Characterisation and Expression Analysis of LdSERK1, a Somatic Embryogenesis Gene in Lilium davidii var. unicolor
Abstract
1. Introduction
2. Results
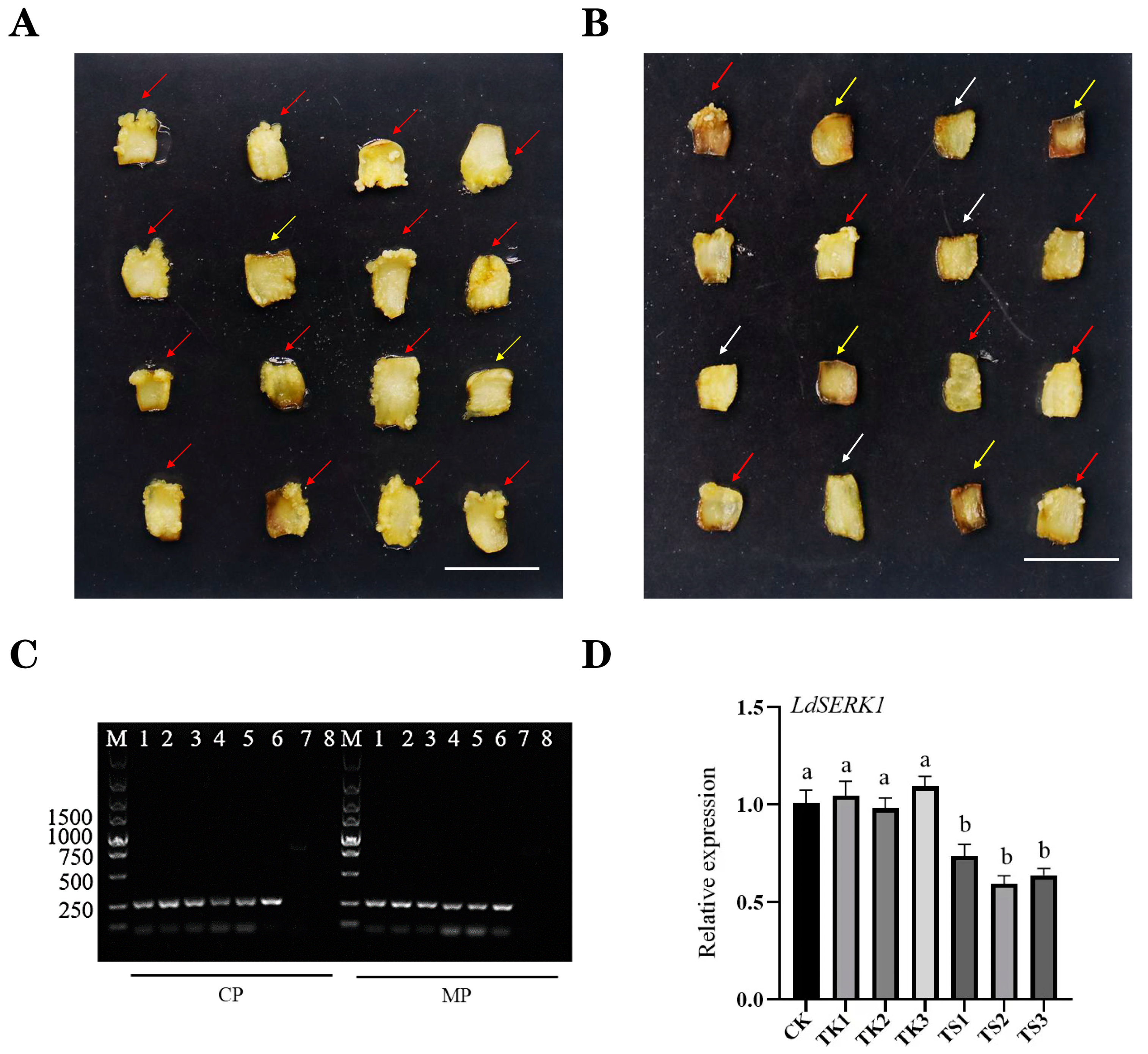
2.1. Somatic Embryo Induction
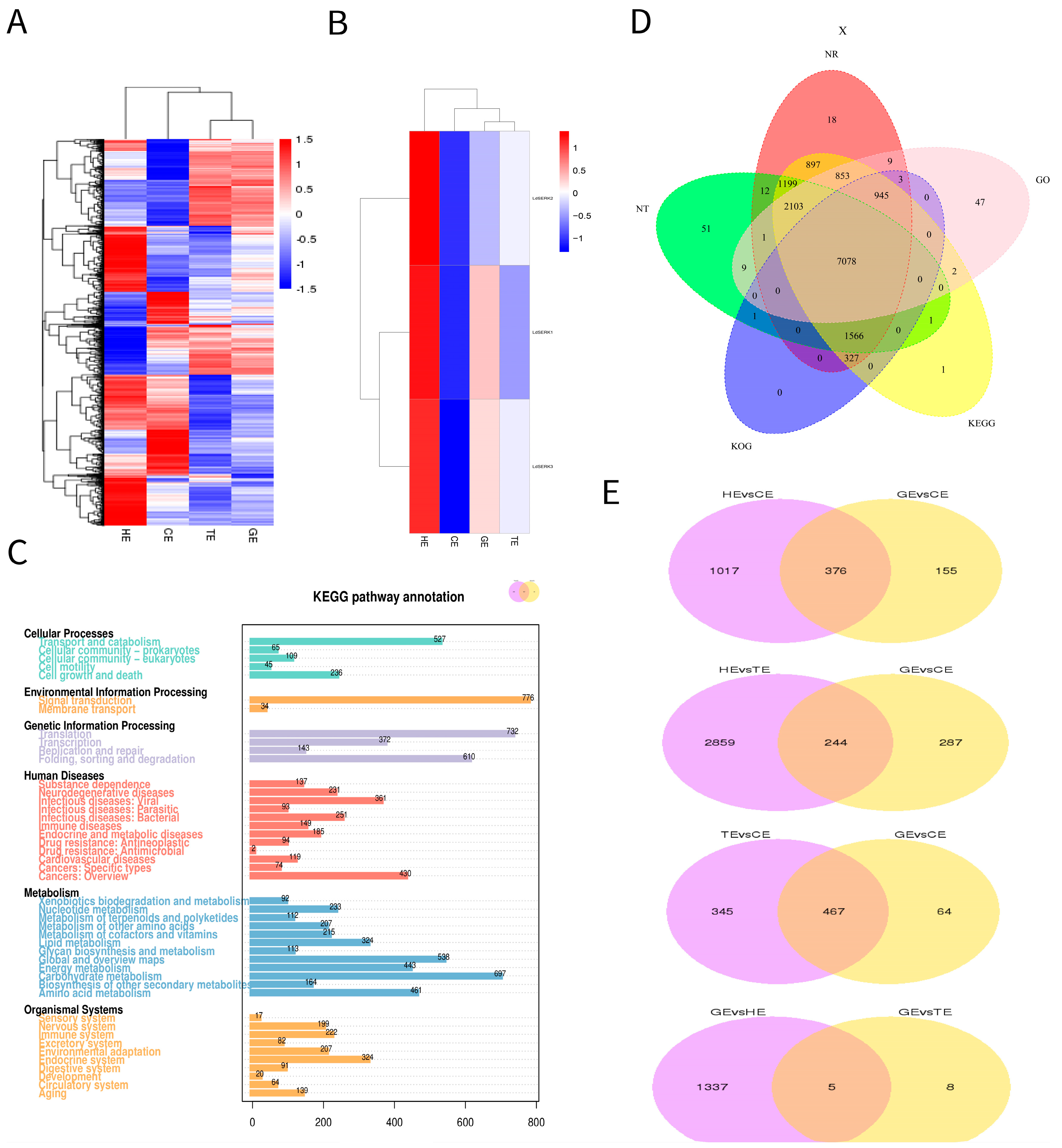
2.2. Differentially Expressed Genes and Analysis of Somatic Embryogenesis Receptor-like Kinase Transcript Level
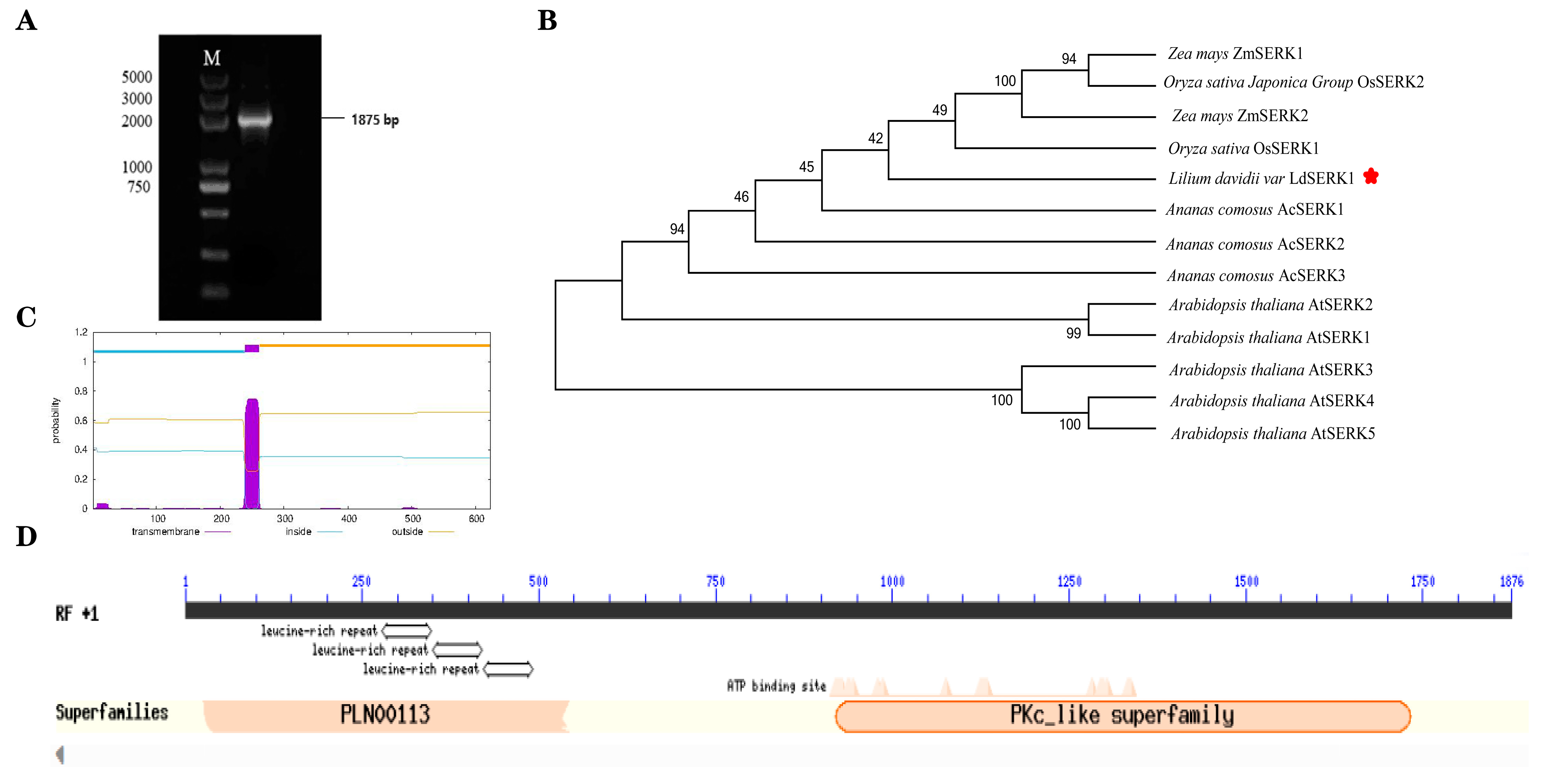
2.3. Gene Clone and Bioinformatics Analysis
2.4. Subcellular Localisation of LdSERK1
2.5. Virus-Induced Gene Silencing in Lilium Scales
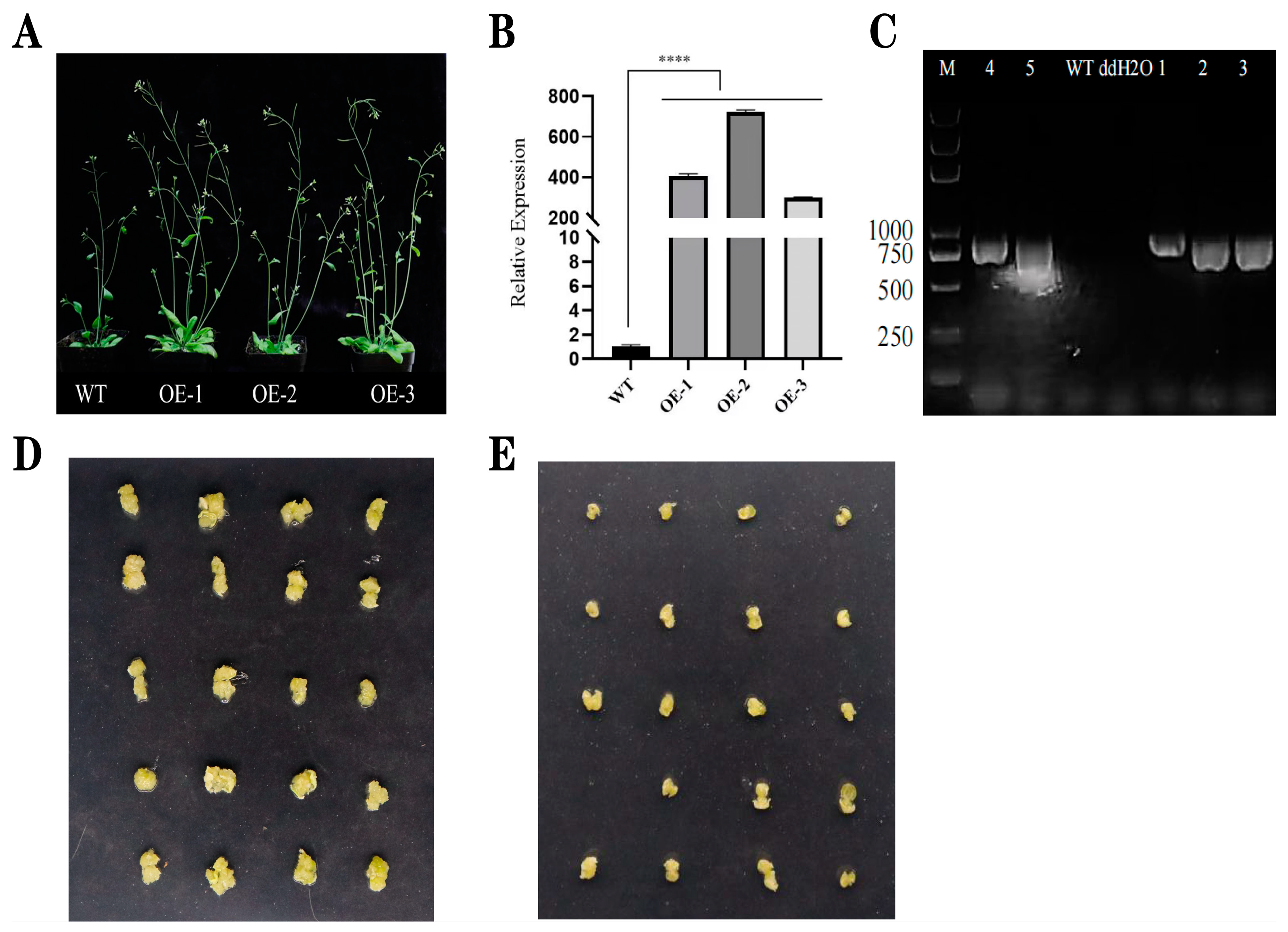
2.6. Phenotypic Analysis and Induction of Seed Callus Tissue in T2-Generation Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Somatic Embryo Induction
4.2. Observation of Paraffin Sections and Tissue Staining
4.3. RNA Extraction
4.4. RNA-Seq Library Preparation and Sequencing
4.5. Differentially Expressed Gene Analysis
4.6. QRT-PCR Verification
4.7. Cloning of the LdSERK1 Gene
4.8. Vector Construction and Agrobacterium Transformation
4.9. Establishment of the Virus-Induced Gene-Silencing System in Lily Scales
4.10. Subcellular Localisation
4.11. Overexpression Vectors for Arabidopsis Transformation
4.12. Quantitative Real-Time PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vries, S.C.D.; Weijers, D. Plant embryogenesis. Curr. Biol. 2017, 27, R870–R873. [Google Scholar] [CrossRef]
- Palovaara, J.; Zeeuw, T.D.; Weijers, D. Tissue and Organ Initiation in the Plant Embryo: A First Time for Everything. Annu. Rev. Cell. Dev. Biol. 2016, 32, 47–75. [Google Scholar] [CrossRef]
- Tian, R.; Paul, P.; Joshi, S.; Perry, S. Genetic activity during early plant embryogenesis. Biochem. J. 2020, 477, 3743–3767. [Google Scholar] [CrossRef]
- Armenta-Medina, A.; Gillmor, C.S.; Gao, P.; Mora-Macias, J.; Kochian, L.V.; Xiang, D.Q.; Datla, R. Developmental and genomic architecture of plant embryogenesis: From model plant to crops. Plant Commun. 2020, 2, 100136. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Weijers, D. Flowering plant embryos: How did we end up here? Plant Reprod. 2021, 34, 365–371. [Google Scholar] [CrossRef]
- Sharma, V.; Clark, A.J.; Kawashima, T. Insights into the molecular evolution of fertilization mechanism in land plants. Plant Reprod. 2021, 34, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M. Historical review of research on plant cell dedifferentiation. J. Plant Res. 2015, 128, 349–359. [Google Scholar] [CrossRef]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A. Overview of Somatic Embryogenesis. In Somatic Embryogenesis. Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2527, pp. 1–8. [Google Scholar]
- Backs-Hüsemann, D.; Reinert, J. Embryobildung durch isolierte Einzelzellen aus Gewebekulturen vonDaucus carota. Protoplasma 1970, 70, 49–60. [Google Scholar] [CrossRef]
- Marinangeli, P. Somatic Embryogenesis of Lilium from Microbulb Transverse Thin Cell Layers. In Vitro Embryogenesis in Higher Plants. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1359, pp. 387–394. [Google Scholar]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Pencik, A.; Tureková, V.; Paulisiç, S.; Rolcìk, J.; Strnad, M.; Mihaljevic, S. Ammonium regulates embryogenic potential in Cucurbita pepo through pH-mediated changes in endogenous auxin and abscisic acid. Plant Cell Tissue Organ Cult. 2015, 122, 89–100. [Google Scholar] [CrossRef]
- Zhang, S.T.; Zhu, C.; Zhang, X.Y.; Liu, M.Y.; Xue, X.D.; Lai, C.W.; Xuhan, X.; Chen, Y.K.; Zhang, Z.H.; Lai, Z.X.; et al. Single-cell RNA sequencing analysis of the embryogenic callus clarifies the spatiotemporal developmental trajectories of the early somatic embryo in Dimocarpus longan. Plant J. 2023, 115, 1277–1297. [Google Scholar] [CrossRef] [PubMed]
- Gaj, M.D. Somatic embryogenesis and plant regeneration in the culture of Arabidopsis thaliana (L.) Heynh. immature zygotic embryos. Methods Mol. Biol. 2011, 10, 257–265. [Google Scholar]
- Calabuig-Serna, A.; Mir, R.; Arjona, P.; Seguí-Simarro, J.M. Calcium dynamics and modulation in carrot somatic embryogenesis. Front. Plant Sci. 2023, 14, 1150198. [Google Scholar] [CrossRef]
- Sangra, A.; Shahin, L.; Dhir, S.K. Long-Term Maintainable Somatic Embryogenesis System in Alfalfa (Medicago sativa) Using Leaf Explants: Embryogenic Sustainability Approach. Plants 2019, 8, 278. [Google Scholar] [CrossRef]
- Garrocho-Villegas, V.; Jesús-Olivera, M.T.D.; Quintanar, E.S. Maize somatic embryogenesis: Recent features to improve plant regeneration. Methods Mol. Biol. 2012, 877, 173–182. [Google Scholar]
- Khanday, I.; Santos-Medellín, C.; Sundarensan, V. Somatic embryo initiation by rice BABY BOOM1 involves activation of zygote-expressed auxin biosynthesis genes. New Phytol. 2023, 238, 673–687. [Google Scholar] [CrossRef]
- Tribulato, A.; Remotti, P.C.; Löffler, H.J.M.; van Tuyl, J.M.V. Somatic embryogenesis and plant regeneration in Lilium longiflorum Thunb. Plant Cell Rep. 1997, 17, 113–118. [Google Scholar] [CrossRef]
- Song, S.L.; Wang, Z.P.; Ren, Y.M.; Sun, H.M. Full-Length Transcriptome Analysis of the ABCB, PIN/PIN-LIKE S, and AUX/LAX Families Involved in Somatic Embryogenesis of Lilium pumilum DC. Fisch. Int. J. Mol. Sci. 2020, 21, 453. [Google Scholar] [CrossRef]
- Zhang, J.; Gai, M.Z.; Li, X.Y.; Li, T.L.; Sun, H.M. Somatic embryogenesis and direct as well as indirect organogenesis in Lilium pumilum DC. Fisch., an endangered ornamental and medicinal plant. Biosci. Biotechnol. Biochem. 2016, 80, 1898–1906. [Google Scholar] [CrossRef]
- Nhut, D.T.; Van, L.B.; Minh, N.T.; de Silva, J.T.; Fukai, S.; Tanaka, M.; Van, K.T.T. Somatic embryogenesis through pseudo-bulblet transverse thin cell layer of Lilium longiflorum. Plant Growth Regul. 2002, 37, 193–198. [Google Scholar] [CrossRef]
- Khosravi, S.; Azghandi, A.V.; Mojtahedi, N.; Haddad, R. In vitro propagation of Lilium longiflorum var. ceb-dazzle through direct somatic embryogenesis. Pak. J. Biol. Sci. 2007, 10, 2517–2521. [Google Scholar] [CrossRef]
- Ho, C.-W.; Jian, W.-T.; Lai, H.-C. Plant regeneration via somatic embryogenesis from suspension cell cultures of Lilium × formolongi Hort. using a bioreactor system. Vitr. Cell. Dev. Biol. Plant 2006, 42, 240–246. [Google Scholar] [CrossRef]
- Mehdi, B.; Mesbah, B.; Masoud, M.; Ahmad, K. Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell Tiss Organ Cult. 2010, 102, 229–235. [Google Scholar]
- Yan, R.; Song, S.; Li, H.Y.; Sun, H.M. Functional analysis of the eTM-miR171-SCL6 module regulating somatic embryogenesis in Lilium pumilum DC. Fisch. Hortic. Res. 2022, 9, Uhac045. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, J.; Yan, R.; Wang, C.X.; Sun, H.M. Functional characterization of the MiR171a promoter and endogenous target mimics identifcation in Lilium pumilum DC. Fisch. during somatic embryogenesis. Plant Cell Tissue Organ Cult. 2020, 144, 345–357. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Santos, M.O.; Aragão, F.J.L. Role of SERK genes in plant environmental response. Plant Signal. Behav. 2009, 4, 1111–1113. [Google Scholar] [CrossRef]
- Gulzar, B.; Mujib, A.; Malik, M.Q.; Sayeed, R.; Mamgain, J.; Ejaz, B. Genes, proteins and other networks regulating somatic embryogenesis in plants. J. Genet. Eng. Biotechnol. 2020, 18, 31. [Google Scholar] [CrossRef]
- Somleva, M.N.; Schmidt, E.D.L.; Vriers, S.C.D. Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Rep. 2000, 19, 718–726. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Shan, L. SERKs. Curr. Biol. 2020, 30, R293–R294. [Google Scholar] [CrossRef]
- Chinchilla, D.; Shan, L.B.; He, P.; Vries, S.D.; Kemmerling, B. One for all: The receptor-associated kinase BAK1. Trends Plant Sci. 2009, 14, 535–541. [Google Scholar] [CrossRef]
- Ma, X.Y.; Xu, G.Y.; He, P.; Shan, L.B. SERKing Coreceptors for Receptors. Trends Plant Sci. 2016, 21, 1017–1033. [Google Scholar] [CrossRef]
- Li, J. Multi-tasking of somatic embryogenesis receptor-like protein kinases. Curr. Opin. Plant Biol. 2010, 13, 509–514. [Google Scholar] [CrossRef]
- Hecht, V.; Vielle-Calzada, J.P.V.; Hartog, M.V.; Schmidt, E.D.; Boutilier, K.; Grossniklaus, U.; Vries, S.C.D. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001, 27, 803–816. [Google Scholar] [CrossRef]
- Ito, Y.; Takaya, K.; Kurata, N. Expression of SERK family receptor-like protein kinase genes in rice. Biochim. Biophys. Acta 2005, 1730, 253–258. [Google Scholar] [CrossRef]
- Pérez-Núñez, M.T.; Souza, R.; Sáenz, L.; Chan, J.L.; Zúñiga-Aguilar, J.J.; Oropeza, C. Detection of a SERK-like gene in coconut and analysis of its expression during the formation of embryogenic callus and somatic embryos. Plant Cell Rep. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L.; Yang, Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 2005, 222, 107–117. [Google Scholar] [CrossRef]
- Sharma, S.K.; Millam, S.; Hein, I.; Bryan, G.J. Cloning and molecular characterisation of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta 2008, 228, 319–330. [Google Scholar] [CrossRef]
- Santos, M.O.; Romano, E.; Vieira, L.S.; Baldoni, A.B.; Aragão, F.J.L. Suppression of SERK gene expression affects fungus tolerance and somatic embryogenesis in transgenic lettuce. Plant Biol. 2009, 11, 83–89. [Google Scholar] [CrossRef]
- Karlova, R.; Boeren, S.; Russinova, E.; Aker, J.; Vervoort, J.; Vries, S.D. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell 2006, 18, 626–638. [Google Scholar] [CrossRef]
- Kwaaitaal, M.A.C.J.; Vries, S.C.D.; Russinova, E. Arabidopsis thaliana Somatic Embryogenesis Receptor Kinase 1 protein is present in sporophytic and gametophytic cells and undergoes endocytosis. Protoplasma 2005, 226, 55–65. [Google Scholar] [CrossRef]
- Zuo, S.M.; Zhou, X.G.; Chen, M.S.; Zhang, S.L.; Schwessinger, B.; Ruan, D.L.; Yuan, C.; Wang, J.; Chen, X.W.; Ronald, P.C. OsSERK1 regulates rice development but not immunity to Xanthomonas oryzae pv. oryzae or Magnaporthe oryzae. J. Integr. Plant Biol. 2014, 56, 1179–1192. [Google Scholar] [CrossRef]
- Baudino, S.; Hansen, S.; Brettschneider, R.; Hecht, V.F.; Dresselhaus, T.; Lörz, H.; Dumas, C.; Rogowsky, P.M. Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta 2001, 213, 1–10. [Google Scholar] [CrossRef]
- Galaz-Ávalos, R.M.; Martínez-Sánchez, H.G.; Loyola-Vargas, V.M. Induction of Somatic Embryogenesis in Jatropha curcas. Methods Mol. Biol. 2018, 1815, 207–214. [Google Scholar]
- Salaün, C.; Lepiniec, L.; Dubreucq, B. Genetic and Molecular Control of Somatic Embryogenesis. Plants 2021, 10, 1467. [Google Scholar] [CrossRef]
- Radoeva, T.; Weijers, D. A roadmap to embryo identity in plants. Trends Plant Sci. 2014, 19, 709–716. [Google Scholar] [CrossRef]
- Nakano, M.; Sakakibara, T.; Suzuki, S.; Saito, H. Decrease in the regeneration potential of long-term cell suspension cultures of Lilium formosanum Wallace and its restoration by the auxin transport inhibitor, 2,3,5-triiodobenzoic acid. Plant Sci. 2000, 158, 129–137. [Google Scholar] [CrossRef]
- Lu, R.; Martin-Hernandez, A.M.; Peart, J.R.; Malcuit, I.; Baulcombe, D.C. Virus-induced gene silencing in plants. Methods 2003, 30, 296–303. [Google Scholar] [CrossRef]
- Tasaki, K.; Terada, H.; Masuta, C.; Yamagishi, M. Virus-induced gene silencing (VIGS) in Lilium leichtlinii using the Cucumber mosaic virus vector. Plant Biotechnol. 2016, 33, 373–381. [Google Scholar] [CrossRef]
- Liu, X.P.; Yan, W.X.; Liu, S.J.; Wu, J.; Leng, P.S.; Hu, Z.H. LiNAC100 contributes to linalool biosynthesis by directly regulating LiLiS in Lilium ‘Siberia’. Planta 2024, 259, 73. [Google Scholar] [CrossRef] [PubMed]
- Carlquist, S. The use of ethylenediamine in softening hard plant structures for paraffin sectioning. Stain. Technol. 1982, 57, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Z.; Lin, M.; Ali, M.M.; Zheng, Y.P.; Yi, X.Y.; Wang, S.J.; Chen, F.X.; Lin, Z.M. LhANS-rr1, LhDFR, and LhMYB114 Regulate Anthocyanin Biosynthesis in Flower Buds of Lilium ‘Siberia’. Genes 2023, 14, 559. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yi, X.; Zhang, L.; Ali, M.M.; Ke, M.; Lu, Y.; Zheng, Y.; Cai, X.; Fang, S.; Wu, J.; et al. Characterisation and Expression Analysis of LdSERK1, a Somatic Embryogenesis Gene in Lilium davidii var. unicolor. Plants 2024, 13, 1495. https://doi.org/10.3390/plants13111495
Wang S, Yi X, Zhang L, Ali MM, Ke M, Lu Y, Zheng Y, Cai X, Fang S, Wu J, et al. Characterisation and Expression Analysis of LdSERK1, a Somatic Embryogenesis Gene in Lilium davidii var. unicolor. Plants. 2024; 13(11):1495. https://doi.org/10.3390/plants13111495
Chicago/Turabian StyleWang, Shaojuan, Xiaoyan Yi, Lijuan Zhang, Muhammad Moaaz Ali, Mingli Ke, Yuxian Lu, Yiping Zheng, Xuanmei Cai, Shaozhong Fang, Jian Wu, and et al. 2024. "Characterisation and Expression Analysis of LdSERK1, a Somatic Embryogenesis Gene in Lilium davidii var. unicolor" Plants 13, no. 11: 1495. https://doi.org/10.3390/plants13111495
APA StyleWang, S., Yi, X., Zhang, L., Ali, M. M., Ke, M., Lu, Y., Zheng, Y., Cai, X., Fang, S., Wu, J., Lin, Z., & Chen, F. (2024). Characterisation and Expression Analysis of LdSERK1, a Somatic Embryogenesis Gene in Lilium davidii var. unicolor. Plants, 13(11), 1495. https://doi.org/10.3390/plants13111495





