Genome-Wide Identification Analysis of GST Gene Family in Wild Blueberry Vaccinium duclouxii and Their Impact on Anthocyanin Accumulation
Abstract
:1. Introduction
2. Result
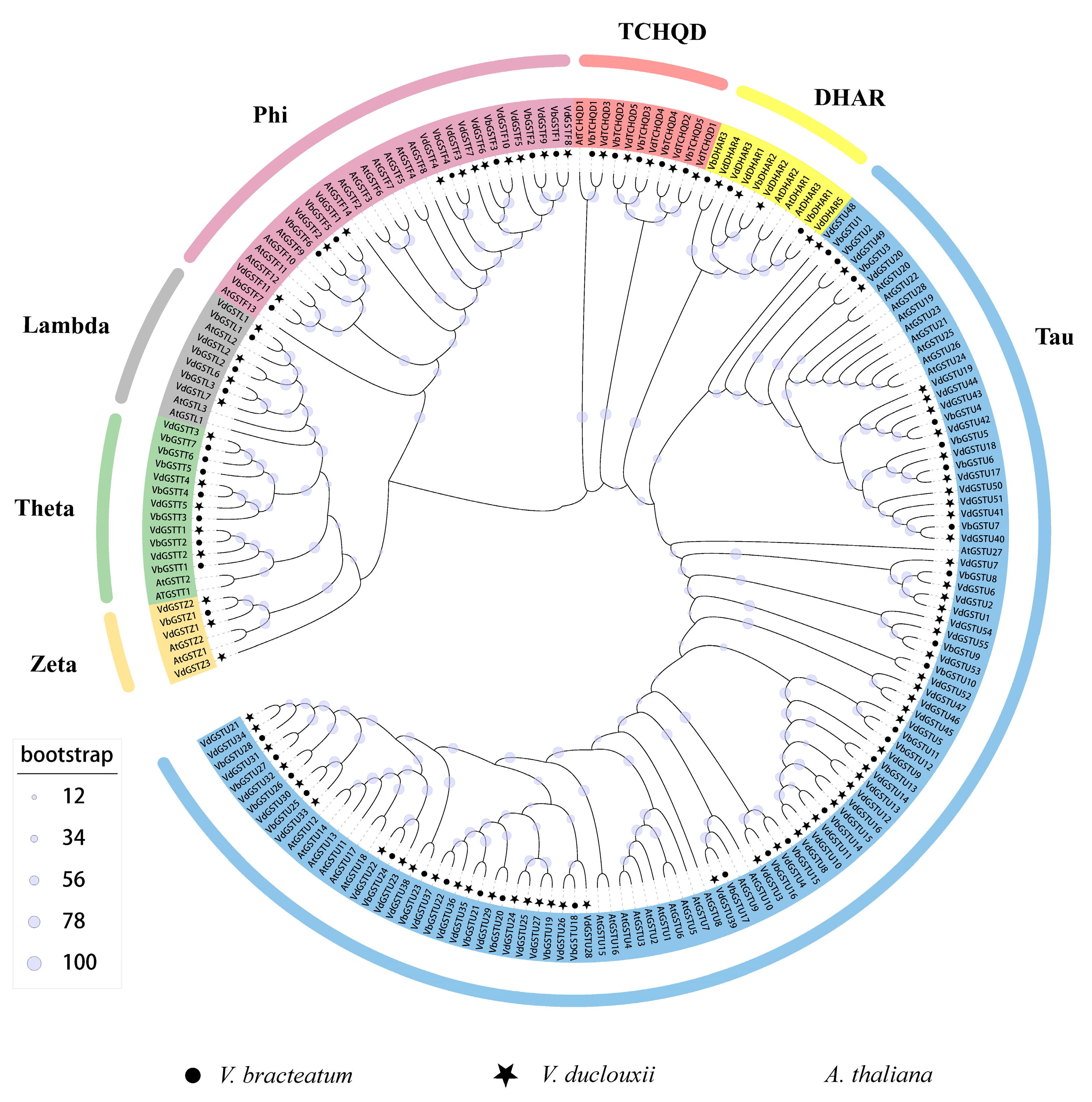
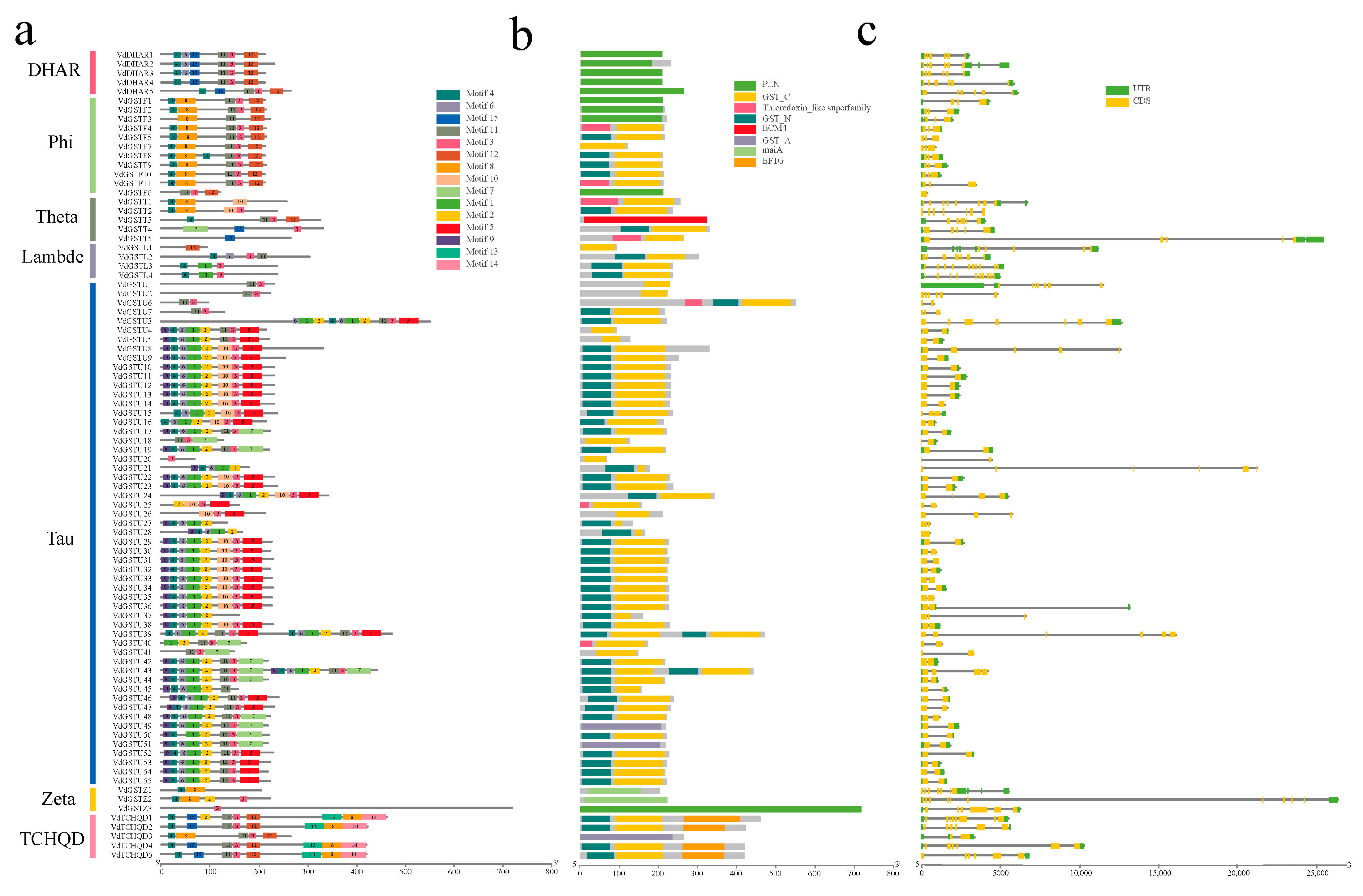
2.1. Identification Bioinformatics, and Phylogenetic Analysis of the VdGSTs Gene Family
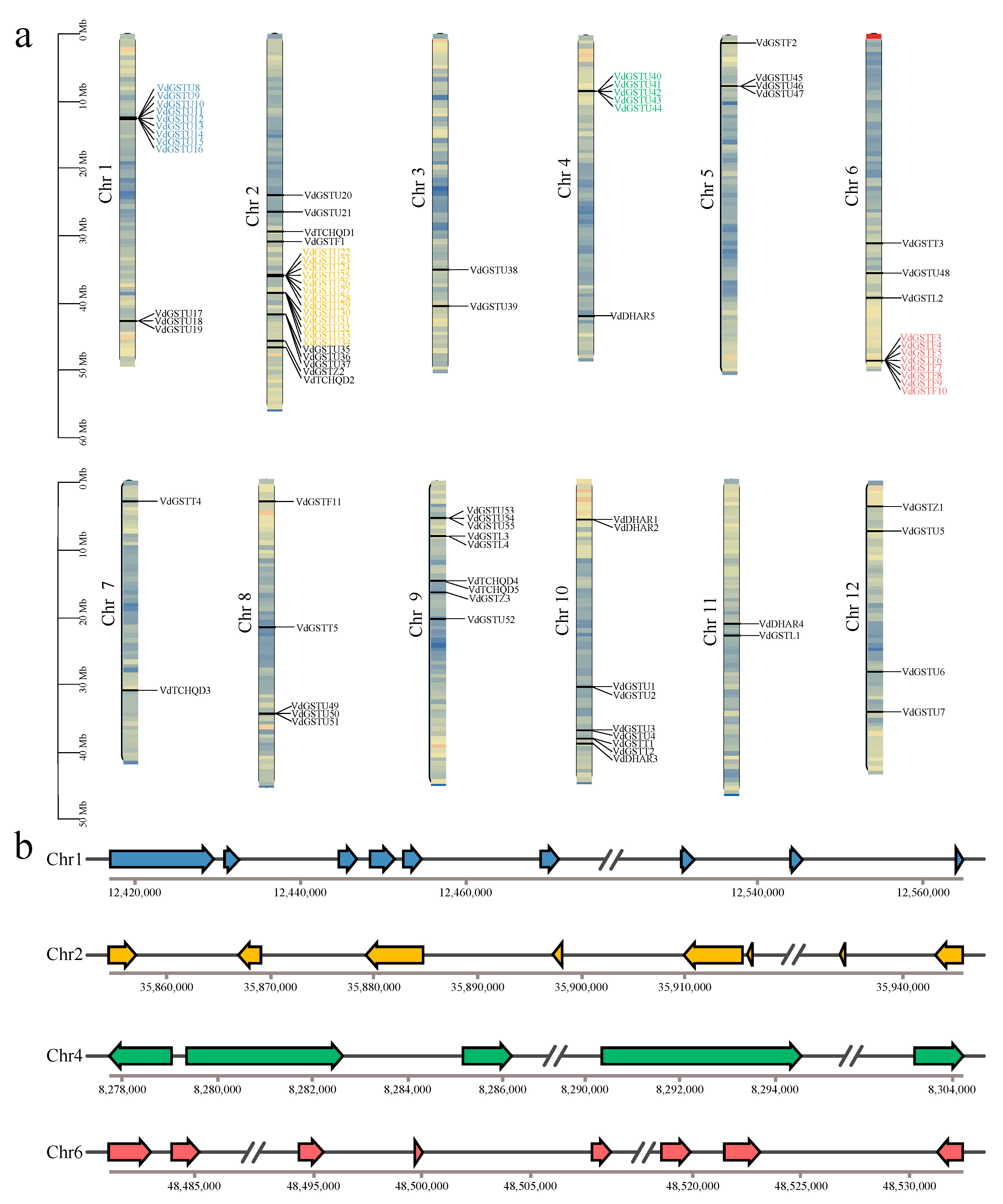
2.2. Chromosomal Localization and Synteny Analysis of the VdGSTs Gene Family
2.3. Duplication, Synthesis, and Evolutionary Analysis of VdGSTs Genes Family
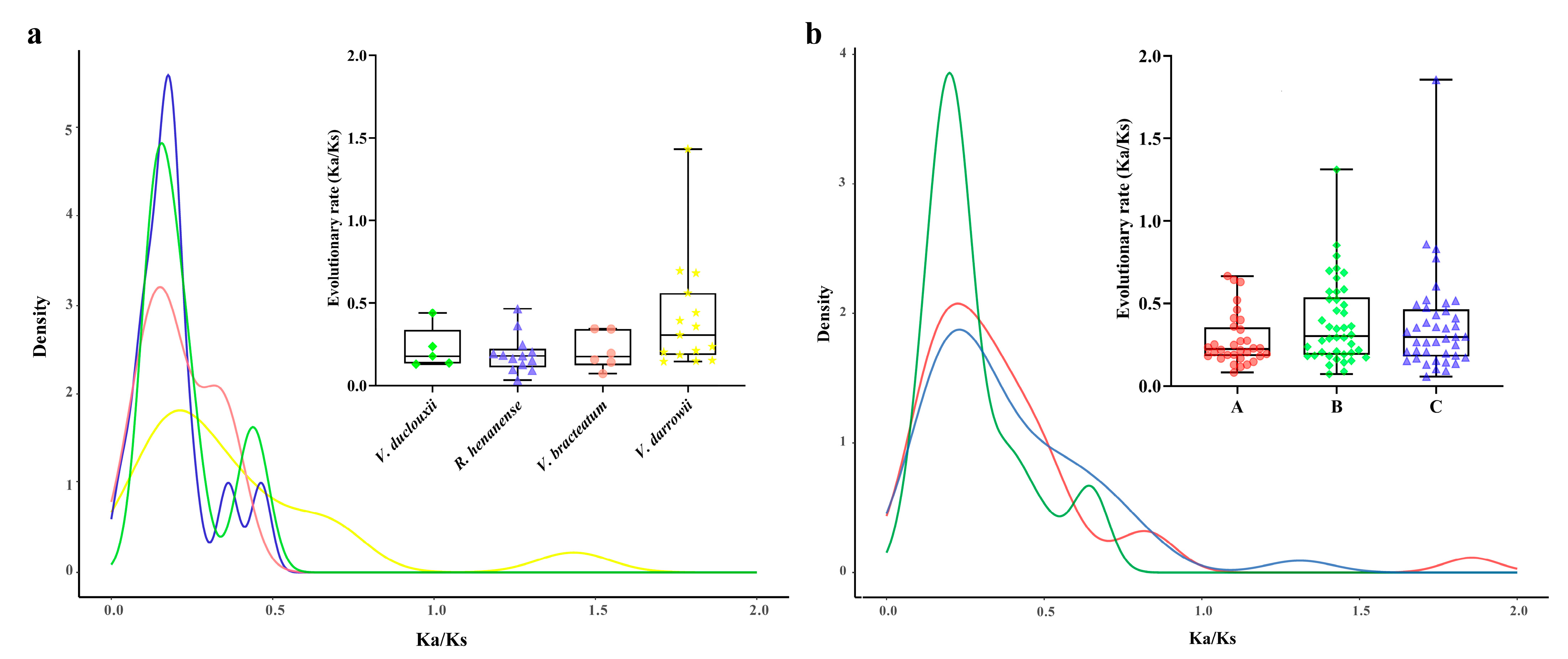
2.4. Analysis of Non-Synonymous (Ka) and Synonymous (Ks) Substitution Patterns in Protein-Coding Genes
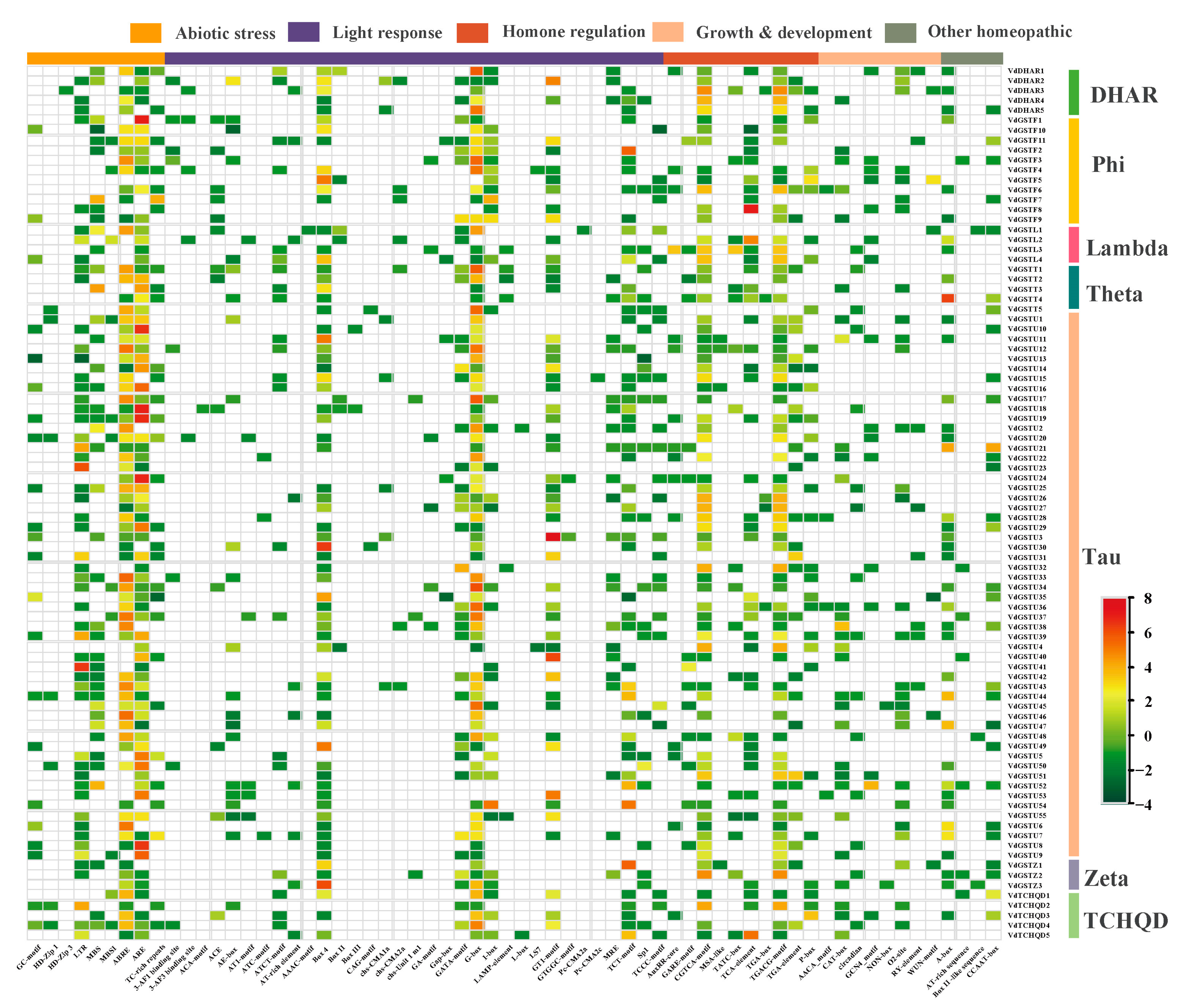
2.5. Identification of Cis-Acting Regulatory Elements in the Promoter Regions of the VdGSTs
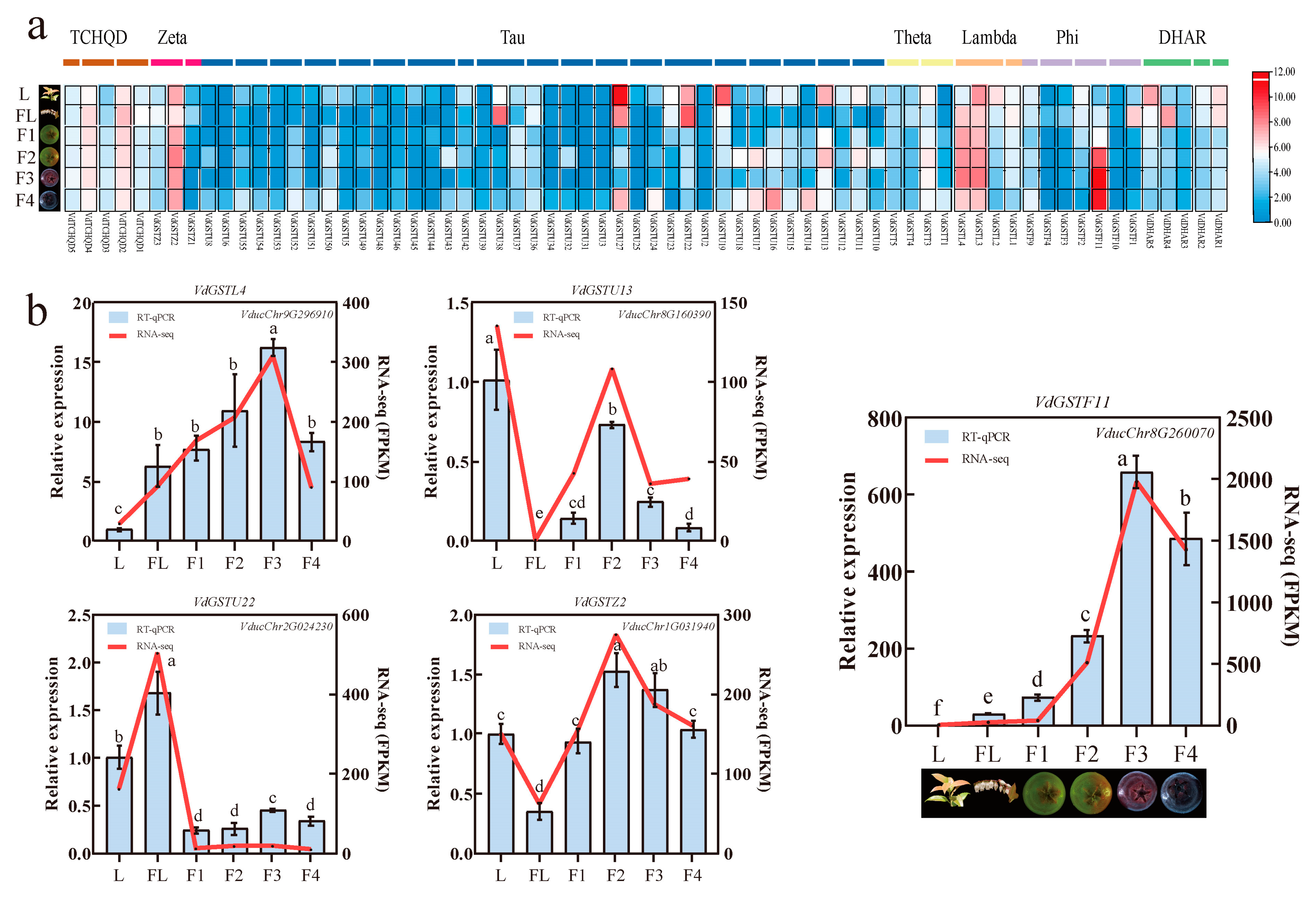
2.6. Transcriptome Data Analysis and Expression Analysis of VdGSTs Genes in Various Tissues
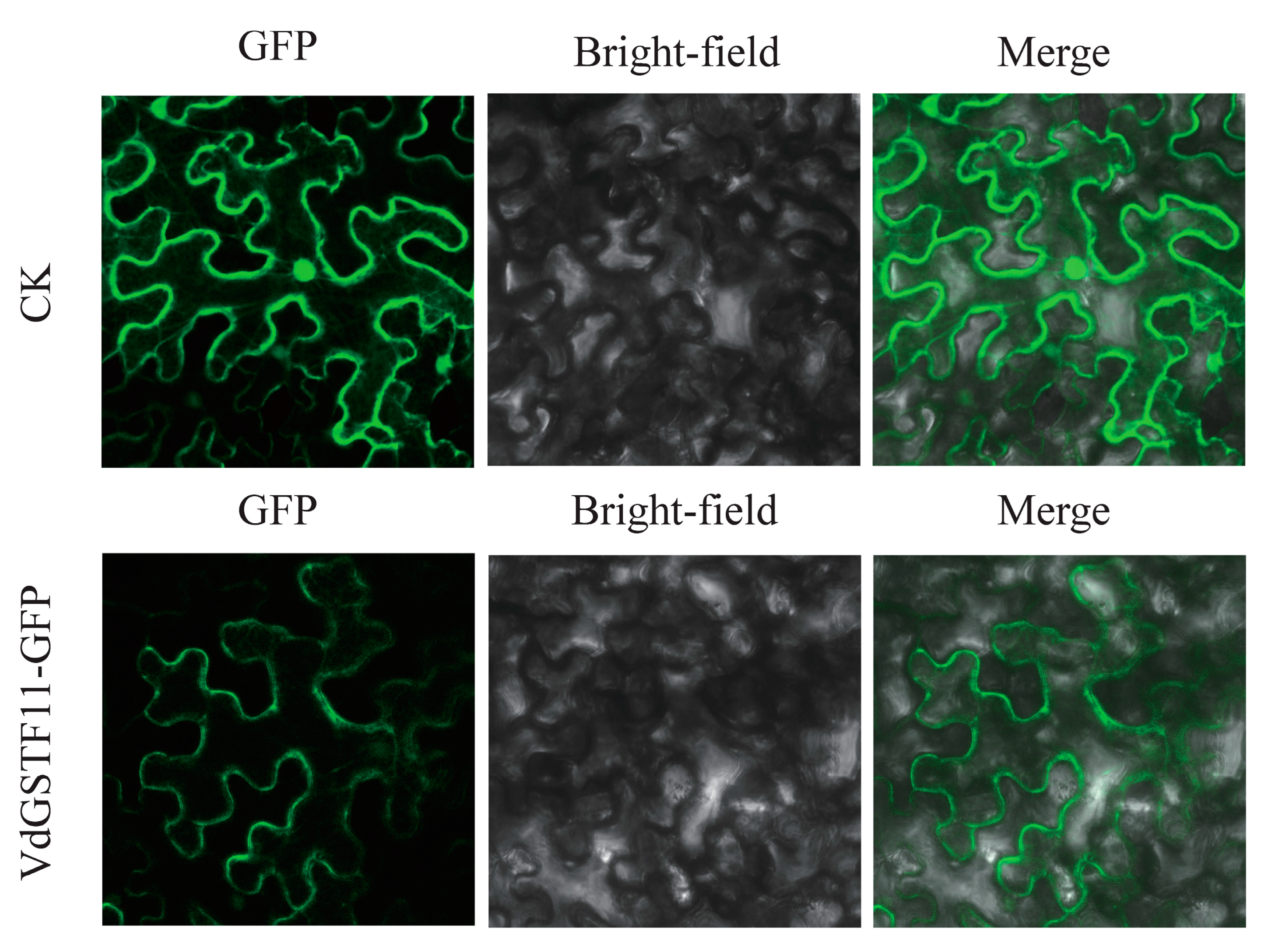
2.7. Validation of VdGSTF11 Expression by qRT-PCR and Its Subcellular Localization
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Identification and Phylogenetic Analysis of VdGST Gene Family in V. duclouxii
4.3. Chromosomal Localization, Gene Structure Prediction, and Motif Composition of VdGSTs
4.4. Comparative Genomics Analysis of V. duclouxii with Four Other Species
4.5. Prediction of Cis-Regulatory Elements in VdGSTs Gene Promoters
4.6. Expression Analysis of VdGSTs Genes Based on RNA-Seq Data
4.7. Validation of Transcriptome Data with qRT-PCR
4.8. Subcellular Localization Analysis of VdGSTF11
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Lawand, C.; Ryan, D.A.J.; McDonald, J.E.; Donner, H.; Forney, C.F. Oxygen radical absorbing capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum L.) during ripening and storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. [Google Scholar] [CrossRef]
- Moze, S.; Polak, T.; Gasperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.a.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium species (Ericaceae): Phytochemistry and biological properties of medicinal plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Yu, J.; Chen, X.; Wang, L.S. Potential of dietary supplementation with berries to enhance immunity in humans. J. Food Bioact. 2021, 16, 19–24. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, A.T., Jr.; Chau, H.K.; Strahan, G.D.; Nuñez, A.; Simon, S.; White, A.K.; Dieng, S.; Heuberger, E.R.; Yadav, M.P.; Hirsch, J. Structure and composition of blueberry fiber pectin and xyloglucan that bind anthocyanins during fruit puree processing. Food Hydrocoll. 2021, 116, 106572. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From mechanisms of regulation in plants to health benefits in foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar] [CrossRef] [PubMed]
- Vaish, S.; Gupta, D.; Mehrotra, R.; Mehrotra, S.; Basantani, M.K. Glutathione S-transferase: A versatile protein family. 3 Biotech 2020, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-mediated regulation of anthocyanin biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-W.; Wang, C.-K.; Huang, X.-Y.; Hu, D.-G. Genome-wide analysis of the glutathione s-transferase (GST) genes and functional identification of MdGSTU12 reveals the involvement in the regulation of anthocyanin accumulation in apple. Genes 2021, 12, 1733. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, Y.; Zhang, A.; Wu, H.; Liu, Z.; Ren, X. Molecular cloning and functional characterization of AcGST1, an anthocyanin-related glutathione S-transferase gene in kiwifruit (Actinidia chinensis). Plant Mol. Biol. 2019, 100, 451–465. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Liu, T.; Zhao, Y.; Zhao, X.; Liu, J.; Zhang, J. Functional analysis of the anthocyanin-associated glutathione s-transferase gene StGST1 in potato. Potato Res. 2022, 66, 215–230. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, H.; Pan, L.; Niu, L.; Wei, B.; Cui, G.; Wang, L.; Yao, J.L.; Zeng, W.; Wang, Z. Two loss-of-function alleles of the glutathione S-transferase (GST) gene cause anthocyanin deficiency in flower and fruit skin of peach (Prunus persica). Plant J. 2021, 107, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- He, Z.J.; He, J.W.; Yang, Z.S.; He, G.Q.; Yang, Y.L.; Wang, C.W.; Yang, H.T. Investigation and studying on nutrition composition of two species of Vaccinium L. in Yunnan. Chin. Agric. Sci. Bull. 2014, 30, 242–245. [Google Scholar]
- Lei, L.; Liu, G.; Yan, D.; Zhang, M.; Cui, Q.; Zhao, Q.; Chu, L.; Wen, L.; Wang, L.; Du, Q. Manipulation of ploidy for blueberry breeding: In vitro chromosome doubling of diploid Vaccinium duclouxii (Lévl.) Hand.-Mazz by trifluralin. Sci. Hortic. 2023, 317, 112056. [Google Scholar] [CrossRef]
- Zeng, T.; He, Z.J.; He, J.F.; Lv, W.; Huang, S.X.; Li, J.W.; Zhu, L.Y.; Wan, S.; Zhou, W.F.; Yang, Z.S.; et al. The Telomere-to-telomere gap-free reference genome of wild blueberry (Vaccinium duclouxii) provides its high soluble sugar and anthocyanin accumulation. Hortic. Res. 2023, 10, uhad209. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon–intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Han, L.; Zou, H.; Zhou, L.; Wang, Y. Transcriptome-based identification and expression analysis of the glutathione S-transferase(GST)family in tree peony reveals a likely role in anthocyanin transport. Hortic. Plant J. 2022, 8, 787–802. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, F.; Xiang, L.; Chen, K. The isolation and identification of anthocyanin-related GSTs in Chrysanthemum. Horticulturae 2021, 7, 231. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, W.; Zhu, Y.; Allan, A.C.; Lin-Wang, K.; Xu, C. PpGST1, an anthocyanin-related glutathione S-transferase gene, is essential for fruit coloration in peach. Plant Biotechnol. J. 2019, 18, 1284–1295. [Google Scholar] [CrossRef]
- Han, L.; Zhou, L.; Zou, H.; Yuan, M.; Wang, Y. PsGSTF3, an anthocyanin-related glutathione s-transferase gene, is essential for petal coloration in tree peony. Int. J. Mol. Sci. 2022, 23, 1423. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, R.; Madrid-Espinoza, J.; Salinas-Cornejo, J.; González-Villanueva, E.; Ruiz-Lara, S. Differential roles for VviGST1, VviGST3, and VviGST4 in proanthocyanidin and anthocyanin transport in Vitis vinífera. Front. Plant Sci. 2016, 7, 1166. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, L.; Zhang, J.; Zhang, Y.; Wang, Y.; Chen, Q.; Luo, Y.; Zhang, Y.; Li, M.; Wang, X. Identification of anthocyanins-related glutathione S-transferase (GST) genes in the genome of cultivated strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2020, 21, 8708. [Google Scholar] [CrossRef] [PubMed]
- Smit, S.J.; Lichman, B.R. Plant biosynthetic gene clusters in the context of metabolic evolution. Nat. Prod. Rep. 2022, 39, 1465–1482. [Google Scholar] [CrossRef] [PubMed]
- Estévez, I.H.; Hernández, M.R. Plant glutathione S-transferases: An overview. Plant Gene 2020, 23, 100233. [Google Scholar] [CrossRef]
- Zuo, D.; Lei, S.; Qian, F.; Gu, L.; Wang, H.; Du, X.; Zeng, T.; Zhu, B. Genome-wide identification and stress response analysis of BcaCPK gene family in amphidiploid Brassica carinata. BMC Plant Biol. 2024, 24, 296. [Google Scholar] [CrossRef]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 364396. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhao, S.-W.; Shi, T.-L.; Zhao, W.; Zhang, R.-G.; Tian, X.-C.; Guo, J.-F.; Yan, X.-M.; Bao, Y.-T.; Li, Z.-C. Gapless genome assembly of azalea and multi-omics investigation into divergence between two species with distinct flower color. Hortic. Res. 2023, 10, uhac241. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, M.; Shen, M.; Bu, S.; Zhu, B.; He, F.; Zhang, X.; Gao, X.; Xiao, J. Chromosome-level genome assembly and annotation of the native Chinese wild blueberry Vaccinium bracteatum. Fruit Res. 2022, 2, 8. [Google Scholar] [CrossRef]
- Yu, J.; Hulse-Kemp, A.M.; Babiker, E.; Staton, M. High-quality reference genome and annotation aids understanding of berry development for evergreen blueberry (Vaccinium darrowii). Hortic. Res. 2021, 8, 228. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.W.; Xu, Z.Z.; Zhou, L.; Li, J.J.; Yu, Q.; Luo, J.; Chan, Z.L.; Jongsma, M.A.; Hu, H.; et al. TcMYC2 regulates pyrethrin biosynthesis in Tanacetum cinerariifolium. Hortic. Res. 2022, 9, uhac178. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 408, 402–408. [Google Scholar] [CrossRef]
- Valenzuela, F.; D’Afonseca, V.; Hernández, R.; Gómez, A.; Arencibia, A.D. Validation of reference genes in a population of blueberry (Vaccinium corymbosum) plants regenerated in colchicine. Plants 2022, 11, 2645. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.; Zhu, L.; Tan, L.; Gu, L.; Wang, H.; Du, X.; Zhu, B.; Zeng, T.; Wang, C. Genome-Wide Identification Analysis of GST Gene Family in Wild Blueberry Vaccinium duclouxii and Their Impact on Anthocyanin Accumulation. Plants 2024, 13, 1497. https://doi.org/10.3390/plants13111497
Lv W, Zhu L, Tan L, Gu L, Wang H, Du X, Zhu B, Zeng T, Wang C. Genome-Wide Identification Analysis of GST Gene Family in Wild Blueberry Vaccinium duclouxii and Their Impact on Anthocyanin Accumulation. Plants. 2024; 13(11):1497. https://doi.org/10.3390/plants13111497
Chicago/Turabian StyleLv, Wei, Liyong Zhu, Lifa Tan, Lei Gu, Hongcheng Wang, Xuye Du, Bin Zhu, Tuo Zeng, and Caiyun Wang. 2024. "Genome-Wide Identification Analysis of GST Gene Family in Wild Blueberry Vaccinium duclouxii and Their Impact on Anthocyanin Accumulation" Plants 13, no. 11: 1497. https://doi.org/10.3390/plants13111497
APA StyleLv, W., Zhu, L., Tan, L., Gu, L., Wang, H., Du, X., Zhu, B., Zeng, T., & Wang, C. (2024). Genome-Wide Identification Analysis of GST Gene Family in Wild Blueberry Vaccinium duclouxii and Their Impact on Anthocyanin Accumulation. Plants, 13(11), 1497. https://doi.org/10.3390/plants13111497






