Fine Root Density Dynamics and Carbon Stock of Eucalyptus spp.: Interplay of Age, Genotype, and Edaphoclimatic Conditions
Abstract
1. Introduction
2. Results
2.1. Compartmentalized Carbon Stock According to Site, Age, and Genotype
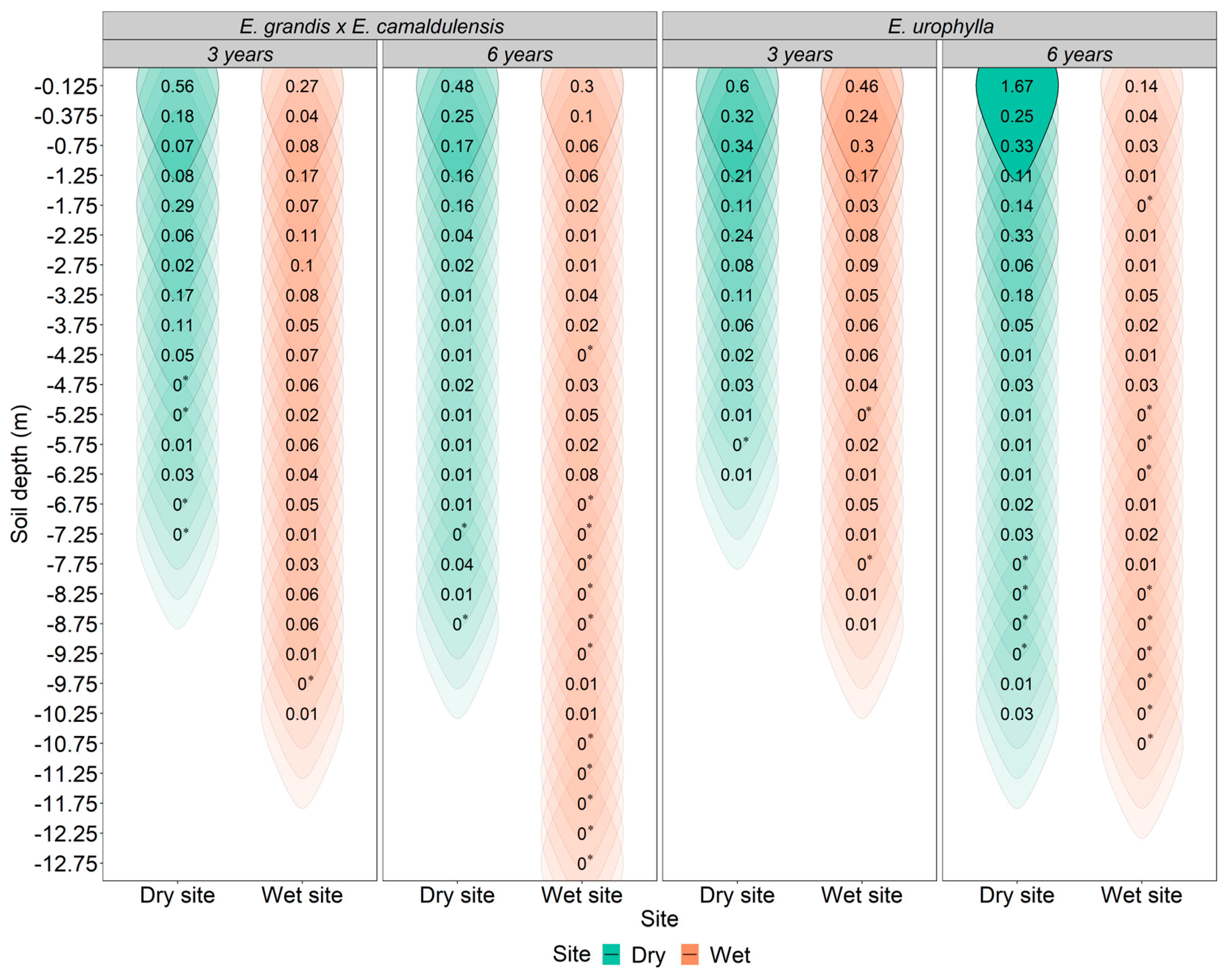
2.2. Vertical Distribution of Fine Root Density
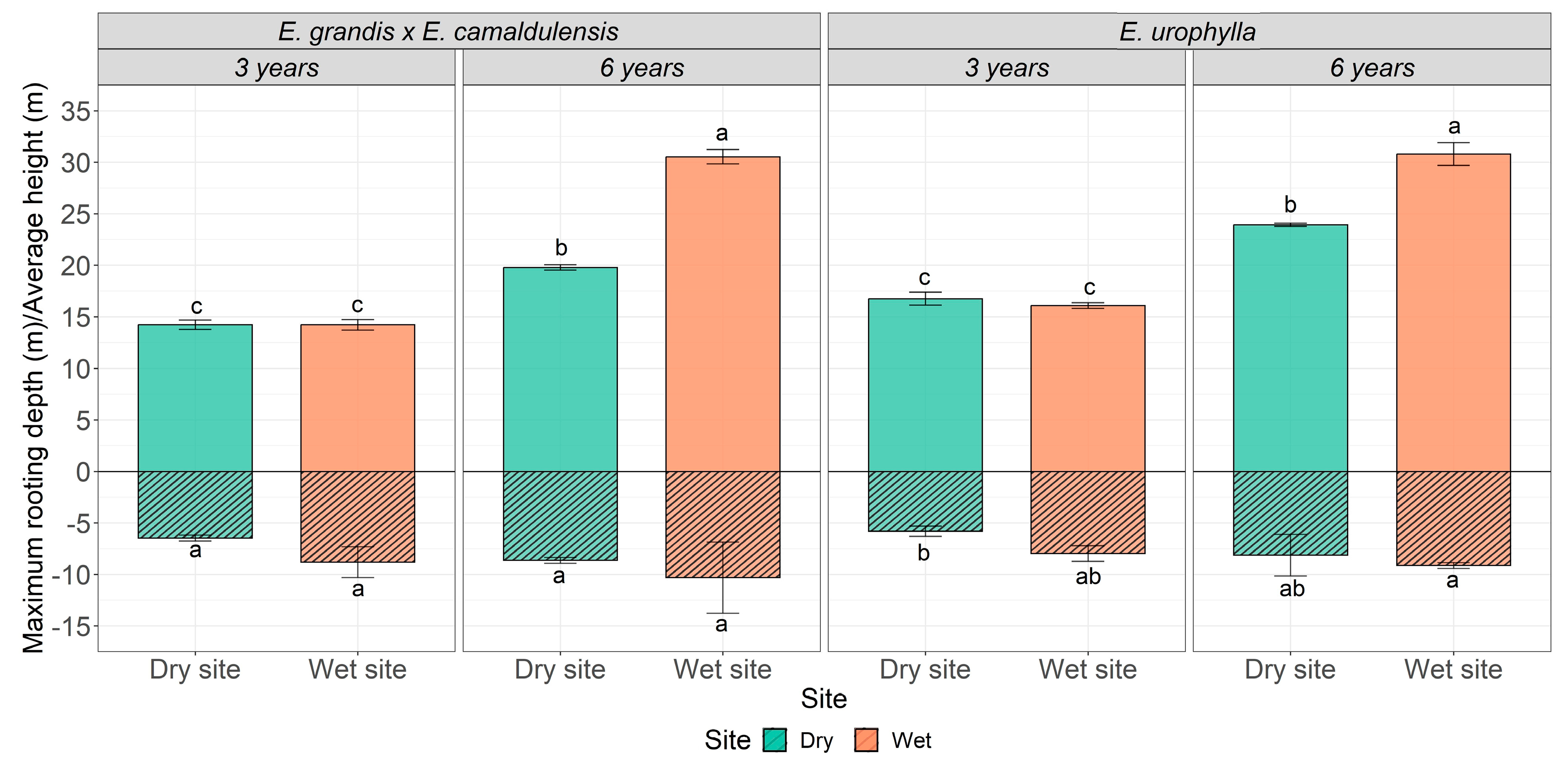
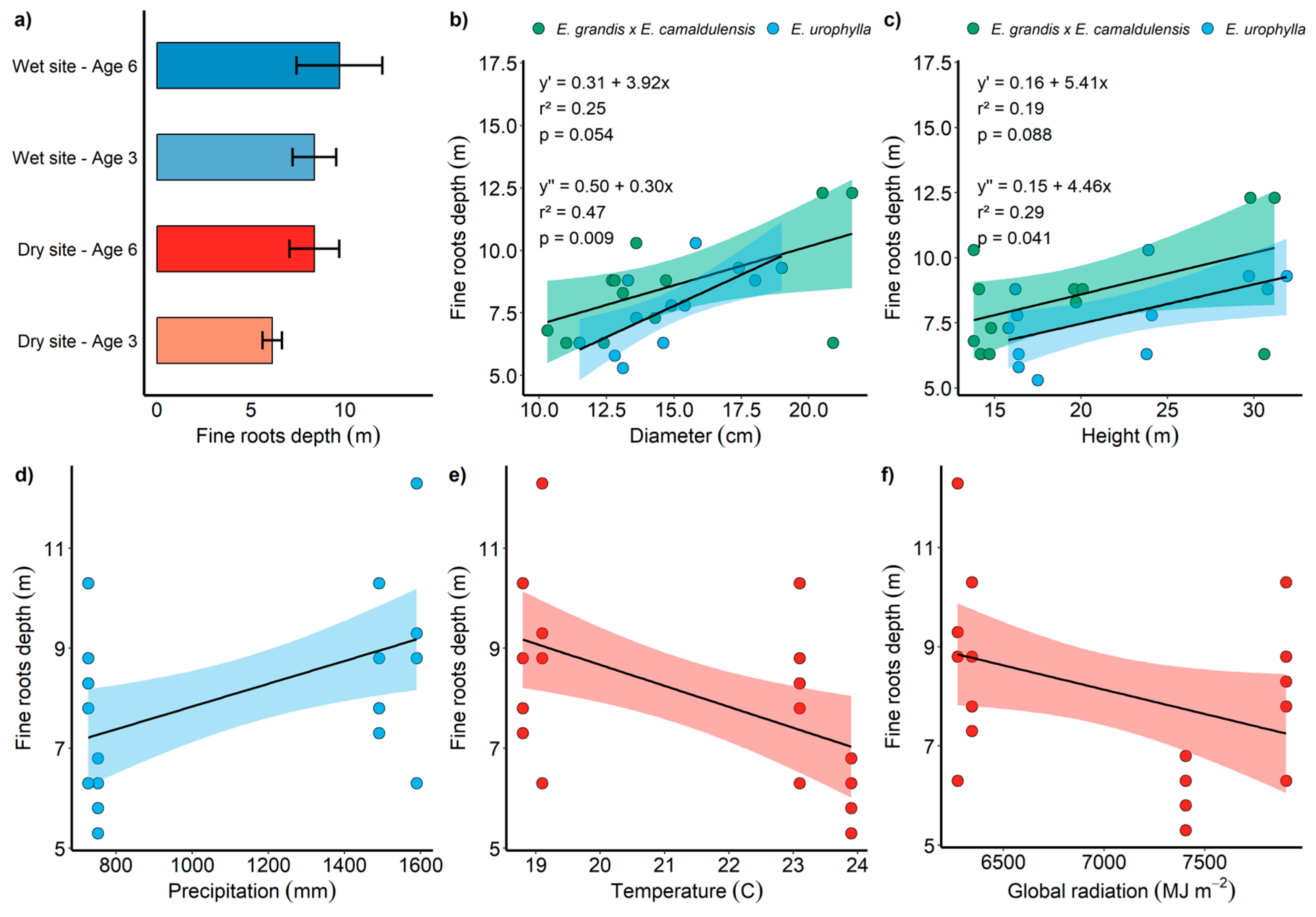
2.3. Root Depth and Height Growth
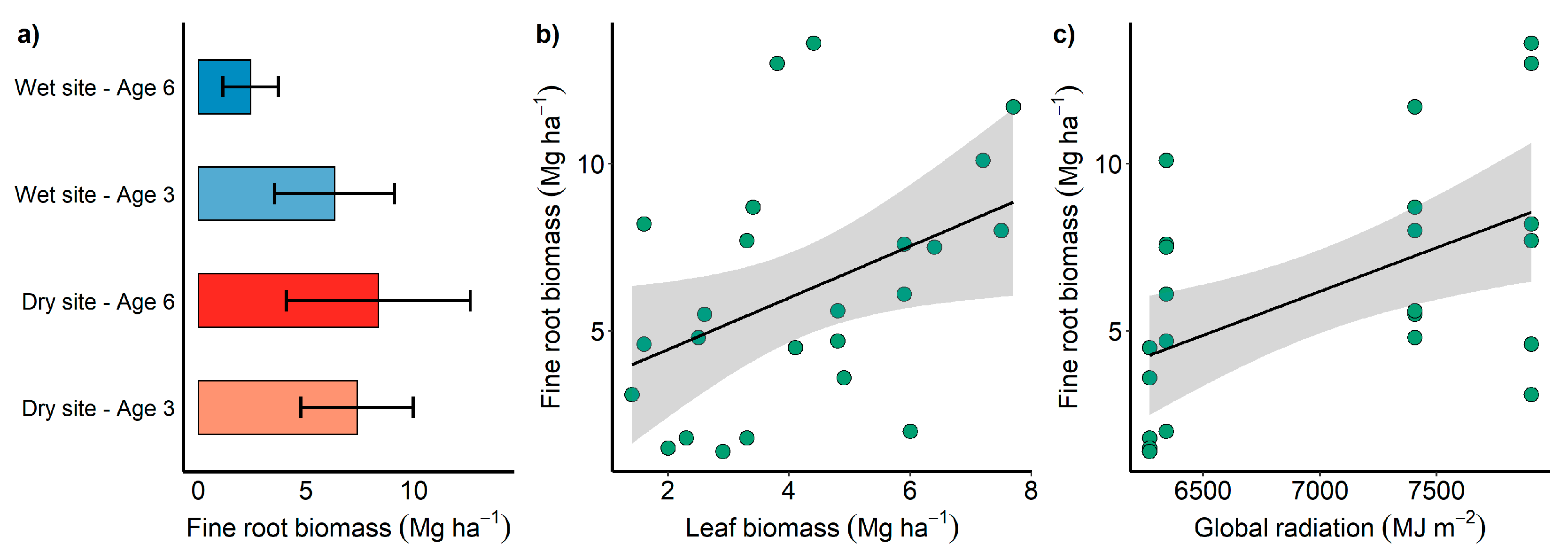
2.4. Climate and Its Influence on the Distribution and Depth of Fine Roots
3. Discussion
4. Materials and Methods
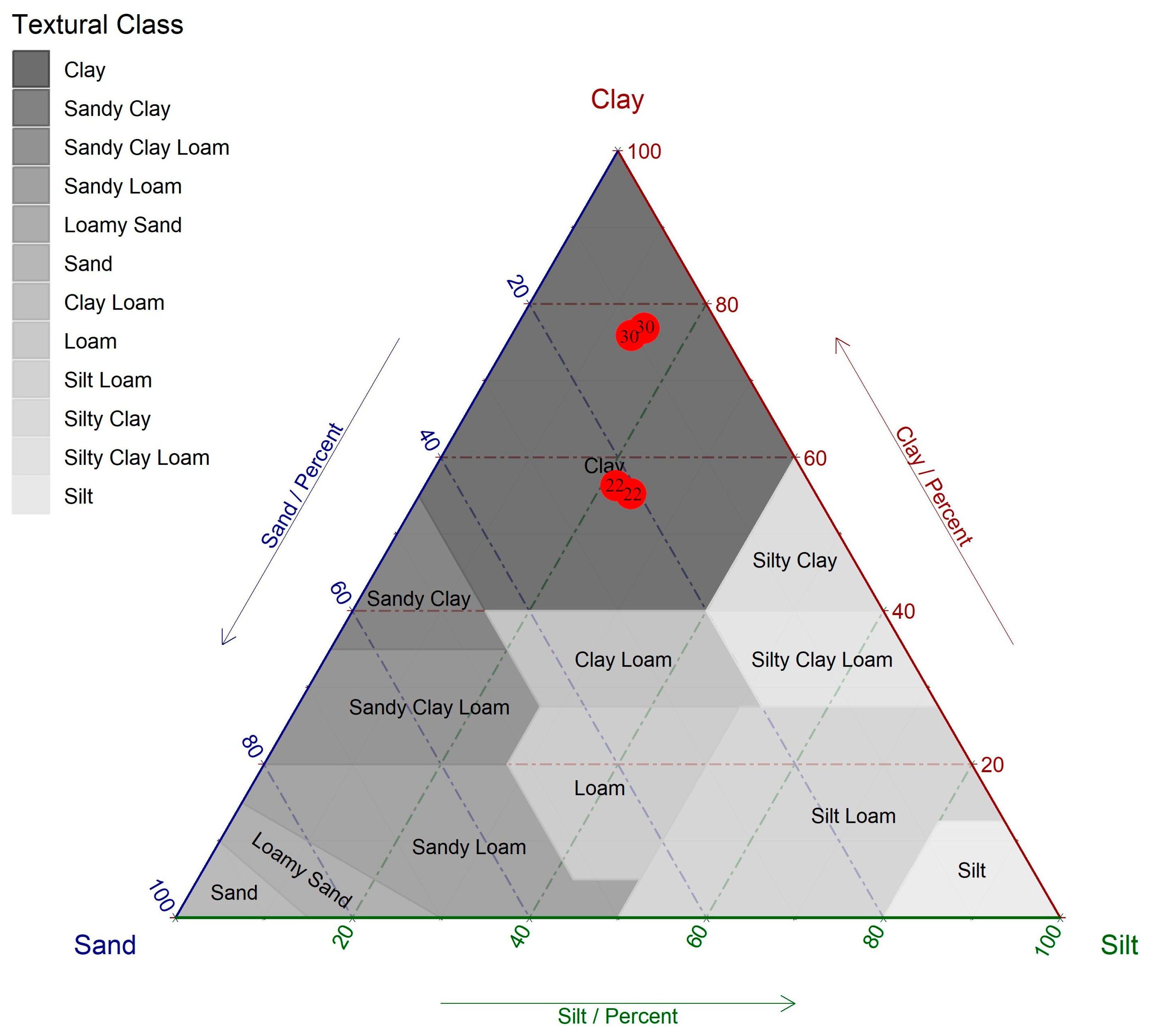
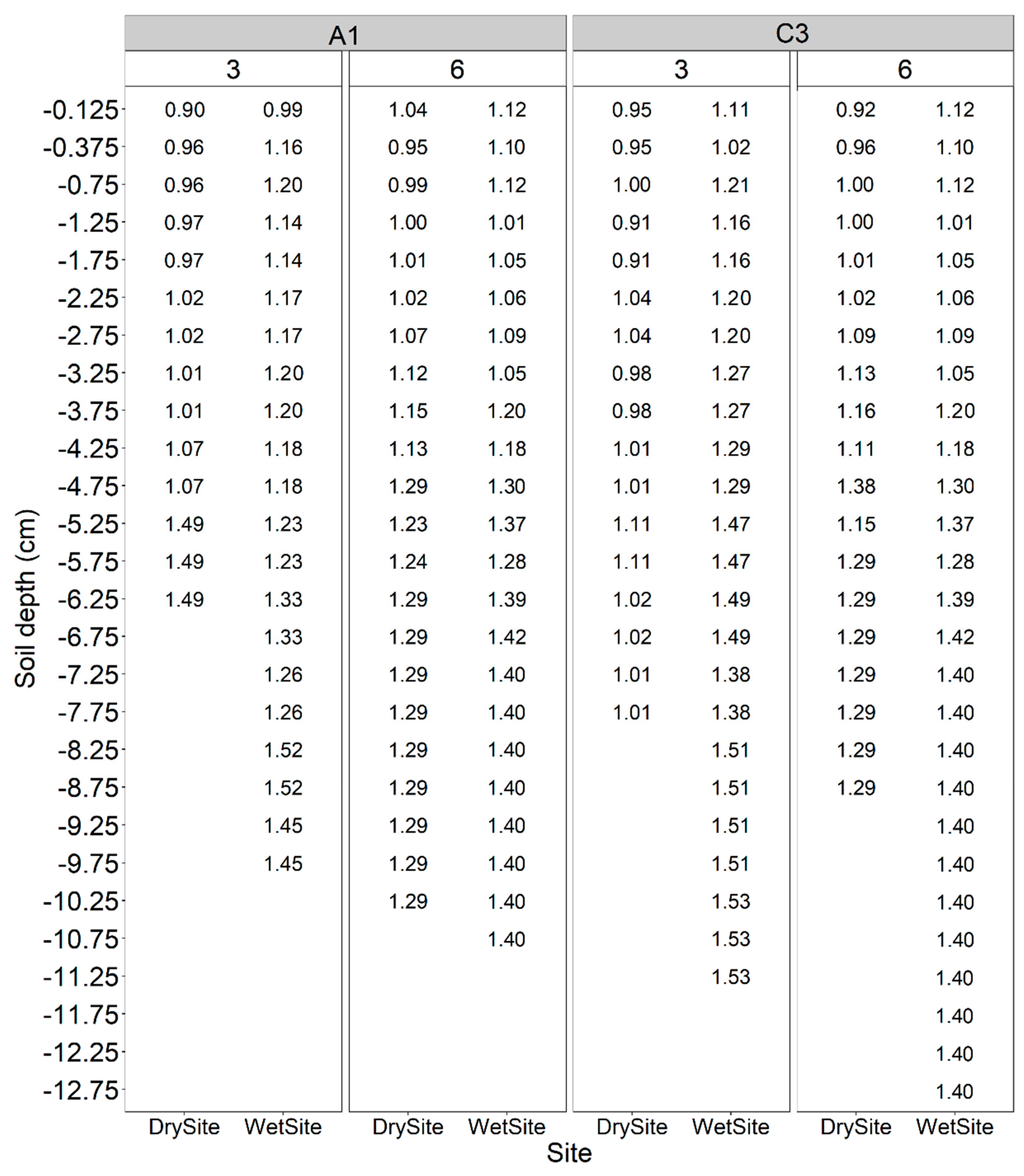
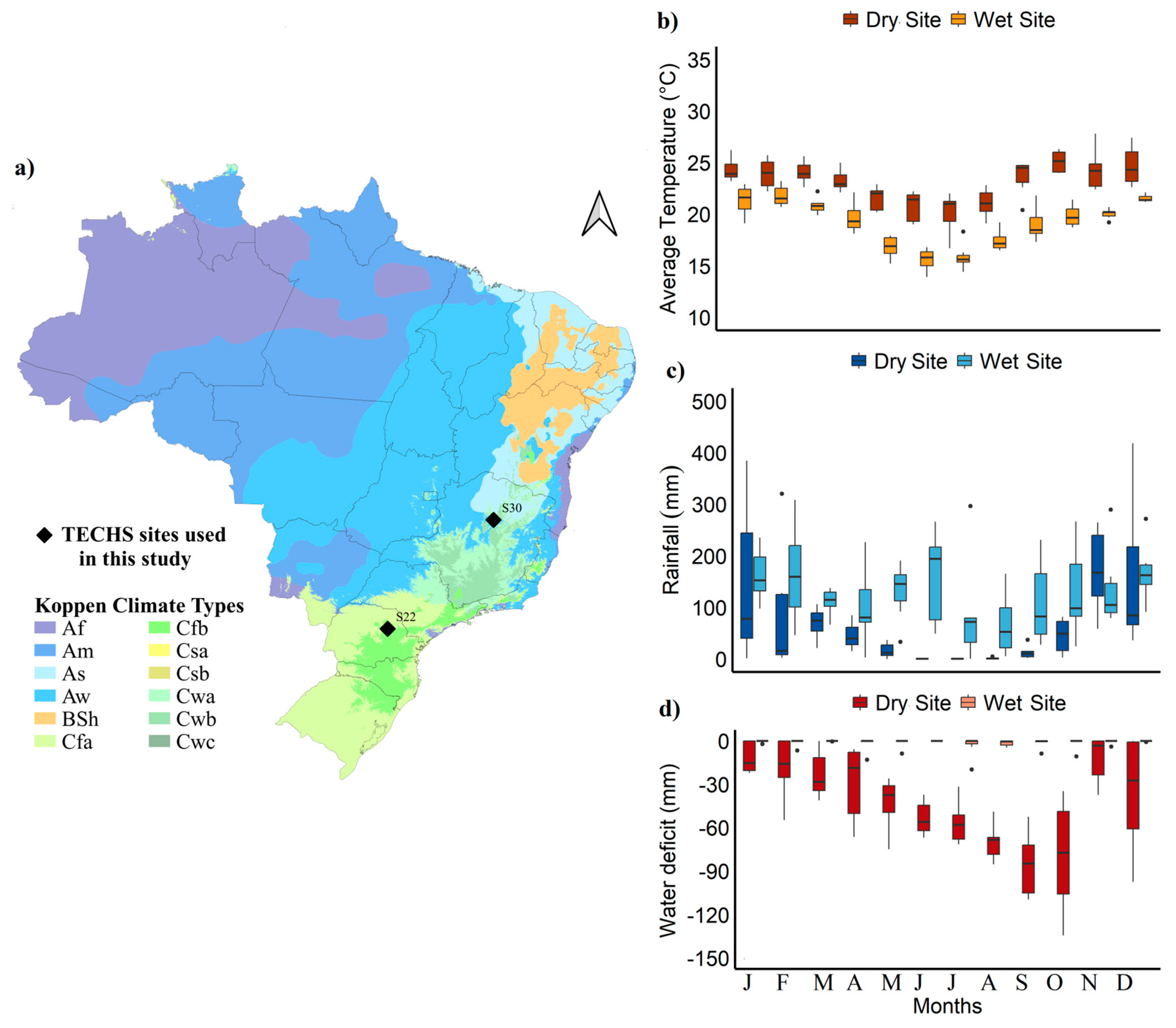
4.1. Description of the Study Sites
4.2. Fine Root Sampling
4.3. Sampling of Coarse Roots, Leaves, Branches, and Stems
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Maroušek, J.; Minofar, B.; Maroušková, A.; Strunecký, O.; Gavurová, B. Environmental and Economic Advantages of Production and Application of Digestate Biochar. Environ. Technol. Innov. 2023, 30, 103109. [Google Scholar] [CrossRef]
- Cardinael, R.; Mao, Z.; Prieto, I.; Stokes, A.; Dupraz, C.; Kim, J.H. Competition with Winter Crops Induces Deeper Rooting of Walnut Trees in a Mediterranean Alley Cropping Agroforestry System. Plant Soil 2015, 391, 219–235. [Google Scholar] [CrossRef]
- Freschet, T.; Valverde-barrantes, O.J.; Tucker, C.M.; Joseph, M.; Mccormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-mead, K.R.; Iversen, C.M.; et al. Climate, Soil and Plant Functional Types as Drivers of Global Fi Ne-Root Trait Variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Maeght, J.L.; Rewald, B.; Pierret, A. How to Study Deep Roots-and Why It Matters. Front. Plant Sci. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining Fine Roots Improves Understanding of Below-Ground Contributions to Terrestrial Biosphere Processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.; Nouvellon, Y.; Epron, D. Relating Coarse Root Respiration to Root Diameter in Clonal Eucalyptus. Tree Physiol. 2008, 28, 1245–1254. [Google Scholar] [CrossRef]
- Fort, F.; Volaire, F.; Guilioni, L.; Barkaoui, K.; Navas, M.L.; Roumet, C. Root Traits Are Related to Plant Water-Use among Rangeland Mediterranean Species. Funct. Ecol. 2017, 31, 1700–1709. [Google Scholar] [CrossRef]
- Laclau, J.P.; da Silva, E.A.; Rodrigues Lambais, G.; Bernoux, M.; le Maire, G.; Stape, J.L.; Bouillet, J.P.; de Moraes Gonçalves, J.L.; Jourdan, C.; Nouvellon, Y. Dynamics of Soil Exploration by Fine Roots down to a Depth of 10 m throughout the Entire Rotation in Eucalyptus Grandis Plantations. Front. Plant Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.C.; de Deus, J.C.; Nouvellon, Y.; Campoe, O.C.; Stape, J.L.; Aló, L.L.; Guerrini, I.A.; Jourdan, C.; Laclau, J.P. A Fast Exploration of Very Deep Soil Layers by Eucalyptus Seedlings and Clones in Brazil. For. Ecol. Manag. 2016, 366, 143–152. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A Global Analysis of Root Distributions for Terrestrial Biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Christina, M.; Nouvellon, Y.; Laclau, J.P.; Stape, J.L.; Bouillet, J.P.; Lambais, G.R.; le Maire, G. Importance of Deep Water Uptake in Tropical Eucalypt Forest. Funct. Ecol. 2017, 31, 509–519. [Google Scholar] [CrossRef]
- Bordron, B.; Robin, A.; Oliveira, I.R.; Guillemot, J.; Laclau, J.P.; Jourdan, C.; Nouvellon, Y.; Abreu-Junior, C.H.; Trivelin, P.C.O.; Gonçalves, J.L.M.; et al. Fertilization Increases the Functional Specialization of Fine Roots in Deep Soil Layers for Young Eucalyptus Grandis Trees. For. Ecol. Manag. 2019, 431, 6–16. [Google Scholar] [CrossRef]
- Fan, J.; McConkey, B.; Wang, H.; Janzen, H. Root Distribution by Depth for Temperate Agricultural Crops. Field Crops Res. 2016, 189, 68–74. [Google Scholar] [CrossRef]
- Germon, A.; Laclau, J.P.; Robin, A.; Jourdan, C. Tamm Review: Deep Fine Roots in Forest Ecosystems: Why Dig Deeper? For. Ecol. Manag. 2020, 466, 118135. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Q. Groundwater Influences on Soil Moisture and Surface Evaporation Q. J. Hidrol. 2004, 297, 285–300. [Google Scholar] [CrossRef]
- Al Afas, N.; Marron, N.; Zavalloni, C.; Ceulemans, R. Growth and Production of a Short-Rotation Coppice Culture of Poplar—IV: Fine Root Characteristics of Five Poplar Clones. Biomass Bioenergy 2008, 32, 494–502. [Google Scholar] [CrossRef]
- Campoe, O.C.; Alvares, C.A.; Carneiro, R.L.; Binkley, D.; Ryan, M.G.; Hubbard, R.M.; Stahl, J.; Moreira, G.; Moraes, L.F.; Stape, J.L. Climate and Genotype Influences on Carbon Fluxes and Partitioning in Eucalyptus Plantations. For. Ecol. Manag. 2020, 475, 118445. [Google Scholar] [CrossRef]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon Allocation in Forest Ecosystems. Glob. Change Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; Ferreira, J.M.d.A.; et al. The Brazil Eucalyptus Potential Productivity Project: Influence of Water, Nutrients and Stand Uniformity on Wood Production. For. Ecol. Manag. 2010, 259, 1684–1694. [Google Scholar] [CrossRef]
- IBÁ (Industria Brasileira de ÁRvores). 2023. Available online: https://www.iba.org/ (accessed on 3 April 2024).
- Binkley, D.; Campoe, O.; Alvares, C.A.; Carneiro, R.L.; Stape, J.L. Variation in Whole-Rotation Yield among Eucalyptus Genotypes in Response to Water and Heat Stresses: The TECHS Project. For. Ecol. Manag. 2020, 462, 117953. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G. Thinking about Efficiency of Resource Use in Forests. For. Ecol. Manag. 2004, 193, 5–16. [Google Scholar] [CrossRef]
- De Mattos, E.M.; Binkley, D.; Campoe, O.C.; Alvares, C.A.; Stape, J.L. Variation in Canopy Structure, Leaf Area, Light Interception and Light Use e Ffi Ciency among Eucalyptus Clones. For. Ecol. Manag. 2020, 463, 118038. [Google Scholar] [CrossRef]
- Scolforo, H.F.; McTague, J.P.; Burkhart, H.; Roise, J.; Alvares, C.A.; Stape, J.L. Site Index Estimation for Clonal Eucalypt Plantations in Brazil: A Modeling Approach Refined by Environmental Variables. For. Ecol. Manag. 2020, 466, 118079. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Makkonen, K.; Kellomäki, S.; Valtonen, E.; Mälkönen, E. Below- and above-Ground Biomass, Production and Nitrogen Use in Scots Pine Stands in Eastern Finland. For. Ecol. Manag. 2002, 165, 317–326. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological Limits to Plant Phenotypic Plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.C.G. Caracterização de Procedências Para a Formação de Populações Base de Eucalyptus Camaldulensis Dehn, Escola Superior de Agricultura “Luiz de Queiroz”; USP: North Bethesda, MD, USA, 1996. [Google Scholar]
- Hubbard, R.M.; Carneiro, R.L.; Campoe, O.; Alvares, C.A.; Figura, M.A.; Moreira, G.G. Contrasting Water Use of Two Eucalyptus Clones across a Precipitation and Temperature Gradient in Brazil. For. Ecol. Manag. 2020, 475, 118407. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting Depths, Lateral Root Spreads and Below-Ground / Above-Ground Allometries of Plants in Water-Limited Ecosystems Published by: British Ecological Society Linked References Are Available on JSTOR for This Article: Rooting Depths, Lateral Root Spr. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y. Influence of Environmental Variability on Root Dynamics in Northern Forests. Crit. Rev. Plant Sci. 2009, 28, 179–197. [Google Scholar] [CrossRef]
- Laclau, J.P.; Deleporte, P.; Ranger, J.; Bouillet, J.P.; Kazotti, G. Nutrient Dynamics throughout the Rotation of Eucalyptus Clonal Stands in Congo. Ann. Bot. 2003, 91, 879–892. [Google Scholar] [CrossRef]
- Zhou, Z.; Shangguan, Z. Vertical Distribution of Fine Roots in Relation to Soil Factors in Pinus Tabulaeformis Carr. Forest of the Loess Plateau of China. Plant Soil 2007, 291, 119–129. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; Arnaud, M.; M’Bou, A.T.; Saint-André, L.; Jourdan, C. Changes with Age in the Spatial Distribution of Roots of Eucalyptus Clone in Congo Impact on Water and Nutrient Uptake. For. Ecol. Manag. 2002, 171, 43–57. [Google Scholar] [CrossRef]
- Silva, V.E.; Nogueira, T.A.R.; Abreu-Junior, C.H.; He, Z.; Buzetti, S.; Laclau, J.P.; Teixeira Filho, M.C.M.; Grilli, E.; Murgia, I.; Capra, G.F. Influences of Edaphoclimatic Conditions on Deep Rooting and Soil Water Availability in Brazilian Eucalyptus Plantations. For. Ecol. Manag. 2020, 455, 117673. [Google Scholar] [CrossRef]
- Tron, S.; Bodner, G.; Laio, F.; Ridolfi, L.; Leitner, D. Can Diversity in Root Architecture Explain Plant Water Use Efficiency? A Modeling Study. Ecol. Model. 2015, 312, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Laio, F.; D’Odorico, P.; Ridolfi, L. An Analytical Model to Relate the Vertical Root Distribution to Climate and Soil Properties. Geophys. Res. Lett. 2006, 33, 1–5. [Google Scholar] [CrossRef]
- Schenk, H.J. Soil Depth, Plant Rooting Strategies and Species’ Niches. New Phytol. 2008, 178, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root Elongation, Water Stress, and Mechanical Impedance: A Review of Limiting Stresses and Beneficial Root Tip Traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.J.; Jackson, R.B. The Global Biogeography of Roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant Root Growth, Architecture and Function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Christina, M.; Laclau, J.P.; Gonçalves, J.L.M.; Jourdan, C.; Nouvellon, Y.; Bouillet, J.P. Almost Symmetrical Vertical Growth Rates above and below Ground in One of the World’s Most Productive Forests. Ecosphere 2011, 2, 1–10. [Google Scholar] [CrossRef]
- Funk, C.; Peterson, P.; Landsfeld, M.; Pedreros, D.; Verdin, J.; Shukla, S.; Husak, G.; Rowland, J.; Harrison, L.; Hoell, A.; et al. The Climate Hazards Infrared Precipitation with Stations—A New Environmental Record for Monitoring Extremes. Nature 2015, 2, 1–21. [Google Scholar] [CrossRef]
- Xavier, A.C.; King, W.; Scanlon, B.R. Daily Gridded Meteorological Variables in Brazil (1980–2013). R. Meteorol. Soc. 2016, 2659, 2644–2659. [Google Scholar] [CrossRef]
- Stackhouse, P.W.; Westberg, D.; Chandler, W.S.; Zhang, T.; Hoell, J.M. Prediction of Worldwide Energy Resource (POWER): Agro-climatology Methodology (1.0 Latitude by 1.0 Longitude Spatial Resolution); Technical Report; NASA (National Aeronautics and Space Administration): Washington, DC, USA, 2015.
- Elli, E.F.; Sentelhas, P.C.; Freitas, C.H.; Carneiro, R.L.; Alvares, C.A. Assessing the Growth Gaps of Eucalyptus Plantations in Brazil–Magnitudes, Causes and Possible Mitigation Strategies. For. Ecol. Manag. 2019, 451, 117464. [Google Scholar] [CrossRef]
- Camargo, A.P. de Contribuição Para a Determinação Da Evapotranspiração Potencial No Estado de São Paulo. Bragantia 1962, 21, 163–213. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements; FAO: Rome, Italy, 1998; ISBN 9251042195. [Google Scholar]
- Menezes, A.A. Produtividade Do Eucalipto e Dua Relação Com a Qualidade e a Classe de Solo; Universidade Federal de Viçosa: Viçosa, Brazil, 2005. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo 3a Edição Revista e Ampliada. In Man. De Métodos De Análise De Solo—Capítulo 8—Densidade De Partículas; Embrapa: Brasília, Brazil, 2017; p. 574. [Google Scholar]
- Staff, S.S. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 436th ed.; United States Department of Agriculture, Natural Resources Conservation Service, Agriculture Handbook: Washington, DC, USA, 1999.
- Embrapa. Manual de Métodos de Análise de Solo; EMBRAPA: Rio de Janeiro, Brasil, 2011; Volume 2. [Google Scholar]
- Stape, J.L.; Binkley, D.; Ryan, M.G. Eucalyptus Production and the Supply, Use and Efficiency of Use of Water, Light and Nitrogen across a Geographic Gradient in Brazil. For. Ecol. Manag. 2004, 193, 17–31. [Google Scholar] [CrossRef]
- Van Raij, B.; Andrade, J.C.D.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação Da Fertilidade de Solos Tropicais; Instituto Agronomico: Campinas, Brazil, 2001; ISBN 8585564059. [Google Scholar]
- Bartón, K. MuMIn: Multi-Model Inference. Available online: http://r-forge.r-project.org/projects/mumin/ (accessed on 3 April 2024).
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
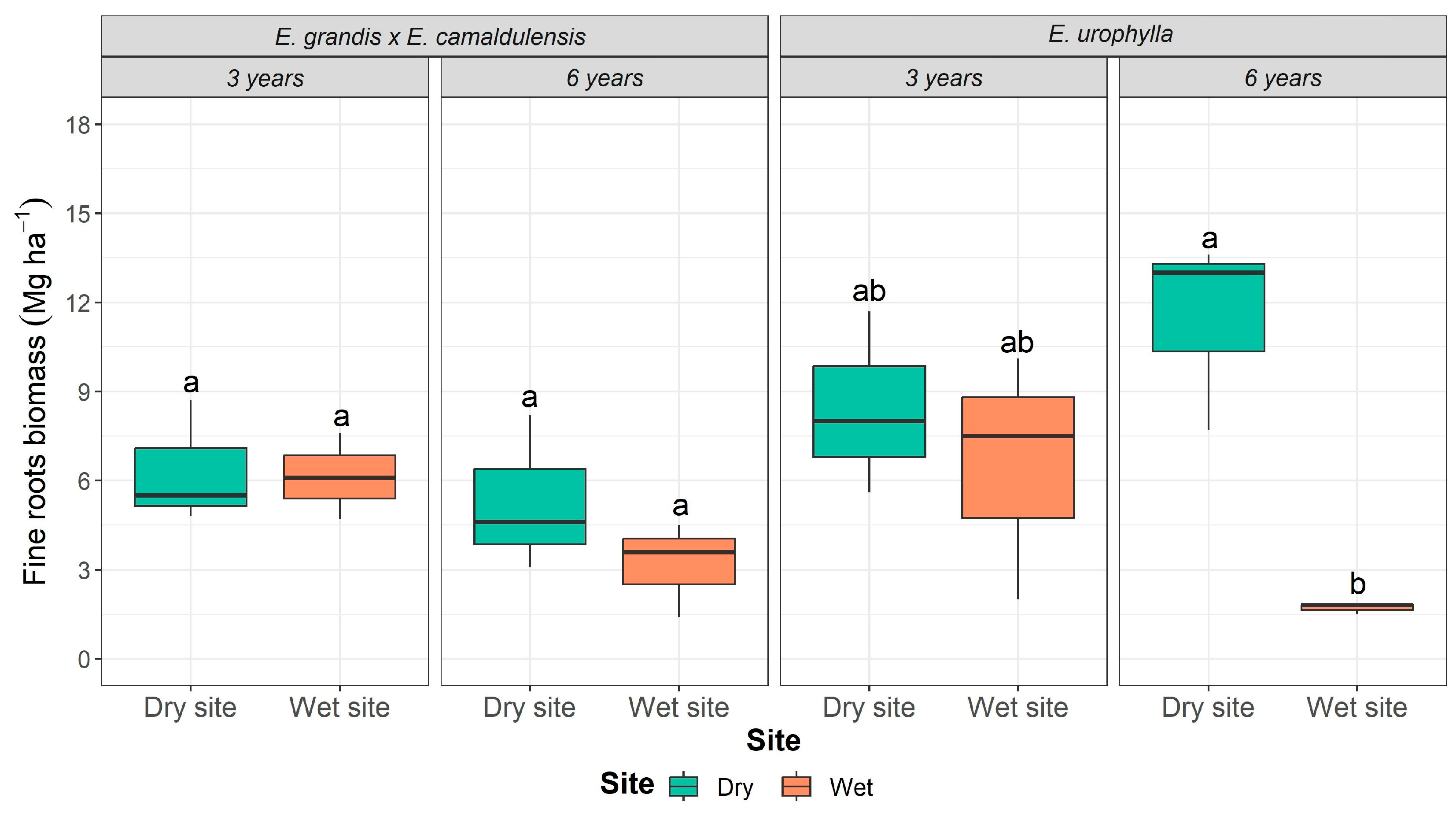
| Genotype | Site | Age | Leaves | Wood | Coarse Roots | Fine Roots | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mg ha−1 | Mg C ha−1 | Mg ha−1 | Mg C ha−1 | Mg ha−1 | Mg C ha−1 | Mg ha−1 | Mg C ha−1 | |||
| E. urophylla | Dry | 3 | 6.7 ± 1.6 a | 3.3 ± 0.8 | 49.7 ± 8.3 c | 24.9 ± 3.7 | 9.7 ± 1.6 c | 4.8 ± 0.7 | 8.4 a ± 3.1 b | 4.2 ± 1.4 |
| 6 | 3.8 ± 0.5 b | 1.9 ± 0.3 | 108.8 ± 9.6 b | 54.4 ± 4.3 | 27.4 ± 7.3 ab | 13.8 ± 3.3 | 11.4 ± 3.2 a | 5.7 ± 1.5 | ||
| Wet | 3 | 6.5 ± 0.6 a | 3.3 ± 0.3 | 75.6 ± 7.1 bc | 37.8 ± 3.1 | 13.2 ± 1.7 bc | 6.6 ± 0.8 | 6.5 ± 4.1 ab | 3.3 ± 1.9 | |
| 6 | 2.5 ± 0.7 b | 1.3 ± 0.3 | 170.6 ± 22.6 a | 85.3 ± 10.0 | 34.3 ± 8.3 a | 17.2 ± 3.8 | 1.7 ± 0.2 b | 0.8 ± 0.1 | ||
| E. grandis × E. camaldulensis | Dry | 3 | 2.8 ± 0.5 bc | 1.4 ± 0.3 | 36.6 ± 4.5 c | 18.3 ± 2.0 | 10.2 ± 2.0 b | 5.1 ± 0.9 | 6.3 ± 2.1 a | 3.2 ± 0.9 |
| 6 | 1.5 ± 0.1 c | 0.8 ± 0.1 | 56.2 ± 7.6 b | 28.1 ± 3.4 | 26.8 ± 11.2 ab | 13.4 ± 5.1 | 5.3 ± 2.6 a | 2.6 ± 1.2 | ||
| Wet | 3 | 5.5 ± 0.6 a | 2.8 ± 0.3 | 53.9 ± 4.7 b | 26.9 ± 2.1 | 11.4 ± 0.9 b | 5.7 ± 0.4 | 6.1 ± 1.5 a | 3.1 ± 0.7 | |
| 6 | 4.0 ± 1.0 ab | 2.0 ± 0.5 | 156.6 ± 4.3 a | 78.3 ± 1.9 | 35.2 ± 7.8 a | 17.6 ± 3.5 | 3.2 ± 1.6 a | 1.6 ± 0.7 | ||
| Site | Depth | Clay | Silt | Sand | OM | WHC | pH | P | K | Ca | Mg | CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | (%) | (%) | (%) | g L−1 | L m−2 | CaCl2 | mg L−1 Soil | mmolc L−3 | ||||
| Dry | 0–20 | 76 | 14 | 10 | 63 | 225 | 3.8 | 4.0 | 1.4 | 47.0 | 25.0 | 223.4 |
| 20–40 | 77 | 15 | 8 | 32 | 225 | 3.9 | 0.0 | 1.8 | 50.0 | 28.0 | 194.8 | |
| Wet | 0–20 | 56 | 22 | 22 | 58 | 214 | 3.9 | 5.0 | 0.9 | 12.0 | 11.0 | 165.9 |
| 20–40 | 55 | 24 | 21 | 46 | 214 | 4.0 | 2.0 | 6.7 | 63.0 | 11.0 | 201.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basílio, J.J.N.; Campoe, O.C.; Queiroz, T.B.; de Souza, C.R.; Carneiro, R.L.; Alvares, C.A.; Figura, M.A. Fine Root Density Dynamics and Carbon Stock of Eucalyptus spp.: Interplay of Age, Genotype, and Edaphoclimatic Conditions. Plants 2024, 13, 1503. https://doi.org/10.3390/plants13111503
Basílio JJN, Campoe OC, Queiroz TB, de Souza CR, Carneiro RL, Alvares CA, Figura MA. Fine Root Density Dynamics and Carbon Stock of Eucalyptus spp.: Interplay of Age, Genotype, and Edaphoclimatic Conditions. Plants. 2024; 13(11):1503. https://doi.org/10.3390/plants13111503
Chicago/Turabian StyleBasílio, Josiana Jussara Nazaré, Otávio Camargo Campoe, Túlio Barroso Queiroz, Cléber Rodrigo de Souza, Rafaela Lorenzato Carneiro, Clayton Alcarde Alvares, and Marco Aurélio Figura. 2024. "Fine Root Density Dynamics and Carbon Stock of Eucalyptus spp.: Interplay of Age, Genotype, and Edaphoclimatic Conditions" Plants 13, no. 11: 1503. https://doi.org/10.3390/plants13111503
APA StyleBasílio, J. J. N., Campoe, O. C., Queiroz, T. B., de Souza, C. R., Carneiro, R. L., Alvares, C. A., & Figura, M. A. (2024). Fine Root Density Dynamics and Carbon Stock of Eucalyptus spp.: Interplay of Age, Genotype, and Edaphoclimatic Conditions. Plants, 13(11), 1503. https://doi.org/10.3390/plants13111503







