tabacum. GFP stands for green fluorescence field; DAPI stands for DAPI field; CHI stands for chloroplast autofluorescence field; DIC stands for bright field; and Merge stands for superimposed field. Excitation light wavelength: GFP field, 488 nm; DAPI field, 358 nm; and CHI field, 488 nm. Note that green fluorescence and chloroplast autofluorescence have the same excitation light but different acquisition light wavelengths. Bar = 20 μM.
2.5. SLJAZ15 Overexpression in A. thaliana Increased Resistance to O. aegyptiaca
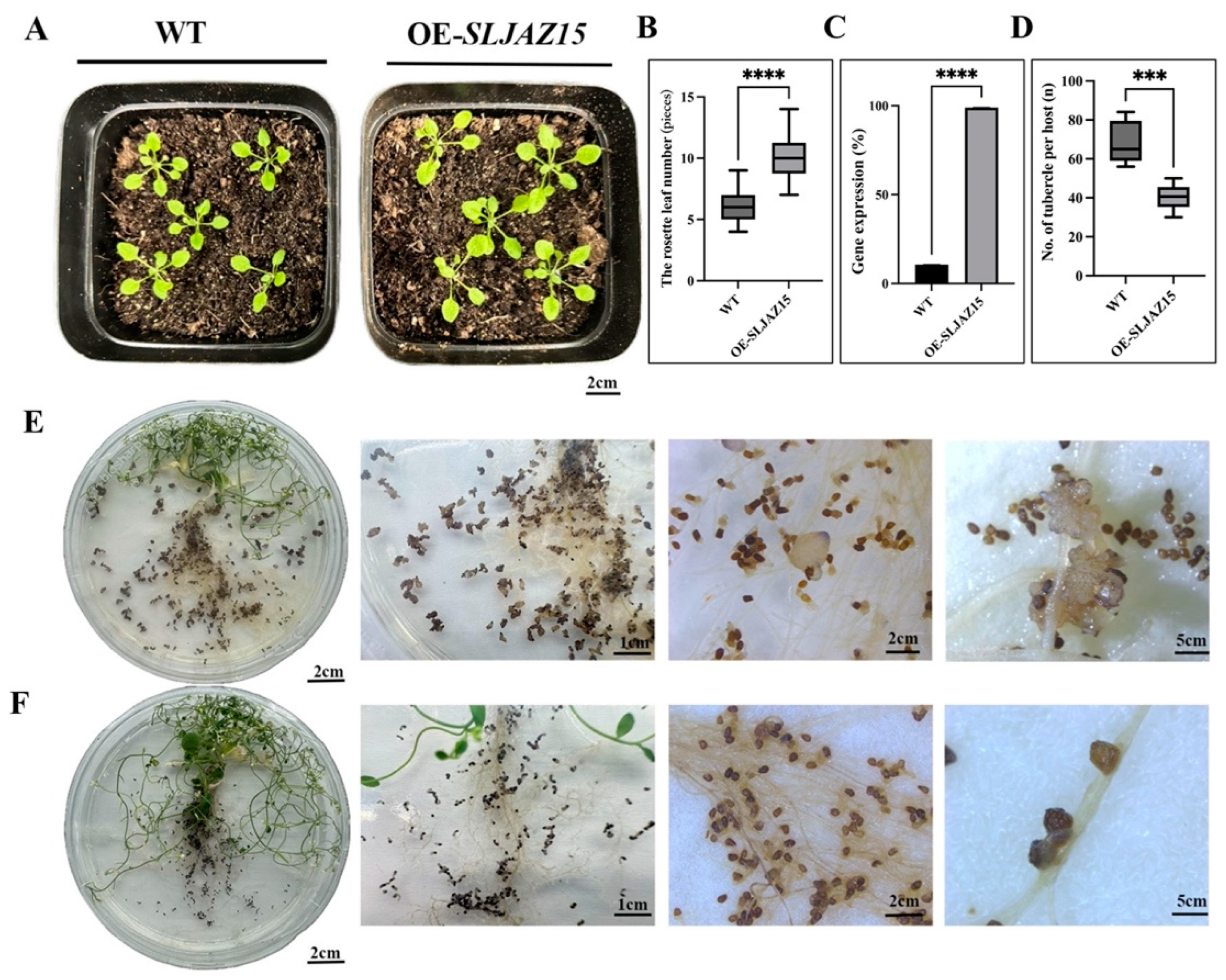
To investigate whether the function of SLJAZ15 is conserved, the A. thaliana Floral Dip method was used to obtain positive Arabidopsis plants overexpressing SLJAZ15. In the root chamber method, after co-cultivating O. aegyptiaca and transgenic A. thaliana for 20 d, the number of O. aegyptiaca parasitizing transgenic plants was significantly reduced compared with that of the control Arabidopsis, with an average 1.5-fold reduction in the number of parasitizing O. aegyptiaca per plant (Figure 5D). The average number of O. aegyptiaca parasitizing WT plants was 68.4 at different times, while the number of O. aegyptiaca parasitizing transgenic plants was only 40.4 at different times, which was a 69% reduction (Figure 5E,F). Moreover, O. aegyptiaca parasitizing overexpressing plants browned and died at a later stage, and their developmental status was significantly worse than that of O. aegyptiaca parasitizing common A. thaliana. Statistical findings from the growth and development of the host A. thaliana showed that the plant height and the growth and development of transgenic plants were significantly better than those of the WT after assessing O. aegyptiaca using the potting method (Figure 5A,B).
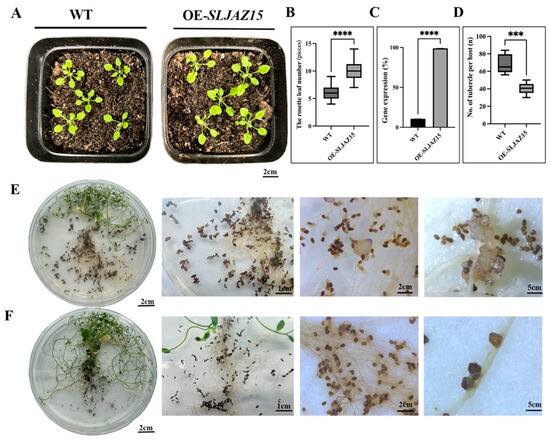
Figure 5.
Heterologous overexpression of SLJAZ15 in Arabidopsis thaliana. (A) Phenotypes of planting after SLJAZ15 overexpression in A. thaliana after 10 d. (B) Data on the rosette leaf number in the wt and SLJAZ15 overexpressing Arabidopsis plants after 10 days. (C) qRT-PCR results after SLJAZ15 gene overexpression. (D) Number of tubercles per A. thaliana plant. (E) Parasitized phenotype of transgenic WT A. thaliana plants co-cultured with O. aegyptiaca for 20 days with the root chamber method. (F) Parasitized phenotype of transgenic A. thaliana plants OE-SLJAZ15 co-cultured with O. aegyptiaca for 20 days with the root chamber method. *** Represents p < 0.001, and **** represents p < 0.0001.
2.6. Physiological and Biochemical Analyses
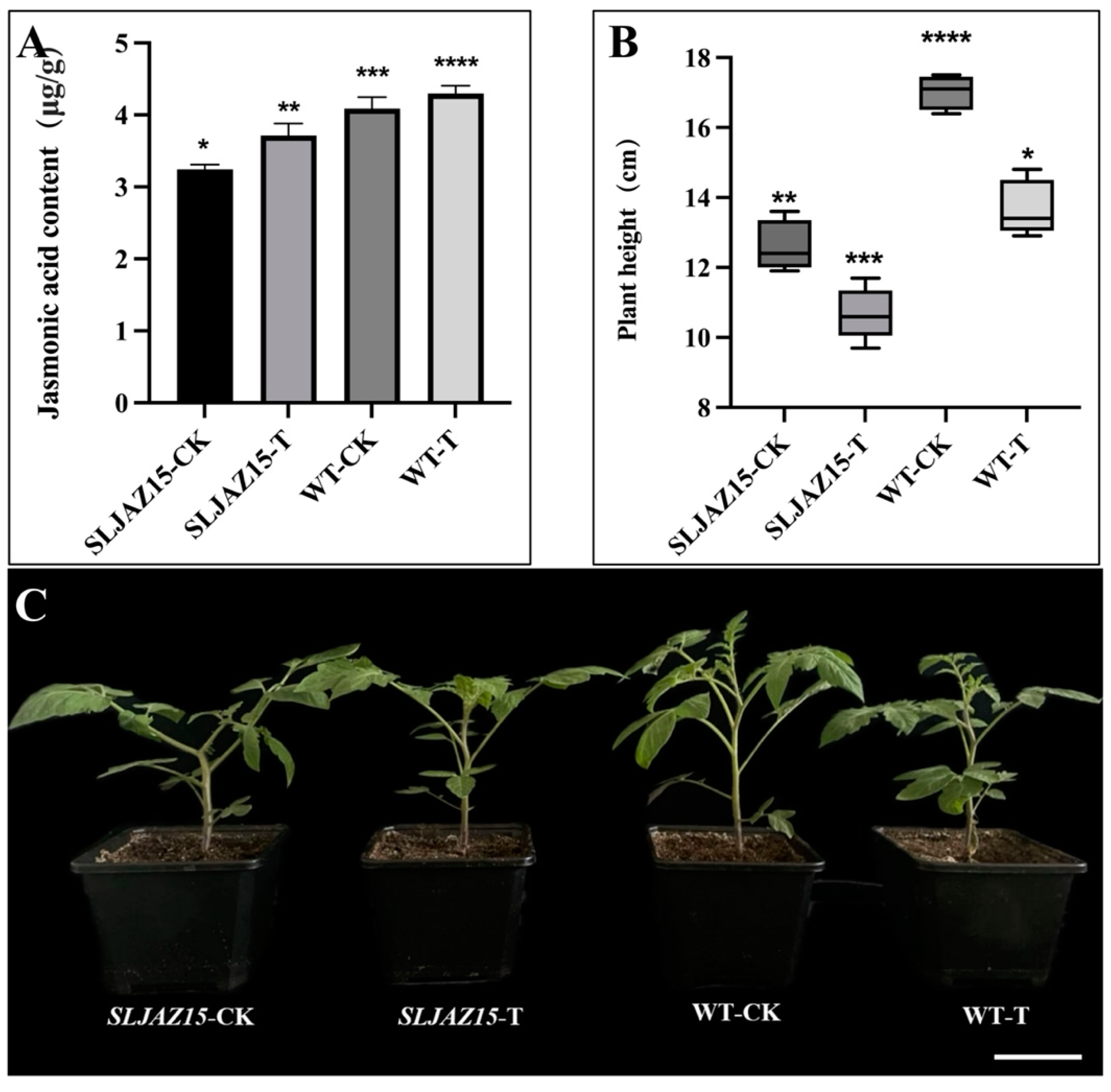
JA is an important hormone that regulates plant growth and development, and it plays a very important role in the plant body. SLJAZ15 is one of the genes of the JAZ family involved in the regulation of plant growth and development, as well as in the defense against biotic and abiotic stressors. Therefore, to further analyze the role of this gene, we measured the JA content of tomato plants and analyzed the function of the gene according to changes in content. Based on the measured data (Figure 6), we observed that the JA content in normal tomato plants was significantly higher than that in SLJAZ15-silenced plants, with 3.72 and 3.24 levels, respectively. Both normal and SLJAZ15-silenced transgenic plants showed a certain increase in JA content after 20 d of parasitization with O. aegyptiaca, but the increase was small.
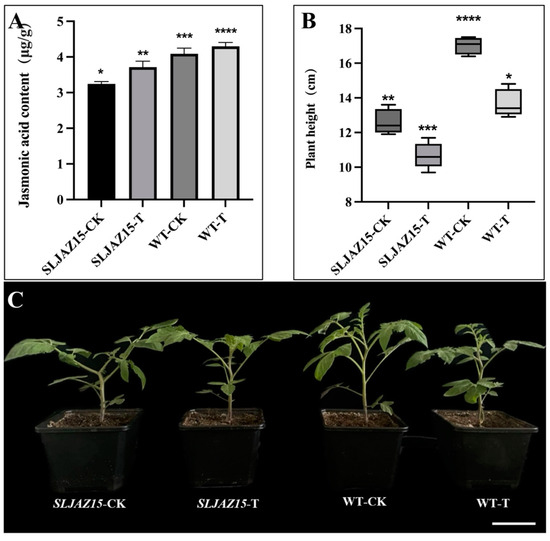
Figure 6.
Results of physiological and biochemical analyses of SLJAZ15 and morphological phenotype of transgenic tomato plants. (A) Jasmonic acid content in different tomato plants. (B,C) Height data and phenotypes of different tomato plants. (B) Height data for different tomato plants. (C) Phenotypes of different tomato plants. SLJAZ15-CK, SLJAZ15-silenced plants after 15 d; SLJAZ15-T, co-cultivation of SLJAZ15-silenced plants with O. aegyptiaca after 15 d; WT-CK, common tomato plants after 15 d; and WT-T, co-cultivation of common tomato plants with O. aegyptiaca after 15 d. * Represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001, and **** represents p < 0.0001.
According to these experiments, with either VIGS or overexpression, the height and growth status of transgenic SLJAZ15 plants were altered to different degrees. In the SLJAZ15 silencing system, the silenced plants grew poorly, became shorter in height, and even became deformed; however, in the overexpression system, the growth of A. thaliana accelerated to different degrees.
3. Discussion
Tomato (S. lycopersicum) is widely grown all over the world and is a very important cash crop. However, during its growth and development, it is susceptible to a variety of pests and diseases, which can lead to a decrease in yield. From previous studies, we know that plants have evolved very complex defense mechanisms [35] in the process of developing continuous resistance to biotic stress, including programmed cell death, allergic necrosis, secretion of antimicrobial substances, and constant changes in endogenous hormones [36]. At the same time, the evolution of the genome of a plant is particularly important for it to better adapt to environmental changes [37]. Individual genomes play their own roles and functions, thus forming a network of disease [38,39] resistance results, which is a hotspot of forthcoming genetic research.
Jasmonate zinc finger proteins expressed in inflorescence meristem (ZIM) domain proteins (JAZ) are important negative regulators within the JA signaling pathway and play an important role in the plant defense response [40]. When plants are subjected to biotic or abiotic stresses, the natural signaling molecule JA can activate the expression of downstream response genes and give them the ability to cope with the stress [41]. Furthermore, JA participates in plant growth and development and defense responses, such as pathogen infection and insect injury [42,43,44]. The JA content is dynamically balanced in plants. When the JA content in plants is low, JAZ proteins bind to MYC2 transcription factors (TFs) to inhibit the transcription of downstream response genes [45]. In contrast, JAZ proteins bind JA (-Ile) receptor complexes for subsequent degradation by the 26S proteasome, enabling the transcriptional activity of MYC2 TFs and ultimately activating the JA signaling pathway [46]. Twelve members of the JAZ gene family have been identified in Arabidopsis thaliana [47]. In addition, they have been reported in rice, maize, soybean, and tobacco crop species [48,49,50]. In tomato, some attention has been devoted to the identification, characterization, and functional analysis of JAZ gene family members [30,31,51].
3.1. Analysis of the JAZ Gene Family in Tomato
The JAZ gene family is present in a wide range of plants. There are 23 JAZs in A. thaliana [51], 15 in rice [52], 16 in maize [48], 36 in turnip [53], 30 in cotton [54], and 11 in grape [55]. We found that SLJAZ5 was located on chromosome 3; SLJAZ6 and SLJAZ7 on chromosome 6; SLJAZ8 on chromosome 7; SLJAZ9, SLJAZ10, SLJAZ11, and SLJAZ12 on chromosome 8; SLJAZ13 on chromosome 9; SLJAZ14 on chromosome 11; and SLJAZ15 and SLJAZ16 on chromosome 12. We grouped these sixteen JAZ genes into five groups according to their grouping pattern in the JAZ gene family in Arabidopsis thaliana, and the genes in the same branch could have similar or complementary physiological functions.
According to the results of previous studies, the structures of introns and exons represent different biological functions of genes, and genes with the same structure of identical introns and exons may have the same gene function [56]. In previous studies, JAZ family members were divided into five subfamilies, and genes on the same branch have similar structures and functions [57]. In the present study, we divided the sixteen SLJAZ genes into four subfamilies. Most of the SLJAZ genes contained different numbers of introns and exons, and only the SLJAZ2 gene did not contain introns, suggesting that introns have been deleted in these genes, which led us to note that the precise loss and gain of introns may be an important factor in promoting de novo gene birth [58]. Based on gene theme analysis, we aimed to find nine themes in sixteen SLJAZ genes. Among them, we found the shared Jas motif, which is contained in all SLJAZ genes. Within the same clade, some motifs are unique and form the basis of gene family classification and functional differentiation.
Plant evolution and gene family expansion require the advancement of gene duplicate expression [57]. Studies have shown that 70–80% of angiosperms experience gene duplication or polyploidy events [59]. Effective means of generating new genes and developing resistance to foreign invaders would include methods such as gene fragment duplication, base shifting, and replication, as mentioned previously [60]. In this study, we found that there were four pairs of duplicated genes in the SLJAZ genes we screened. To continue exploring the phylogenetic relationships between the SLJAZ genes and the SLJAZ genes of other plant species, we then determined the co-lineage relationships between tomato and A. thaliana. Finally, eight pairs of co-linear JAZ genes were identified between tomato and A. thaliana.
3.2. Functional Analysis of the SLJAZ15 Gene
To gain a more detailed understanding of the resistance of genes in this gene family to O. aegyptiaca, we screened the key gene SLJAZ15, which is 756 bp across the field and located on chromosome 12. The resistant tomato variety “H1015” appeared to be weakened by the phenomenon of reduced resistance, and the rate of parasitization increased by 47.23–91.13%; along with the increase in the rate of parasitization, the plants appeared to be short and misshapen. This demonstrates that SLJAZ15 is in a critical position for plant resistance to biotic stress, and silencing this gene affects the ability of plants to resist parasitism by O. aegyptiaca. Moreover, impaired plant growth and development demonstrated that this gene is related to plant growth and development.
To further understand the function of the SLJAZ15 gene family, we analyzed it using subcellular localization, which, as predicted, showed that SLJAZ15 was localized in the nucleus. This is consistent with the expectation that the SLJAZ15 gene is involved in the plant resistance stress response.
Next, we obtained A. thaliana plants heterologously overexpressing SLJAZ15. The results were in line with our expectation that Arabidopsis plants overexpressing the SLJAZ15 gene would grow better, with better stem and leaf development, as well as enhanced O. aegyptiaca resistance, with a significant reduction in O. aegyptiaca parasitism to approximately 59.06% of the WT. Moreover, transgenic A. thaliana root development was better in the absence of O. aegyptiaca inoculation, with a longer root length and larger leaf development.
3.3. Physiological and Biochemical Analyses of SLJAZ15
Physiological and biochemical analyses are an important way of revealing the interactions of various substances and molecules in an organism, and analyzing the content, structure, and function of various types of substances in an organism allows us to understand its metabolic processes and the differences between organisms in different states. In this study, we found that the JA content in transgenic plants after SLJAZ15 silencing was significantly reduced. Side-by-side comparisons also revealed that after parasitizing O. aegyptiaca, both SLJAZ15-silenced plants and control plants showed a significant increase in JA content in vivo, suggesting, in agreement with our results, that JA has a positive role in defense against external biotic stress.
Interestingly, we also found a role for JA in the plant growth state at the same time. In previous studies, we found that large amounts of exogenous JA cause stunted plant growth [61], while moderate amounts promote plant growth [62]. Even in the JAZ family, there are important genes that can regulate the morphology of the plant and accelerate its flowering [33]. In this experiment, the key gene, SLJAZ15, was silenced, and the plants were dwarfed. The dwarfing phenomenon was more significant after parasitism by O. aegyptiaca, and the silenced plants were more prone to aberrant growth.
3.4. Conclusions
O.aegyptiaca is a holoparasitic plant. It mainly parasitizes plant roots and severely reduces the yield of tomato in China. However, conventional control methods are ineffective, and there are few known sources of resistance genes. As an important part of the JA signal transduction pathway, JAZs play a prominent role in plant disease resistance. First, we identified 16 SLJAZ genes in tomato. These genes clustered into four branches, and their gene structure and motif distribution in the same group were similar. Based on previous research, the promoter regions of these genes might contain many cis-acting elements associated with disease resistance. We subsequently confirmed the negative regulatory effect of the SLJAZ15 gene on tomato resistance to O.aegyptiaca. Additionally, we also observed that following the allogeneic overexpression of the SLJAZ15 gene, A. thaliana’s growth and development were notably improved, the parasite load was significantly reduced, and the nodules were susceptible to necrosis. Lastly, we also confirmed that the SLJAZ15 gene plays a significant role in the regulation of hormone levels in plants and the subsequent impact on plant growth. These results not only show the importance of SLJAZs in tomato disease resistance but also lay a good foundation for further study of the potential regulatory mode of SLJAZ genes.
4. Materials and Methods
4.1. Identification of SLJAZ
The hidden Markov model of contours (HMMER) (http://www.hmmer.org/, accessed on 23 December 2023) of known JAZ structural domains was downloaded from the Protein Family of Structural Domains (Pfam) database (http://pfam.xfam.org/, accessed on 23 December 2023). Tomato genome and A. thaliana genome sequence data were obtained from the NCBI database (https://www.ncbi.nlm.nih.gov, accessed on 24 December 2023). To eliminate pseudogenes, the REtrotransposed Gene EXPlorer was used for filtration. Structural domains were validated using the Simple Module Architecture Research Tool (SMART) (http://smart.embl-heidelberg.de/, accessed on 24 December 2023) and the Conserved Structural Domains Database (CDD) (https://www.ncbi.nlm.nih.gov/cdd/, accessed on 24 December 2023) online tools; the data were visualized using TBtools.
4.2. Chromosomal Mapping, Phylogenetic Analysis, and Gene Sequence Analysis of JAZ Gene Family Members in Tomato
The chromosome positions of SLJAZ genes were retrieved using the Solanaceae Genomics Network (https://solgenomics.net/, accessed on 28 December 2023), and the chromosomal distribution of SLJAZ genes was mapped using TBtools. The phylogenetic trees of Arabidopsis thaliana and tomato JAZ sequences were then constructed after multiple sequence alignment using the MEGA X software ClustalW (default parameters), and finally, the constructed evolutionary trees were optimized using the Evolution View online tool (https://www.evolgenius.info/evolview/, accessed on 28 December 2023). Protein sequences were analyzed using the Multi EM for Motif Elicitation online program (http:/mememe.nbcr.net/meme/intro.html, accessed on 29 December 2023), with the analysis parameters set to the default values and Motif set to 9.
4.3. Plant Material and Treatments
“Heng Shi 1015” is a processed tomato variety that has proven resistance to O. aegyptiaca in our laboratory. The tomato variety H1015 was planted as experimental material at the experimental station at Shihezi University. A 16/8 h light/dark cycle, 35,000 lx of light intensity, 45% relative humidity, and a diurnal temperature range of 20–25 °C were all applied to the growth of the seedlings. The tomato seedlings were injected with bacterial suspensions mediated by Agrobacterium (TRV-SLJAZ15) after they had developed two true leaves. Following therapy, qRT-PCR assays were carried out. Ten seedlings were selected for each treatment, and all treatments were repeated three times. Leaves of SLJAZ15-silenced plants were collected for the subsequent determination of physiological indices, immediately frozen in liquid nitrogen, and stored at −80 °C.
Arabidopsis plants were obtained by Agrobacterium-mediated floral dipping. Independent transgenic lines were identified by evaluating kanamycin resistance. The T2 homozygous progenies of transgenic lines were selected for further study. Arabidopsis seeds of the wild type (WT) and transgenic SLJAZ15 were surface sterilized for seven minutes using 30% NaClO and eight minutes using 70% ethanol. The seeds were then washed three times with distilled water. Twenty-one-day-old plants growing in a large culture plate were irrigated with Hoagland nutritional solution in order to analyze resistance and determine physiological indices in mature plants. Additionally, the parasitic ability of O. aegyptiaca was examined. The Arabidopsis plants, both WT and transgenic SLJAZ15, were cultivated in a plant culture chamber with long days (16 h of white light, 8 h of darkness, and 60% relative humidity at 21 °C).
The mass ratio of tomato seeds to culture soil (nutrient soil: vermiculite: 2:1) for the potting procedure was 2000:1. Plants (20 cm height and 15 cm diameter) containing the soil mixture were used for their planting, and they were kept in an artificial climate room (20–28 °C, 40–60%, 16/8 h, 10,000 lx). In this investigation, three duplicates of each species were put up. After 25 days, root wash surveys were carried out to tally the number of nodules and O. aegyptiaca that emerged from each pot. Two biological duplicates were set up with thirty treatments and ten controls. The interaction between O. aegyptiaca and the tomato root system was simulated using the root chamber approach. Initially, the tomato seeds were sterilized with 5% NaClO for 20 min at 160 rpm and 25 °C in a shaker. The shaker then continued to shake, and the germinating seeds were incubated at room temperature. After a period of elongation and development of the canals, they were transferred to a glass Petri dish with a sterile sponge covered with two layers of sterile filter paper (15 cm in diameter) and incubated until new roots grew. When the new roots had grown to approximately 2/3 of the volume of the Petri dish, the new roots, which had been sterilized with 70% C2H5OH for 2 min and 1% NaClO for 20 min, were added uniformly around the new roots and incubated for 25 days. After 25 days of incubation, the roots were washed with pure water to remove ungerminated O. aegyptiaca seeds, and then the parasitism rate was determined at different periods (S4, S5, S6) [63,64]. The S4 period means that O. aegyptiaca produces spider web-like structures when the aspirator expands. The S5 period means that the pre-emergence shoot structure of O. aegyptiaca occurs. The S6 period means that there are post-emergence shoots of O. aegyptiaca.
Thirty treatments and ten controls were set up in three biological replicates.
4.4. Target Fragment Amplification
Total RNA was extracted from sampled leaves using the TRIzol method. Then, RNA was reverse transcribed to cDNA using a reverse transcription kit (EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix, Tran, FL, USA). The target sequence of the SLJAZ15 gene was amplified with specific primers, forward 5′-ATGGGGTCATCGGAAAATATG-3′ and reverse 5′-CTAGAAATATTGCTCAGTTTTAACAA-3′, designed using Primer 5.0 software.
4.5. Agrobacterium-Mediated Virus Infection
Agrobacterium tumefaciens strain GV3101 carrying the TRV vector was cultured in an LB liquid medium (containing 50 mg/mL kanamycin and 100 mg/mL rifampicin) until the OD600 was 0.8–1.0. Transformed Agrobacterium tumefaciens cells were then centrifuged (4 °C, 4000 rpm, 10 min) and resuspended in a buffer (10 mM MES, 10 mM MgCl, 200 μM Acetosyingone) until the OD600 was 0.6–0.8. Agrobacterium tumefaciens cells containing TRV1 were mixed with TRV2-derived constructs or TRV2 empty vectors at a volume ratio of 1:1. The mixed bacterial liquid was allowed to stand for 3 h and was then injected into seedlings at the three-to-four leaf stage using a 1 mL syringe.
4.6. VIGS Vector Construction
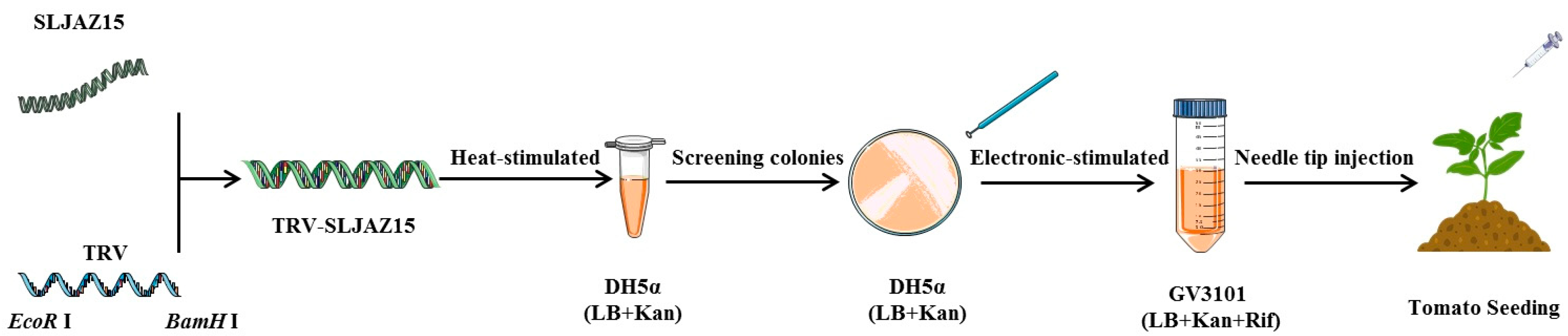
PCR products and the TRV2 empty vector were individually digested with the restriction enzymes EcoR I and BamH I. The correct PCR products were recovered using a product recovery kit (Nanjing Novozymes Biotechnology Co., Ltd., DC301, Shihezi, China). The target fragment was ligated into the empty vector. The vector was heat stimulated into Escherichia coli DH5α cells according to the manufacturer’s instructions. A single positive clone was screened and cultured in liquid LB (containing 50 mg/mL kanamycin). The validated constructs, TRV-SLJAZ15, TRV-PDS, and TRV1:00, were then imported into Agrobacterium GV3101 using the electroshock transformation method. The validated sequences of TRV-SLJAZ15 were as follows: F-primer (5′-3′), GTGAGTAAGGTTACCGAATTCGTGAAACAAATCCTCAAAAACCCATGAATCT; R-primer (5′-3′), CGTGAGCTCGGTACCGATCCTTCTTGAATCAATTTGTTACCAAAACTCACACCAG. When TRV-PDS transgenic plants showed obvious leaf whitening, O. aegyptiaca was co-cultured with other treatments. A schematic diagram of VIGS vector construction is shown below (Figure 7).
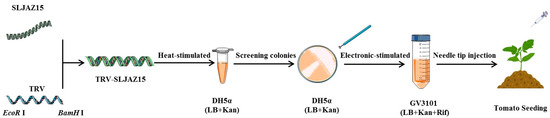
Figure 7.
Schematic diagram of VIGS vector construction. SLJAZ15, target resistance genes; TRV, tobacco rattle virus; EcoR I, the restriction enzymes; BamH I, the restriction enzymes; TRV-SLJAZ15, vehicle for integration; DH5α (LB + Kan), DH5α cultured in an LB medium (with Kan); GV3101 (LB + Kan + Rif), cultured in an LB medium (with Kan and Rif).
4.7. Quantitative Real-Time PCR Analysis
As soon as the seedlings infected with A. tumefaciens carrying the TRV-PDS vector showed visual photobleaching symptoms on the first-grown leaves, samples were collected from the SLJAZ15-silenced and control plants. RNA extraction and cDNA synthesis were performed as described above. qRT-PCR was performed using the iQ5 system. The real-time quantitative PCR mixture for each qRT-PCR consisted of 10 μL of 2 × PerfectStartTM Green qPCR SuperMix, 0.4 μL of each forward and reverse primer, 1 μL of cDNA, and 8 μL of ddH2O in a 20 μL reaction. The reaction was performed as follows: 94 °C for 30 s, followed by 40 cycles of 94 °C for 5 s, 60 °C for 30 s, and 72 °C for 10 s; 3 biological replicates were included. The 2−△△CT method was used for data analysis, and ATActin (Fw: 5′-ATGTCGACAACGGCTCCGGCATG-3′; Rv: 5′-GCTCGGGCACGACAGCACAGCTT-3′) was used as a reference gene. The sequences of gene-specific primers used were as follows: SLJAZ15 Fw, 5′-TGAAGAAGCTGCGTTAGCGT-3′; SLJAZ15 Rv, 5′-TCACCGGCGAACAATTACCA-3′. Three replicates were performed, and plants with more than a 50% reduction in gene expression were selected for subsequent O. aegyptiaca inoculation treatments.
4.8. Subcellular Localization of SLJAZ15
pCAMBIA2300-GFP is a subcellularly localized expression vector with a GFP tag applied in this experiment and kept by the laboratory. The pCAMBIA2300-GFP plasmid and full-length SLJAZ15 were fused into subcellular localized vectors using Sac I and Xba I as the digestion sites and then transferred into DH5α-competent propagating cells using the thermal stimulation method. After obtaining the positive clone, it was transferred into A. tumefaciens to obtain the pCAMBIA2300-SLJAZ15-GFP vector. The specific primers used were Fw: 5′-AGAACACGGGGGACGAGCTCATGGGGTCATCGGAAAATATGGATTC-3′ and Rv: 5′-ACCATGGTGTCGACTCTAGAGAAATATTGCTCAGTTTTAACAAATTGAGCAC-3′. Transient transformation and laser confocal microscopic observation of tobacco leaves were carried out according to the method by Sparkes [25]. Additionally, we eversed three and four leaves, which were earmarked for injection (injection between two leaf veins). A syringe was pressed against the designated area on the posteriorside of the leaf, with one hand applying pressure on the top of the leaf, while the other hand gently depressed the piston until liquid diffusion was observed. Subsequently, following theinfestation, we circled the infected area using a marker, afterwards dousing the leaves with water and placing them in a plastic bag. Finally, we relocated the contaminated tobacco back to its original location. We opened the freshness bag the next day, cut the infested area 2–3 days after injection, tore the epidermal preparation, and observed the results using a fluorescence microscope. The green fluorescence of the GFP fusion protein (excitation wavelength of 488 nm) was observed and visualized with DAPI (excitation wavelength of 340 nm) using a FV10-ASW Laser Confocal Microscope (Leica, Wetzlar, Germany).
4.9. Observations of the Generation and Development of Heterologous Overexpression in Transgenic Plants
A fusion overexpression vector was constructed by inserting SLJAZ15 CDS into a pCNY3 vector digested by Xbal I and Sma I. The vector was used for the construction of the fusion overexpression vector. In this study, A. thaliana was transgenic using the Floral Dip method [65], in which sequences from the T-DNA region of the vector were integrated into the DNA of A. thaliana using the Arabidopsis inflorescence infestation method, resulting in overexpression-positive A. thaliana plants. Kanamycin was used to screen the transgenic lines and was verified with genomic detection PCR. The SLJAZ15 primers used for PCR were as follows: Fw, 5′-TCTAGAATGGGGTCATCGGA-3′; Rv, 5′-CCCGGGGAAATATTGCTC-3′. SLJAZ15 high-expressing strains were screened and cultured to T2 generation, and T2 generation SLJAZ15-overexpressing seeds and Col 0 (WT) were simultaneously seeded in a plant culture room. After culturing to the appropriate stage, we parasitized O. aegyptiaca seeds that had been pretreated with GR24 to grow haustorium in Petri dishes and observed the parasitization results after 20 days of culture.
4.10. Physiological Measurements
Leaf samples were randomly collected from SLJAZ15-CK, SLJAZ15-T, WT-CK, and WT-T after O. aegyptiaca parasitization. Each sample included three replicates. Determination of the JA content in plants was performed using an ELISA Kit (Shanghai Yuanjie Biological Co., Shanghai, China).
4.11. Statistical Analysis
All the experiments were performed using at least three technical replicates or three biological replicates. The data analyses were performed using IBM SPSS Statistics 26 and GraphPad Prism software (v: 9.0.0.121). The differences between various groups were analyzed using the LSD test.
Author Contributions
Conceptualization, S.Z. and X.Z.; methodology, S.C. and L.Z.; software, S.C.; validation, S.C., L.Z. and Q.M.; formal analysis, S.Z.; investigation, S.C.; resources, S.Z.; data curation, S.C.; writing—original draft preparation, S.C.; writing—review and editing, S.Z. and X.Z.; visualization, S.C. and M.C.; supervision, X.C.; project administration, S.Z., X.Z. and X.C.; funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Projects of XPCC (2018CB022) and Huyanghe City (2022C01).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ntoukakis, V.; Gimenez-Ibanez, S. Parasitic plants—A CuRe for what ails thee. Science 2016, 353, 442–443. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Reboud, X.; Gibot-Leclerc, S. Broomrape weeds. Underground mechanisms of parasitism and associated strategies for their control: A review. Front. Plant Sci. 2016, 7, 135. [Google Scholar] [CrossRef]
- Hegenauer, V.; Fürst, U.; Kaiser, B.; Smoker, M.; Zipfel, C.; Felix, G.; Stahl, M.; Albert, M. Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 2016, 353, 478–481. [Google Scholar] [CrossRef]
- Galili, S.; Hershenhorn, J.; Smirnov, E.; Yoneyama, K.; Xie, X.; Amir-Segev, O.; Bellalou, A.; Dor, E. Characterization of a chickpea mutant resistant to Phelipanche aegyptiaca Pers. and Orobanche crenata Forsk. Plants 2021, 10, 2552. [Google Scholar] [CrossRef]
- Weiqiang, L.; Kien Huu, N.; Yasuko, W.; Shinjiro, Y.; Lam-Son, P.T. OaMAX2 of Phelipanche aegyptiacaand Arabidopsis AtMAX2 share conserved functions in both development and drought responses. Biochem. Biophys. Res. Commun. 2016, 478, 521–526. [Google Scholar] [CrossRef]
- Lange, M.; Yellina, A.L.; Orashakova, S.; Becker, A. Virus-induced gene silencing (VIGS) in plants: An overview of target species and the virus-derived vector systems. In Virus-Induced Gene Silencing: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013; Volume 975, pp. 1–14. [Google Scholar] [CrossRef]
- Ryu, C.; Anand, A.; Kang, L.; Mysore, K.S. Agrodrench: A novel and effective agroinoculation method for virus-induced gene silencing in roots and diverse Solanaceous species. Plant J. 2004, 40, 322–331. [Google Scholar] [CrossRef]
- Wroblewski, T.; Tomczak, A.; Michelmore, R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol. J. 2005, 3, 259–273. [Google Scholar] [CrossRef]
- Ratcliff, F.; Martin-Hernandez, A.M.; Baulcombe, D.C. Technical advance: Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 2001, 25, 237–245. [Google Scholar] [CrossRef]
- Ghamsari, M.R.E.; Karimi, F.; Gargari, S.L.M.; Tafreshi, S.A.H.; Salami, S.A. Assessing the tobacco-rattle-virus-based vectors system as an efficient gene silencing technique in Datura stramonium (Solanaceae). Virus Genes 2014, 49, 512–516. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Zhao, J.; Zhang, S.; He, C. Efficient gene silencing mediated by tobacco rattle virus in an emerging model plant Physalis. PLoS ONE 2014, 9, e85534. [Google Scholar] [CrossRef]
- Koschmieder, J.; Fehling-Kaschek, M.; Schaub, P.; Ghisla, S.; Brausemann, A.; Timmer, J.; Beyer, P. Plant-type phytoene desaturase: Functional evaluation of structural implications. PLoS ONE 2017, 12, e0187628. [Google Scholar] [CrossRef]
- Böhm, V. Carotenoids. Antioxidants 2019, 8, 516. [Google Scholar] [CrossRef]
- Qin, G.; Gu, H.; Ma, L.; Peng, Y.; Deng, X.W.; Chen, Z.; Qu, L.-J. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 2007, 17, 471–482. [Google Scholar] [CrossRef]
- Jhu, M.-Y.; Sinha, N.R. Parasitic plants: An overview of mechanisms by which plants perceive and respond to parasites. Annu. Rev. Plant Biol. 2022, 73, 433–455. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Ueda, H.; Sugimoto, Y. Molecular responses of Lotus japonicus to parasitism by the compatible species Phelipanche aegyptiacaand the incompatible species Striga hermonthica. J. Exp. Bot. 2009, 60, 641–650. [Google Scholar] [CrossRef]
- Dos Santos, C.V.; Letousey, P.; Delavault, P.; Thalouarn, P.; Niu, D.-D.; Liu, H.-X.; Jiang, C.-H.; Wang, Y.-P.; Wang, Q.-Y.; Jin, H.-L.; et al. Defense gene expression analysis of Arabidopsis thaliana parasitized by Orobanche ramosa. Phytopathology 2003, 93, 451–457. [Google Scholar] [CrossRef]
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in arabidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Liechti, R.; Farmer, E.E. The jasmonate pathway. Science 2002, 296, 1649–1650. [Google Scholar] [CrossRef]
- Tines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu Nomura, K.; He, S.Y.; Gregg, A.H.; Jonh, B. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Wirhers, J.; Nissan, G.B.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Li, S.-S.; Han, G.-Z. Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiol. 2015, 167, 872–886. [Google Scholar] [CrossRef]
- Singh, A.P.; Pandey, B.K.; Mehra, P.; Heitz, T.; Giri, J. OsJAZ9 overexpression modulates jasmonic acid biosynthesis and potassium deciency responses in rice. Plant Mol. Biol. 2020, 104, 397–410. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, X.; Yu, Y.; Zhu, Y.; Kong, F.; Sun, X.; Wang, F. Overexpression of a TIFY family gene, GsJAZ2, exhibits enhanced tolerance to alkaline stress in soybean. Mol. Breed. 2020, 40, 33. [Google Scholar] [CrossRef]
- Chung, H.S.; Koo, A.J.; Gao, X.; Jayanty, S.; Thines, B.; Jones, A.D.; Howe, G.A. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008, 146, 952–964. [Google Scholar] [CrossRef]
- Hori, Y.; Kurotani, K.-I.; Toda, Y.; Hattori, T.; Takeda, S. Overexpression of the JAZ factors with mutated jas domains causes pleiotropic defects in rice spikelet development. Plant Signal. Behav. 2014, 9, e970414. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, N.; Severing, E.I.; Nijveen, H.; Cheng, F.; Visser, R.G.; Wang, X.; de Ridder, D.; Bonnema, G. Beyond genomic variation—Comparison and functional annotation of three Brassica rapagenomes: A turnip, a rapid cycling and a Chinese cabbage. BMC Genom. 2014, 15, 250. [Google Scholar] [CrossRef]
- Chini, A.; Romdhane, B.W.; Hassairi, A.; Aboul-Soud, M.A. Identification of TIFY/JAZ family genes in Solanum lycopersicum and their regulation in response to abiotic stresses. PLoS ONE 2017, 12, e0177381. [Google Scholar] [CrossRef]
- Ishiga, Y.; Ishiga, T.; Uppalapati, S.R.; Mysore, K.S. Jasmonate ZIM-domain (JAZ) protein regulates host and nonhost pathogen-induced cell death in tomato and Nicotiana benthamiana. PLoS ONE 2013, 8, e75728. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Liu, Z.; Zhao, T.; Jiang, J.; Li, J.; Xu, X.; Yang, H. Genome-wide identification, characterization and expression analysis of the JAZ gene family in resistance to gray leaf spots in tomato. Int. J. Mol. Sci. 2021, 22, 9974. [Google Scholar] [CrossRef]
- Yu, X.; Chen, G.; Tang, B.; Zhang, J.; Zhou, S.; Hu, Z. The Jasmonate Zim-domain protein gene SLJAZ2 regulates plant morphology and accelerates flower initiation in Solanum Lycopersicum Plants. Plant Sci. 2018, 267, 65–73. [Google Scholar] [CrossRef]
- Ohta, T. Evolution of gene families. Gene 2000, 259, 45–52. [Google Scholar] [CrossRef]
- Jander, G.; Clay, N. New synthesis—Plant defense signaling: New opportunities for studying chemical diversity. J. Chem. Ecol. 2011, 37, 429. [Google Scholar] [CrossRef]
- Kud, J.; Wang, W.; Gross, R.; Fan, Y.; Huang, L.; Yuan, Y.; Gray, A.; Duarte, A.; Kuhl, J.C.; Caplan, A.; et al. The potato cyst nematode effector RHA1B is a ubiquitin ligase and uses two distinct mechanisms to suppress plant immune signaling. PLoS Pathog. 2019, 15, e1007720. [Google Scholar] [CrossRef]
- Benoit, M.; Drost, H.-G.; Catoni, M.; Gouil, Q.; Lopez-Gomollon, S.; Baulcombe, D.; Paszkowski, J. Environmental and epigenetic regulation of Rider retrotransposons in tomato. PLoS Genet. 2019, 15, e1008370. [Google Scholar] [CrossRef]
- Lopez-Galiano, M.J.; Gonzalez-Hernandez, A.I.; Crespo-Salvador, O.; Rausell, C.; Real, M.D.; Escamilla, M.; Camañes, G.; Garcia-Agustin, P.; Gonzalez-Bosch, C.; Garcia-Robles, I. Epigenetic regulation of the expression of WRKY75 transcription factor in response to biotic and abiotic stresses in Solanaceae plants. Plant Cell Rep. 2018, 37, 167–176. [Google Scholar] [CrossRef]
- Kaessmann, H. Origins, evolution, and phenotypic impact of new genes. Genome Res. 2010, 20, 1313–1326. [Google Scholar] [CrossRef]
- Major, I.T.; Yoshida, Y.; Campos, M.L.; Kapali, G.; Xin, X.; Sugimoto, K.; Oliveira, F.D.; He, S.Y.; Howe, G.A. Regulation of growth–defense balance by the JASMONATE ZIM-DOMAIN (JAZ)-MYC transcriptional module. New Phytol. 2017, 215, 1533–1547. [Google Scholar] [CrossRef]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Demianski, A.J.; Chung, K.M.; Kunkel, B.N. Analysis of Arabidopsis JAZ gene expression during Pseudomonas syringae pathogenesis. Mol. Plant Pathol. 2012, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, D.; Wang, Y.; Xie, D. Jasmonate action in plant defense against insects. J. Exp. Bot. 2019, 70, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yoshida, Y.; Major, I.T.; Wang, K.; Sugimoto, K.; Kapali, G.; Havko, N.E.; Benning, C.; Howe, G.A. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E10768–E10777. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, A.; Liechti, R.; Farmer, E.E. Arabidopsis jasmonate signaling pathway. Sci. Signal. 2010, 3, cm4. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G. Jasmonates: Biosynthesis, perception and signal transduction. Essays Biochem. 2020, 64, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luthe, D. Identification and evolution analysis of the JAZ gene family in maize. BMC Genom. 2021, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, L.; Wei, X.; Zhou, Y.; Dai, Y.; Zhu, Z. OsJAZ13 negatively regulates Jasmonate signaling and activates hypersensitive cell death response in rice. Int. J. Mol. Sci. 2020, 21, 4379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Niu, D.; Wang, Z.; Yan, X.; Yang, X.; Yang, Y.; Cui, H. Tobacco transcription repressors NtJAZ: Potential involvement in abiotic stress response and glandular trichome induction. Plant Physiol. Biochem. 2019, 141, 388–397. [Google Scholar] [CrossRef]
- Sun, J.-Q.; Jiang, H.-L.; Li, C.-Y. Systemin/Jasmonate-mediated systemic defense signaling in tomato. Mol. Plant 2011, 4, 607–615. [Google Scholar] [CrossRef]
- Tian, J.; Cao, L.; Chen, X.; Chen, M.; Zhang, P.; Cao, L.; Persson, S.; Zhang, D.; Yuan, Z. The OsJAZ1 degron modulates jasmonate signaling sensitivity during rice development. Res. Rep. 2019, 146, dev173419. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Yan, C.; Zhang, J.; Cheng, Y.; Li, W.; Yan, H.; Gao, J. Genome-wide identifcation and expression analysis of the JAZ gene family in turnip. Sci. Rep. 2021, 11, 21330. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xia, X.-C.; Han, L.-H.; Ni, P.; Yan, J.-Q.; Tao, M.; Huang, G.-Q.; Li, X.-B. Genome-wide identification and characterization of JAZ gene family in upland cotton (Gossypium hirsutum). Sci. Rep. 2017, 7, 2788. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Singer, S.D.; Fei, Z.; Wang, H.; Wang, X. Genome-wide identification and analysis of the TIFY gene family in grape. PLoS ONE 2012, 7, e44465. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; de Souza, S.J.; Gilbert, W. Evolution of the intron-exon structure of eukaryotic genes. Sci. Direct 1995, 5, 774–778. [Google Scholar] [CrossRef]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tarrío, R.; Ayala, F.J.; Rodríguez-Trelles, F. Alternative splicing: A missing piece in the puzzle of intron gain. Proc. Natl. Acad. Sci. USA 2008, 105, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of gene duplication in plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Krokene, P.; Kohmann, K.; Huynh, N.B.; Mageroy, M.H. Methyl jasmonate, salicylic acid, and oxalic acid affects growth, inducible defenses, and pine weevil resistance in Norway spruce. Front. Plant Sci. 2023, 14, 1155170. [Google Scholar] [CrossRef]
- Al-Mohammad, M.H.; Sachet, T.F.; Al-Dulaimi, Z.S. Effect of phenylalanine, Jasmonic acid and biofertilizer on growth, yield and anthocyanin pigments of roselle calyces. IOP Conf. Ser. Earth Environ. Sci. 2021, 910, 012077. [Google Scholar] [CrossRef]
- Joel, D.M. Functional Structure of the Mature Haustorium. Parasitic Orobanchaceae; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–60. [Google Scholar] [CrossRef]
- Goyet, V.; Billard, E.; Pouvreau, J.-B.; Lechat, M.-M.; Pelletier, S.; Bahut, M.; Monteau, F.; Spíchal, L.; Delavault, P.; Montiel, G.; et al. Haustorium initiation in the obligate parasitic plant Phelipanche ramosa involves a host-exudated cytokinin signal. J. Exp. Bot. 2017, 68, 5539–5552. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Sher, H.; Ali, A.; Hussain, S.; Ullah, Z. Simplified floral dip transformation method of Arabidopsis thaliana. J. Microbiol. Methods 2022, 197, 106492. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).