Composition Characterization of Cinnamomum osmophloeum Kanehira Hydrosol and Its Enhanced Effects on Erectile Function
Abstract
:1. Introduction
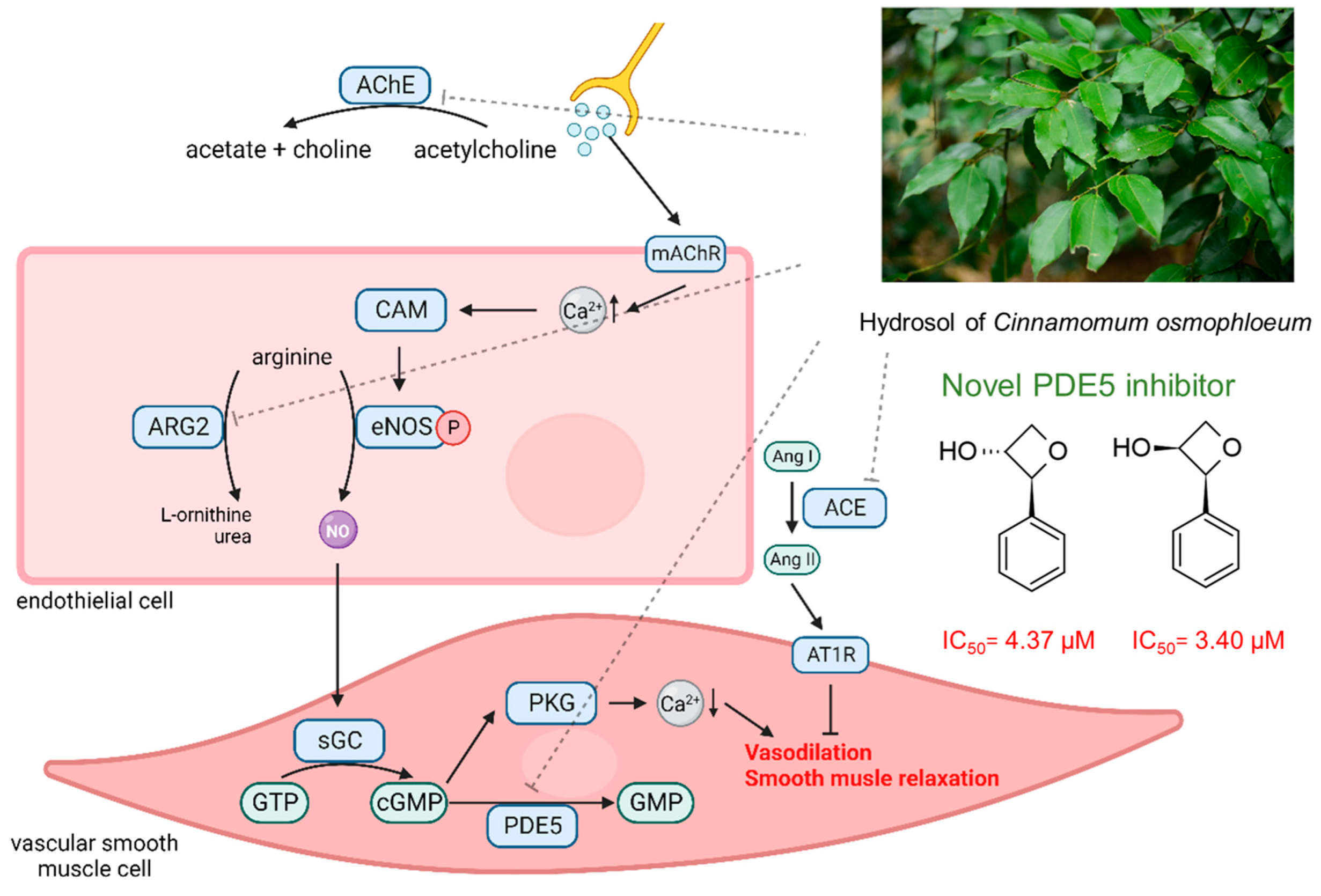
2. Results and Discussion
2.1. Chemical Composition of CO Essential Oil and Hydrosol
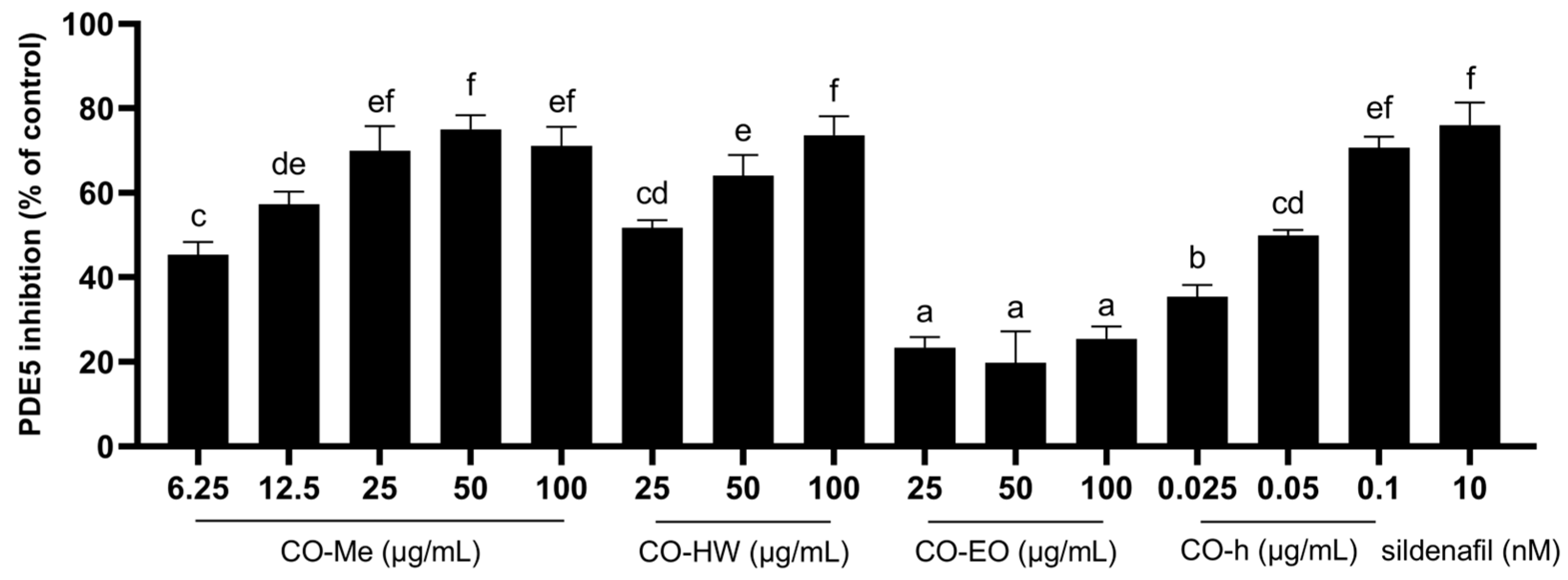
2.2. PDE5 Inhibition Activity of Different CO Extracts
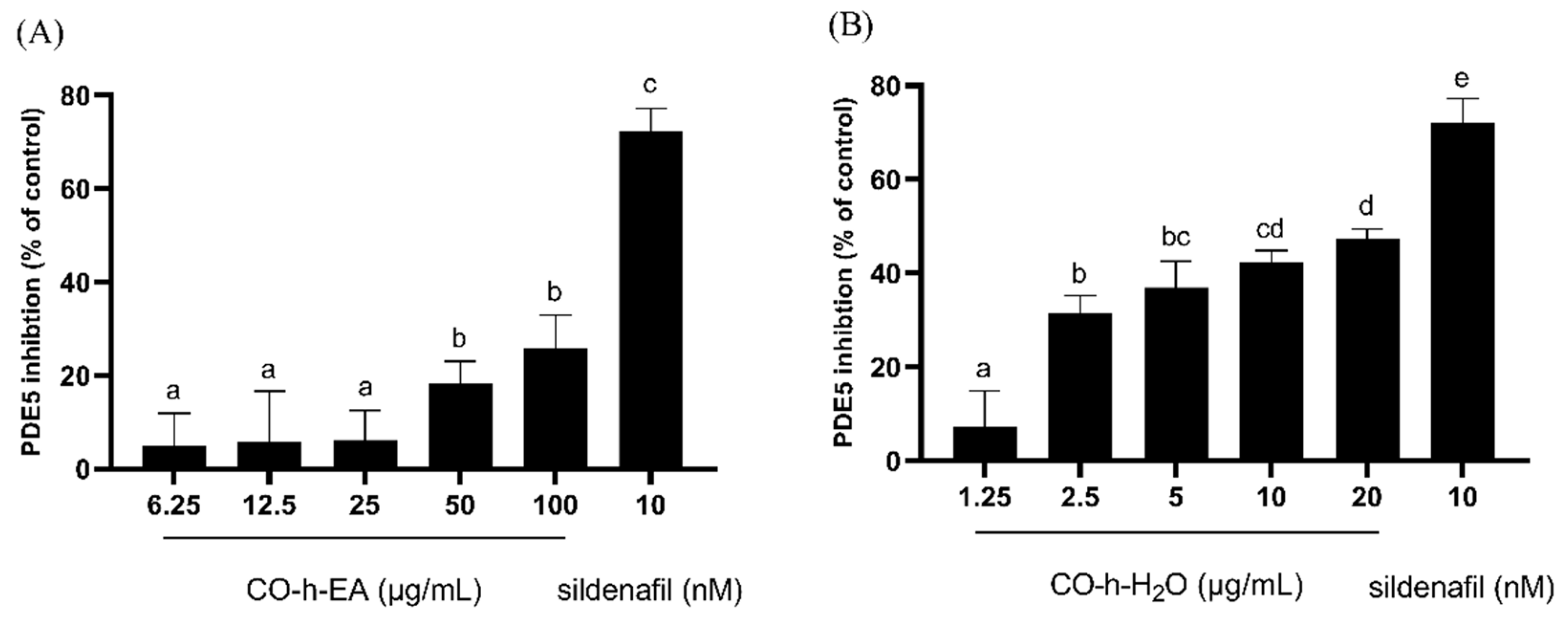
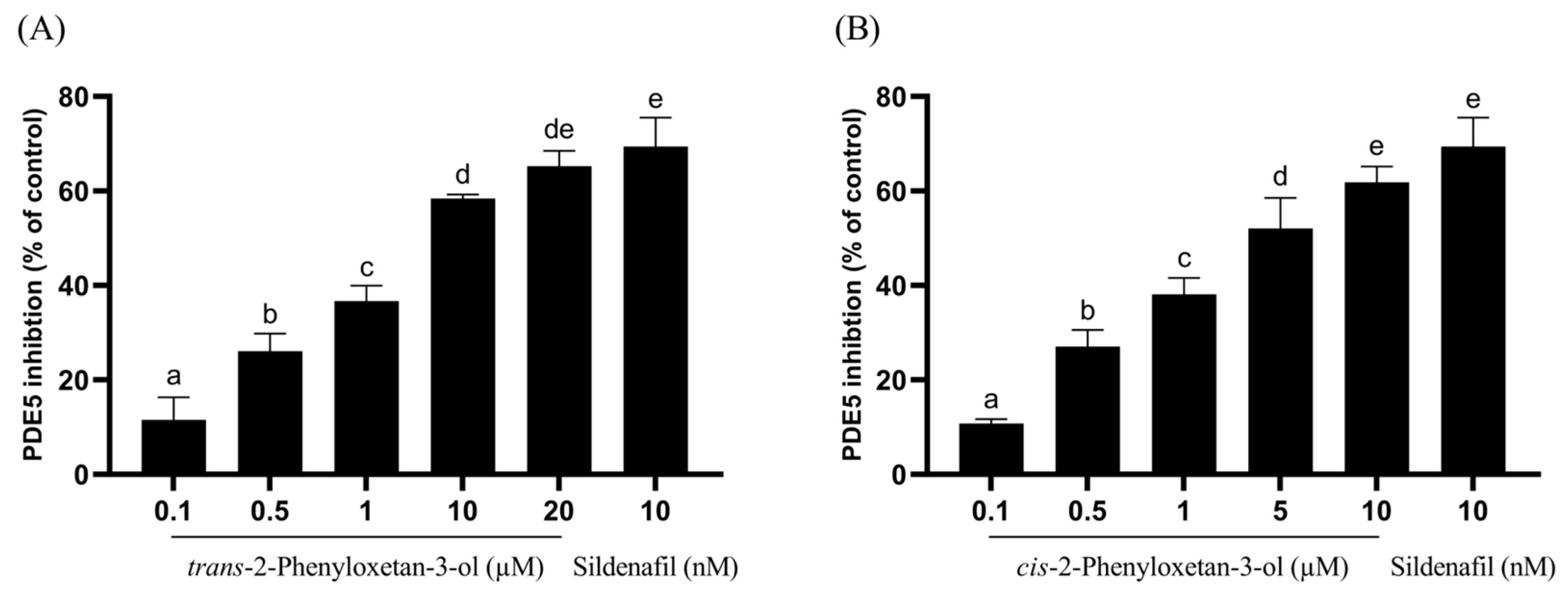
2.3. PDE5 Inhibition Activity of CO Hydrosol and Its Active Compounds
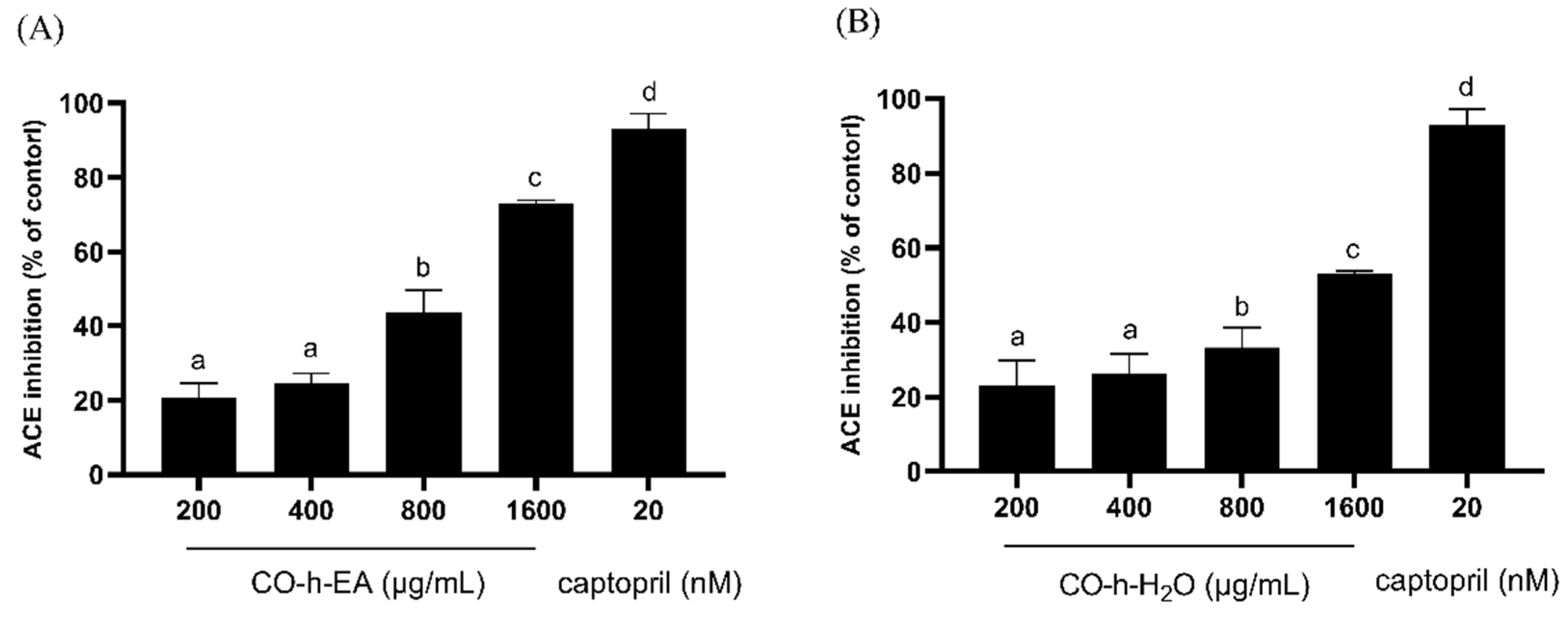
2.4. ACE Inhibition Activity of CO Hydrosol
2.5. AChE Inhibition Activity of CO Hydrosol
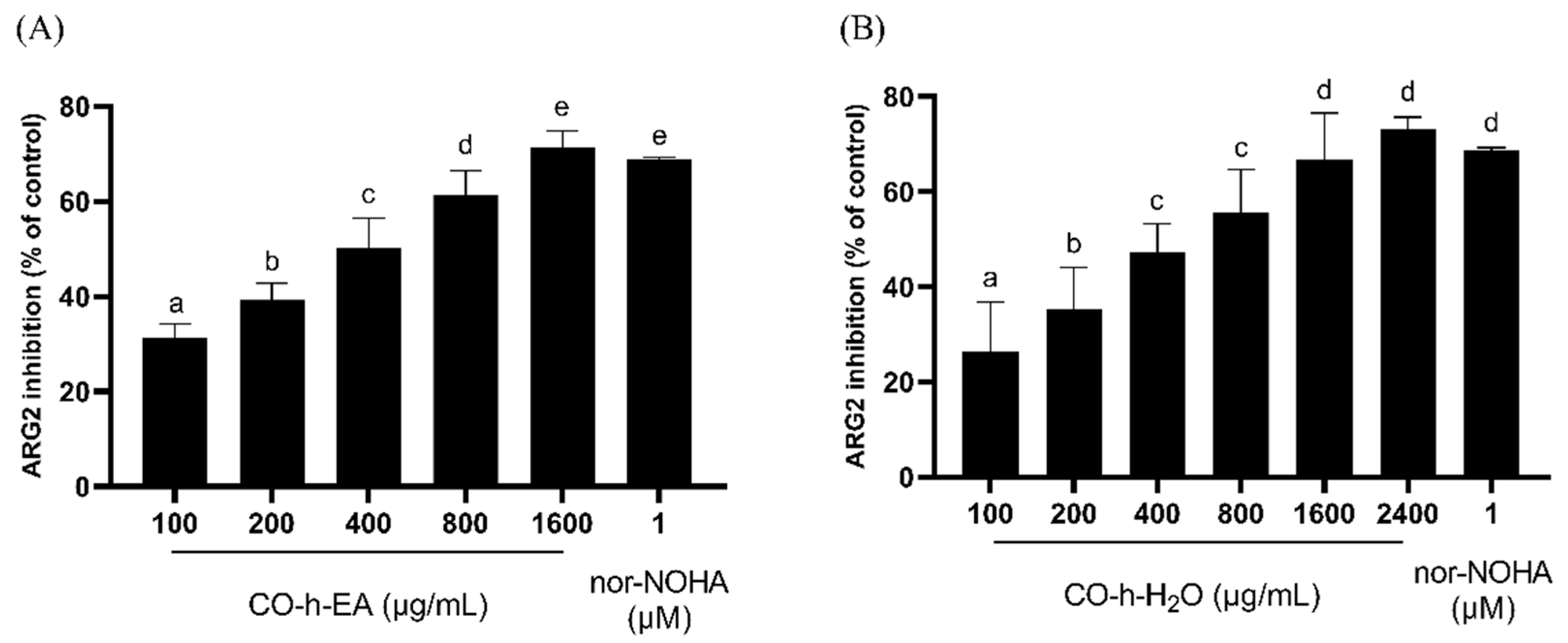
2.6. ARG2 Inhibition Activity of CO Hydrosol
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Preparation of CO Leaf Essential Oil and Hydrosol
3.4. Compounds of CO Hydrosol Water Fraction Identification
3.5. GC/MS Analysis
3.6. PDE5 Inhibition Assay
3.7. ACE Inhibition Assay
3.8. AChE Inhibition Assay
3.9. Arginase 2 Inhibition Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; De Leo, M. Reconsidering Hydrosols as Main Products of Aromatic Plants Manufactory: The Lavandin (Lavandula × intermedia) Case Study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef] [PubMed]
- Belabbes, R.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Tabti, B.; Costa, J.; Muselli, A. Chemical Variability, Antioxidant and Antifungal Activities of Essential Oils and Hydrosol Extract of Calendula arvensis L. from Western Algeria. Chem. Biodivers. 2017, 14, e1600482. [Google Scholar] [CrossRef] [PubMed]
- Diop, S.M.; Diop, M.B.; Guèye, M.T.; Ndiaye, I.; Ndiaye, E.H.B.; Thiam, A.; Fauconnier, M.-L.; Lognay, G. Chemical Composition of Essential Oils and Floral Waters of Ocimum basilicum L. from Dakar and Kaolack Regions of Senegal. J. Essent. Oil Bear. Plants 2018, 21, 540–547. [Google Scholar] [CrossRef]
- Francezon, N.; Stevanovic, T. Chemical Composition of Essential Oil and Hydrosol from Picea mariana Bark Residue. Bioresources 2017, 12, 2635–2645. [Google Scholar] [CrossRef]
- Moukhles, A.; Belcadi, H.; Raissouni, I.; Ben driss, A.; Mansour, A.I. Chemical Composition, in vitro Antibacterial Activity and Corrosion Inhibition of Essential Oil and Hydrolat Extract from Aerial Parts of Thymbra capitata (L.) Cav Harvested at Northern Morocco. J. Essent. Oil Bear. Plants 2020, 23, 375–389. [Google Scholar] [CrossRef]
- Nanashima, N.; Kitajima, M.; Takamagi, S.; Fujioka, M.; Tomisawa, T. Comparison of Chemical Composition between Kuromoji (Lindera umbellata) Essential Oil and Hydrosol and Determination of the Deodorizing Effect. Molecules 2020, 25, 4195. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, E.H.B.; Diop, M.B.; Gueye, M.T.; Ndiaye, I.; Diop, S.M.; Fauconnier, M.-L.; Lognay, G. Characterization of essential oils and hydrosols from senegalese Eucalyptus camaldulensis Dehnh. J. Essent. Oil Res. 2018, 30, 131–141. [Google Scholar] [CrossRef]
- Pardavella, I.; Daferera, D.; Tselios, T.; Skiada, P.; Giannakou, I. The Use of Essential Oil and Hydrosol Extracted from Cuminum cyminum Seeds for the Control of Meloidogyne incognita and Meloidogyne javanica. Plants 2020, 10, 46. [Google Scholar] [CrossRef]
- Merad Boussalah, N. Chemical Composition and Biological Activities of Essential Oil and Hydrosol Extract from Aerial Parts of Cynoglossum cheirifolium L. from Algeria. J. Essent. Oil Bear. Plants 2020, 23, 97–104. [Google Scholar] [CrossRef]
- Shen, X.; Chen, W.; Zheng, Y.; Lei, X.; Tang, M.; Wang, H.; Song, F. Chemical composition, antibacterial and antioxidant activities of hydrosols from different parts of Areca catechu L. and Cocos nucifera L. Ind. Crops Prod. 2017, 96, 110–119. [Google Scholar] [CrossRef]
- Tabet Zatla, A.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Costa, J.; Muselli, A. Antifungal activities of essential oils and hydrosol extracts of Daucus carota subsp. sativus for the control of fungal pathogens, in particular gray rot of strawberry during storage. J. Essent. Oil Res. 2017, 29, 391–399. [Google Scholar] [CrossRef]
- Törnük, F.; Dertli, E. Decontamination of Escherichia coli O157:H7 and Staphylococcus aureus from Fresh-Cut Parsley with Natural Plant Hydrosols. J. Food Process. Preserv. 2015, 39, 1587–1594. [Google Scholar] [CrossRef]
- Xiao, Y.; He, J.; Zeng, J.; Yuan, X.; Zhang, Z.; Wang, B. Application of citronella and rose hydrosols reduced enzymatic browning of fresh-cut taro. J. Food Biochem. 2020, 44, e13283. [Google Scholar] [CrossRef] [PubMed]
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and Antimicrobial Activity of Mexican Oregano (Poliomintha longiflora) Essential Oil, Hydrosol and Extracts from Waste Solid Residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Lis-Balchin, M.; Steyrl, H.; Krenn, E. The comparative effect of novel Pelargonium essential oils and their corresponding hydrosols as antimicrobial agents in a model food system. Phytother. Res. 2003, 17, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, I. Antifungal Activity of Propolis, Thyme Essential Oil and Hydrosol on Natural Mycobiota of Sucuk, a Turkish Fermented Sausage: Monitoring of Their Effects on Microbiological, Color and Aroma Properties. J. Food Process. Preserv. 2015, 39, 1148–1158. [Google Scholar] [CrossRef]
- Hussain, R.A.; Kim, J.; Hu, T.W.; Pezzuto, J.M.; Soejarto, D.D.; Kinghorn, A.D. Isolation of a Highly Sweet Constituent from Cinnamomum osmophloeum Leaves1. Planta Med. 1986, 52, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Liu, J.Y.; Tsai, K.H.; Chen, W.J.; Chang, S.T. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J. Agric. Food Chem. 2004, 52, 4395–4400. [Google Scholar] [CrossRef]
- Chang, S.T.; Chen, P.F.; Chang, S.C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Chen, P.; Chang, S. Application of essential oils from wood on the manufacture of environment-friendly antimicrobial paper products. Quart. J. Chin. 2002, 35, 69–74. [Google Scholar]
- Wang, S.Y.; Chen, P.F.; Chang, S.T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, C.W.; Liao, J.W.; Zhen, W.W.; Chu, F.H.; Chang, S.T. Essential oil from leaves of Cinnamomum osmophloeum acts as a xanthine oxidase inhibitor and reduces the serum uric acid levels in oxonate-induced mice. Phytomedicine 2008, 15, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Liao, J.W.; Chang, S.T.; Wang, S.Y. Antidyslipidemic activity of hot-water extracts from leaves of Cinnamomum osmophloeum Kaneh. Phytother. Res. 2011, 25, 1317–1322. [Google Scholar] [CrossRef]
- Senthil Kumar, K.J.; Hsieh, Y.-H.; Lin, T.-Y.; Chien, S.-C.; Liao, J.-W.; Chu, F.-H.; Chang, S.-T.; Wang, S.-Y. Dietary Indigenous Cinnamon (Cinnamomum osmophloeum) Leaf Powder Reduces Plasma Lipid in Hypercholesterolemia Hamsters. Nat. Prod. Commun. 2019, 14, 1934578X19860667. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsieh, Y.H.; Kumar, K.J.S.; Hsieh, H.W.; Lin, C.C.; Wang, S.Y. The Regulatory Effects of a Formulation of Cinnamomum osmophloeum Kaneh and Taiwanofungus camphoratus on Metabolic Syndrome and the Gut Microbiome. Plants 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Francl, M.M.; Hansell, G.; Patel, B.P.; Swindell, C.S. 1-Oxabicyclobutonium ions can intervene in epoxycarbinyl and 3-oxetanyl solvolyses. J. Am. Chem. Soc. 1990, 112, 3535–3539. [Google Scholar] [CrossRef]
- Cardinal, S.; Paquet-Côté, P.A.; Azelmat, J.; Bouchard, C.; Grenier, D.; Voyer, N. Synthesis and anti-inflammatory activity of isoquebecol. Bioorg. Med. Chem. 2017, 25, 2043–2056. [Google Scholar] [CrossRef]
- Balsamo, A.; Ceccarelli, G.; Crotti, P.; Macchia, F. Mechanism and stereochemistry of oxetane reactions. I. Stereospecific synthesis of the diastereoisomeric 2-phenyl-3-methyloxetanes and study of their configuration and conformation by nuclear magnetic resonance spectroscopy. J. Org. Chem. 1975, 40, 473–476. [Google Scholar] [CrossRef]
- Ruotsalainen, H.; Karki, T. Preparation of 2-aryl-3-oxetanols. Acta Chem. Scand. 1983, 37b, 151–154. [Google Scholar] [CrossRef]
- Julio, L.F.; Barrero, A.F.; Herrador del Pino, M.M.; Arteaga, J.F.; Burillo, J.; Andres, M.F.; Díaz, C.E.; González-Coloma, A. Phytotoxic and Nematicidal Components of Lavandula luisieri. J. Nat. Prod. 2016, 79, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Burnett, A.L.; Rosen, R.C.; Park, P.W.; Stecher, V.J. The Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction. Sex. Med. Rev. 2019, 7, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Galli, G.V.; Dal Cero, E.; Belluti, F.; Matera, R.; Zironi, E.; Pagliuca, G.; Bosisio, E. Potent inhibition of human phosphodiesterase-5 by icariin derivatives. J. Nat. Prod. 2008, 71, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Labazi, H.; Webb, R.C. New insights into hypertension-associated erectile dysfunction. Curr. Opin. Nephrol. Hypertens. 2012, 21, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Clemente, G.S.; van Waarde, A.; Antunes, I.F.; Dömling, A.; Elsinga, P.H. Arginase as a potential biomarker of disease progression: A molecular imaging perspective. Int. J. Mol. Sci. 2020, 21, 5291. [Google Scholar] [CrossRef] [PubMed]
- Lacchini, R.; Muniz, J.J.; Nobre, Y.T.; Cologna, A.J.; Martins, A.C.; Tanus-Santos, J.E. Relationship between Arginase 1 and Arginase 2 levels and genetic polymorphisms with erectile dysfunction. Nitric Oxide 2015, 51, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Thongnopnua, P.; Poeaknapo, C. High-performance liquid chromatographic determination of enalapril in human plasma by enzyme kinetic analytical method. J. Pharm. Biomed. Anal. 2005, 37, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, S.; Borloz, A.; Urbain, A.; Marston, A.; Hostettmann, K.; Carrupt, P.A.; Reist, M. In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: Thin layer chromatography versus microplate methods. Eur. J. Pharm. Sci. 2008, 33, 109–119. [Google Scholar] [CrossRef]
- Badirzadeh, A.; Taheri, T.; Taslimi, Y.; Abdossamadi, Z.; Heidari-Kharaji, M.; Gholami, E.; Sedaghat, B.; Niyyati, M.; Rafati, S. Arginase activity in pathogenic and non-pathogenic species of Leishmania parasites. PLoS Neglected Trop. Dis. 2017, 11, e0005774. [Google Scholar] [CrossRef]
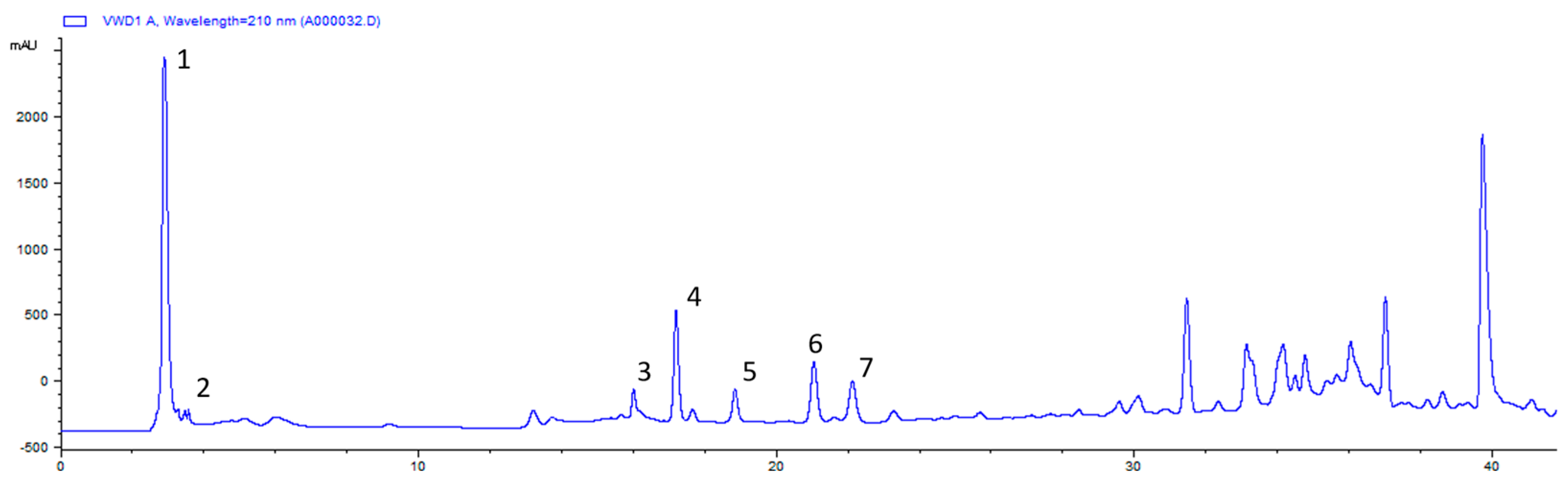
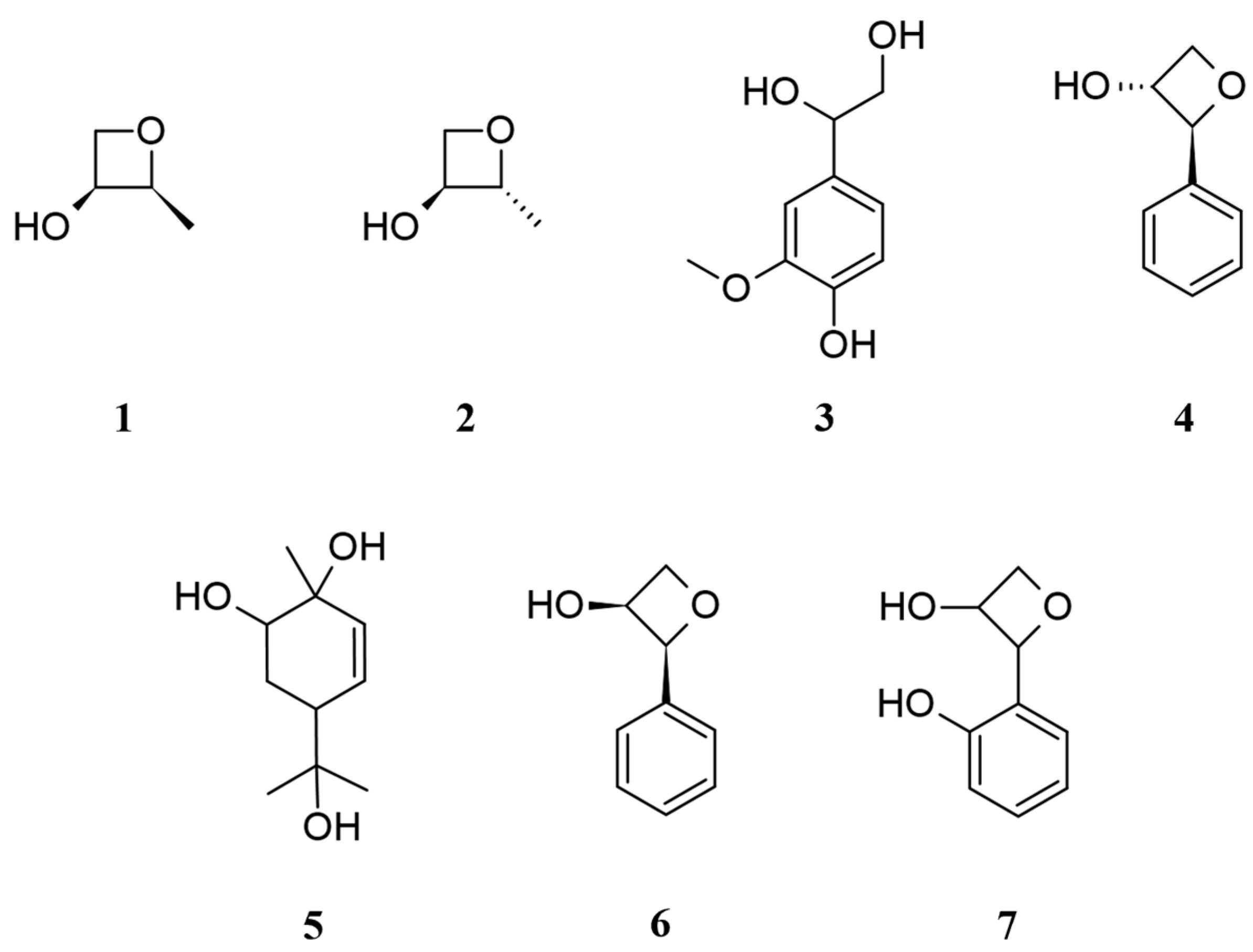

| RT (min) | Compound | Composition (%) | KI | Identification | ||
|---|---|---|---|---|---|---|
| EO | EA | EO | EA | |||
| 9.25 | - | α-Pinene | 1.25 | - | 936 | KI, MS, ST |
| 9.89 | - | Camphene | 0.70 | - | 952 | KI, MS, ST |
| 10.37 | 10.64 | Benzaldehyde | 0.41 | 0.66 | 965 | KI, MS, ST |
| 11.04 | - | β-Pinene | 0.32 | - | 979 | KI, MS, ST |
| 13.32 | - | Limonene | 0.17 | - | 1031 | KI, MS, ST |
| - | 14.13 | Salicylaldehyde | - | 0.21 | 1044 | KI, MS |
| - | 19.05 | Vinylphenylcarbinol | - | 0.35 | 1150 | KI, MS |
| 19.43 | 19.62 | Benzenepropanal | 0.93 | 0.72 | 1162 | KI, MS |
| 20.30 | 20.48 | Terpinen-4-ol | 0.03 | 0.02 | 1179 | KI, MS, ST |
| 20.99 | 21.18 | α-Terpineol | 0.01 | 0.11 | 1193 | KI, MS, ST |
| 21.12 | 21.3 | Estragole | 1.06 | 0.01 | 1196 | KI, MS, ST |
| 22.00 | 22.21 | cis-Cinnamaldehyde | 0.28 | 0.46 | 1216 | KI, MS, ST |
| - | 24.22 | p-Allylphenol | - | 0.34 | 1261 | KI, MS |
| 24.53 | 25.07 | trans-Cinnamaldehyde | 68.38 | 65.03 | 1279 | KI, MS, ST |
| 25.04 | 25.30 | Bornyl acetate | 1.03 | 0.15 | 1283 | KI, MS, ST |
| - | 26.25 | Cinnamyl alcohol | - | 1.94 | 1303 | KI, MS, ST |
| 28.00 | 28.17 | Eugenol | 0.76 | 0.78 | 1351 | KI, MS, ST |
| 29.01 | - | α-Copaene | 0.60 | - | 1374 | KI, MS, ST |
| - | 29.96 | Vanillin | - | 0.13 | 1389 | KI, MS, ST |
| 30.82 | - | β-Caryophyllene | 1.87 | - | 1416 | KI, MS, ST |
| - | 31.68 | Coumarin | - | 4.31 | 1430 | KI, MS, ST |
| 31.94 | 32.26 | trans-Cinnamyl acetate | 18.52 | 7.57 | 1444 | KI, MS, ST |
| 34.88 | - | δ-Cadinene | 1.00 | - | 1515 | KI, MS |
| - | 35.35 | Ethyl 4-ethoxybenzoate | - | 2.76 | 1519 | KI, MS |
| 37.30 | - | Caryophyllene oxide | 0.16 | - | 1577 | KI, MS |
| 48.50 | - | Sclarene | 0.36 | - | 1931 | KI, MS |
| Position | 13C | 1H | HMBC |
|---|---|---|---|
| 2 | 69.1 | 4.47 (d, J = 4.4 Hz, 1H) | |
| 3 | 69.3 | 3.88 (td, J = 4.4, 2.4 Hz, 1H) | |
| 4 | 67.2 | 4.10 (ddd, J = 11.6, 4.8, 1.2 Hz, 1H) | C-3, C-6 |
| 4.21 (dd, J = 11.6, 2.4 Hz, 1H) | |||
| 5 | 124.2 | - | |
| 6 | 155.5 | - | |
| 7 | 117.5 | 7.36 (dd, J = 7.6, 1.6 Hz, 1H) | C-5, C-9 |
| 8 | 130.3 | 7.18 (td, J = 8.4, 1.6 Hz, 1H) | C-6, C-10 |
| 9 | 121.8 | 6.93 (td, J = 7.6, 0.8 Hz, 1H) | C-5, C-7 |
| 10 | 132.0 | 6.81 (dd, J = 8.4, 0.8 Hz, 1H) | C-5, C-9 |
| Retention (min) | Flow (mL/min) | MeOH (%) | H2O (%) |
|---|---|---|---|
| 0 | 1.0 | 1 | 99 |
| 6 | 1.0 | 1 | 99 |
| 10 | 1.0 | 15 | 85 |
| 18 | 1.0 | 18 | 82 |
| 40 | 1.0 | 60 | 40 |
| 41 | 1.0 | 100 | 0 |
| 45 | 1.0 | 100 | 0 |
| 46 | 1.0 | 1 | 99 |
| 50 | 1.0 | 1 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Taso, N.-W.; Chen, C.-J.; Chang, H.-Y.; Wang, S.-Y. Composition Characterization of Cinnamomum osmophloeum Kanehira Hydrosol and Its Enhanced Effects on Erectile Function. Plants 2024, 13, 1518. https://doi.org/10.3390/plants13111518
Wang C-H, Taso N-W, Chen C-J, Chang H-Y, Wang S-Y. Composition Characterization of Cinnamomum osmophloeum Kanehira Hydrosol and Its Enhanced Effects on Erectile Function. Plants. 2024; 13(11):1518. https://doi.org/10.3390/plants13111518
Chicago/Turabian StyleWang, Chung-Hsuan, Nai-Wen Taso, Chi-Jung Chen, Hung-Yi Chang, and Sheng-Yang Wang. 2024. "Composition Characterization of Cinnamomum osmophloeum Kanehira Hydrosol and Its Enhanced Effects on Erectile Function" Plants 13, no. 11: 1518. https://doi.org/10.3390/plants13111518





