Thriving under Salinity: Growth, Ecophysiology and Proteomic Insights into the Tolerance Mechanisms of Obligate Halophyte Suaeda fruticosa
Abstract
:1. Introduction
2. Results
2.1. Plant Growth
2.2. Water Relations
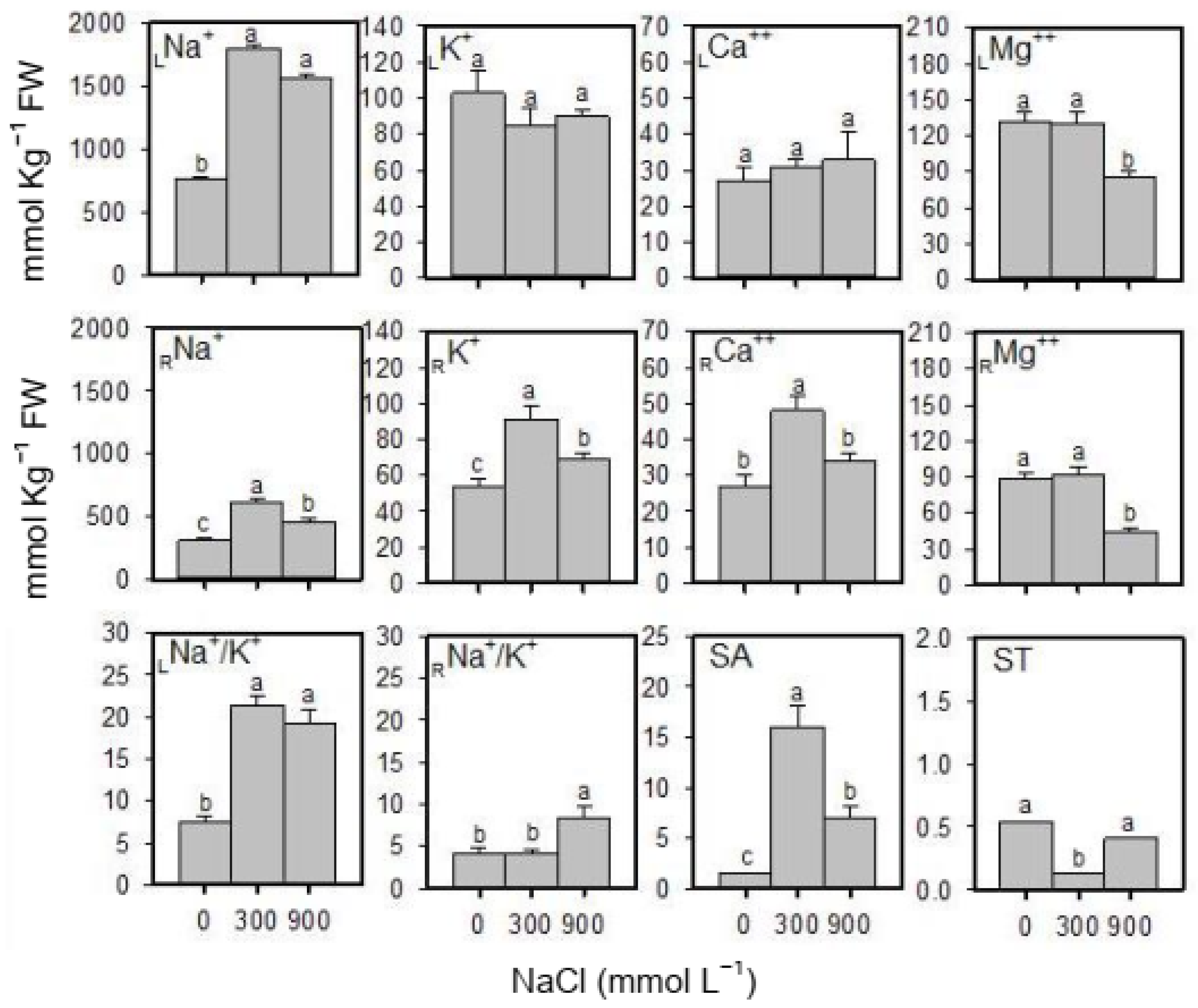
2.3. Cation Content and K+-Selectivity
2.4. Photosynthetic CO2/H2O Gas Exchange, Chlorophyll Fluorescence and Pigments
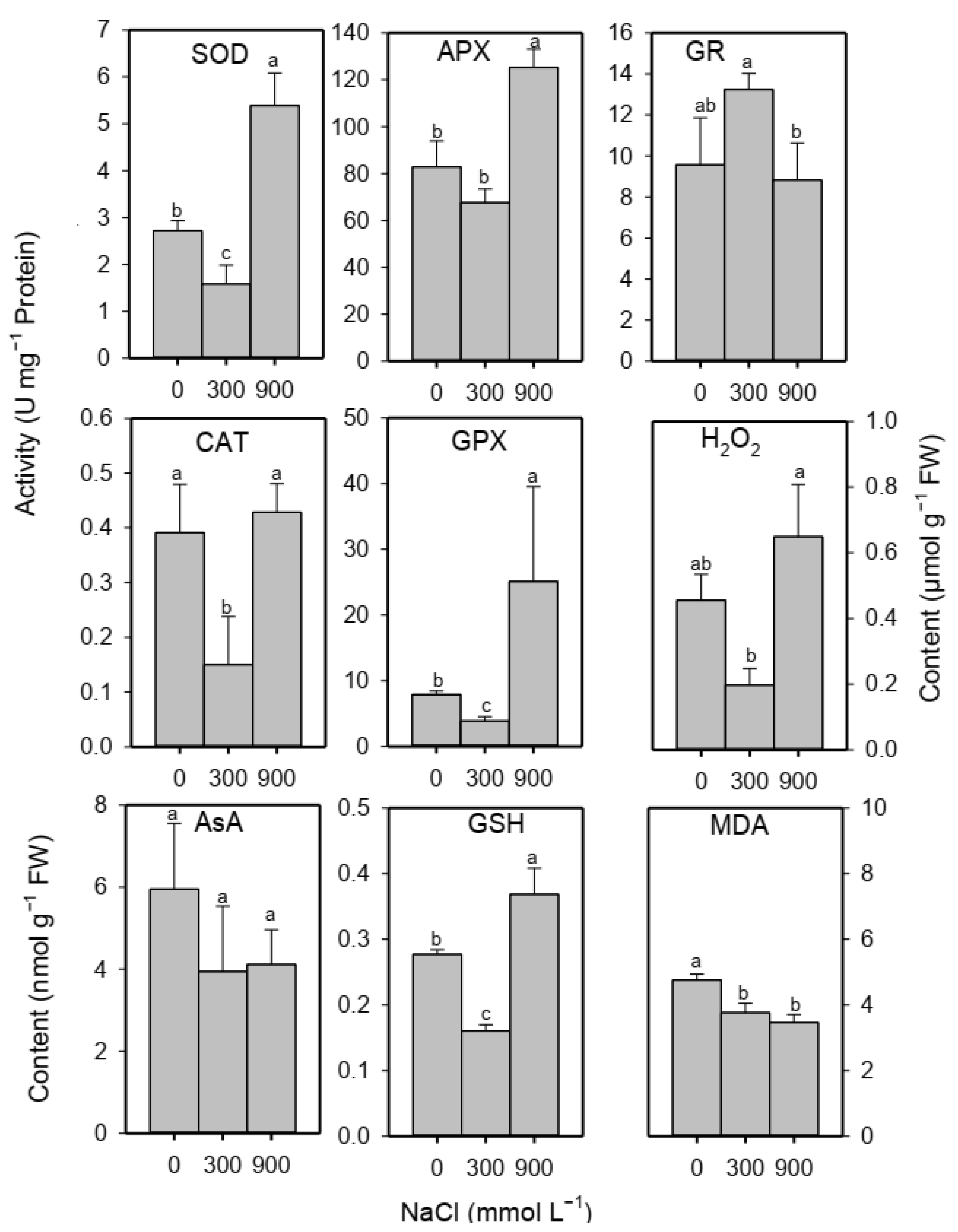
2.5. Oxidative Damage Markers, Enzymatic and Non-Enzymatic Antioxidants
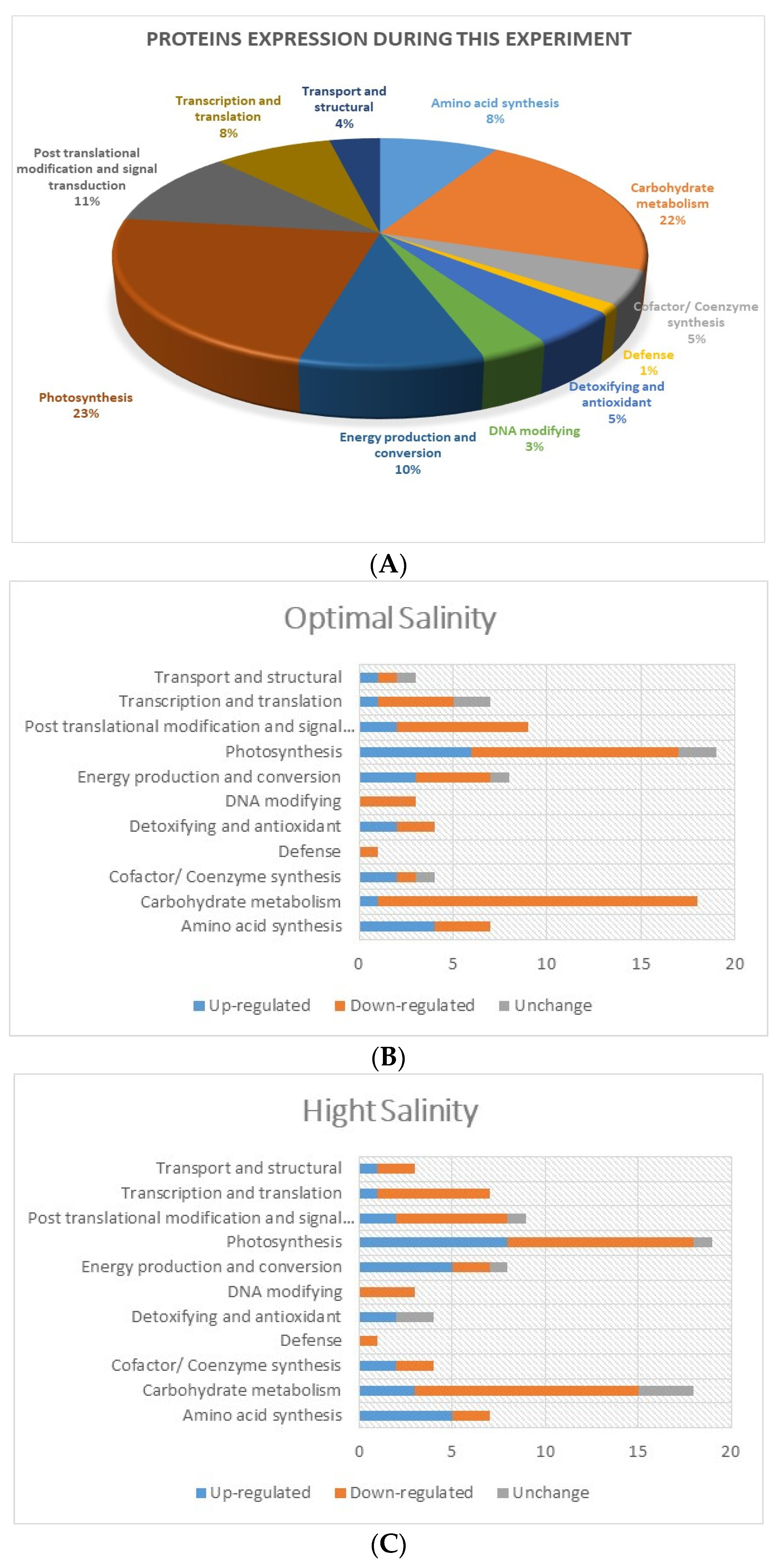
2.6. Differentially Expressed Proteins (DEPs) under Salt Treatments
3. Materials and Methods
3.1. Plant Growth and Culture
3.2. Biomass Measurements
3.3. Water Relations
3.4. Cation Content and Ion Selectivity
3.5. Photosynthetic Gas Exchange, Chlorophyll Fluorescence and Pigments
3.6. Enzymatic and Non-Enzymatic Antioxidants
3.7. Oxidative Damage Markers
3.8. Proteomic Analysis
3.9. Statistical Analysis
4. Discussion
4.1. Growth Stimulation and Physio-Chemical Attributes under Moderate Salinity
4.2. Growth and Physio-Chemical Attributes under High Salinity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Millar, J.; Roots, J. Changes in Australian agriculture and land use: Implications for future food security. Int. J. Agric. Sustain. 2012, 10, 25–39. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Xu, X.; Li, X.; Li, X.; Ciais, P.; Lin, P.; Gong, K.; Ziegler, A.D.; Chen, A. High-spatiotemporal-resolution mapping of global urban change from 1985 to 2015. Nat. Sustain. Nat. Sustain. 2020, 3, 564–570. [Google Scholar] [CrossRef]
- Bologna, M.; Aquino, G. Deforestation and world population sustainability: A quantitative analysis. Sci. Rep. 2020, 10, 7631. [Google Scholar] [CrossRef] [PubMed]
- Lubchenco, J.; Haugan, P.M. The expected impacts of climate change on the ocean economy. In The Blue Compendium: From Knowledge to Action for a Sustainable Ocean Economy; Springer: Berlin/Heidelberg, Germany, 2023; pp. 15–50. [Google Scholar]
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. Soil Salinity and Sodicity. In Growing Crops with Reclaimed Waste Water; Stevens, D., Ed.; CSIRO Publishing: Melbourne, Australia, 2006; pp. 125–138. [Google Scholar]
- Pitman, M.G.; Läuchli, A. Global impact of salinity and agricultural ecosystems. In Salinity: Environment-Plants-Molecules; Springer: Berlin/Heidelberg, Germany, 2002; pp. 3–20. [Google Scholar]
- Ruan, C.-J.; da Silva, J.A.T.; Mopper, S.; Qin, P.; Lutts, S. Halophyte improvement for a salinized world. Crit. Rev. Plant Sci. 2010, 29, 329–359. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Bhaskar, S.D.; Yadav, K.; Penna, S. Halophytes: Prospective plants for future. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 221–234. [Google Scholar]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Pagter, M.; Bragato, C.; Malagoli, M.; Brix, H. Osmotic and ionic effects of NaCl and Na2SO4 salinity on Phragmites australis. Aquat. Bot. 2009, 90, 43–51. [Google Scholar] [CrossRef]
- Rozentsvet, O.; Kosobryukhov, A.; Zakhozhiy, I.; Tabalenkova, G.; Nesterov, V.; Bogdanova, E. Photosynthetic parameters and redox homeostasis of Artemisia santonica L. under conditions of Elton region. Plant Physiol. Biochem. 2017, 118, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Khan, M.A. Halophytes: Biology and economic potentials. Karachi Univ. J. Sci. 2011, 39, 40–44. [Google Scholar]
- Zhang, J.; Zhang, Y.; Quan, X. Electricity assisted anaerobic treatment of salinity wastewater and its effects on microbial communities. Water Res. 2012, 46, 3535–3543. [Google Scholar] [CrossRef] [PubMed]
- Pompeiano, A.; Landi, M.; Meloni, G.; Vita, F.; Guglielminetti, L.; Guidi, L. Allocation pattern, ion partitioning, and chlorophyll a fluorescence in Arundo donax L. in responses to salinity stress. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 151, 613–622. [Google Scholar] [CrossRef]
- Webster, R.J.; Driever, S.M.; Kromdijk, J.; McGrath, J.; Leakey, A.D.; Siebke, K.; Demetriades-Shah, T.; Bonnage, S.; Peloe, T.; Lawson, T. High C3 photosynthetic capacity and high intrinsic water use efficiency underlies the high productivity of the bioenergy grass Arundo donax. Sci. Rep. 2016, 6, 20694. [Google Scholar] [CrossRef] [PubMed]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Asrar, H.; Hussain, T.; Hadi, S.M.S.; Gul, B.; Nielsen, B.L.; Khan, M.A. Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) Staph. Environ. Exp. Bot. 2017, 135, 86–95. [Google Scholar] [CrossRef]
- Gulzar, S.; Hussain, T.; Gul, B.; Hameed, A. Photosynthetic adaptations and oxidative stress tolerance in halophytes from warm subtropical region. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–31. [Google Scholar]
- Fall, F.; Diouf, D.; Fall, D.; Bakhoum, N.; Thioye, B.; Kane, A.; Ndiaye, C.; Ndoye, I.; Bâ, A.M. Growth and physiological responses of Sporobolus robustus kunth seedlings to salt stress. Arid Land Res. Manag. 2017, 31, 46–56. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Wang, K.; Huang, B.; Wang, Z. Differential photosynthetic responses to salinity stress between two perennial grass species contrasting in salinity tolerance. HortScience 2011, 46, 311–316. [Google Scholar] [CrossRef]
- Eller, F.; Lambertini, C.; Nguyen, L.X.; Brix, H. Increased invasive potential of non-native Phragmites australis: Elevated CO2 and temperature alleviate salinity effects on photosynthesis and growth. Glob. Change Biol. 2014, 20, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Percey, W.J.; McMinn, A.; Bose, J.; Breadmore, M.C.; Guijt, R.M.; Shabala, S. Salinity effects on chloroplast PSII performance in glycophytes and halophytes1. Funct. Plant Biol. 2016, 43, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Chen, S.; Dai, S.; Chen, Y.; Wang, Y.; Yan, X. Comparative proteomics of salt tolerance in Arabidopsis thaliana and Thellungiella halophila. J. Proteome Res. 2010, 9, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Venkatesalu, V.; Chellappan, K. Photosynthetic characteristic of Sesuvium portulacastrum L. under salt stress. Photosynthetica 1993, 28, 313–316. [Google Scholar]
- Li, R.; Zhang, J.; Wu, G.; Wang, H.; Chen, Y.; Wei, J. HbCIPK2, a novel CBL-interacting protein kinase from halophyte Hordeum brevisubulatum, confers salt and osmotic stress tolerance. Plant Cell Environ. 2012, 35, 1582–1600. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Koyro, H.-W.; Zhang, W.; Liu, X.; Gul, B.; Liu, X. Low salinity improves photosynthetic performance in Panicum antidotale under drought stress. Front. Plant Sci. 2020, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, M.; Castagna, A.; Remorini, D.; Scattino, C.; Smaoui, A.; Ranieri, A.; Abdelly, C. Photosynthetic responses to salinity in two obligate halophytes: Sesuvium portulacastrum and Tecticornia indica. S. Afr. J. Bot. 2012, 79, 39–47. [Google Scholar] [CrossRef]
- Jithesh, M.; Prashanth, S.; Sivaprakash, K.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237. [Google Scholar] [CrossRef]
- Khan, M.A.; Qaiser, M. Halophytes of Pakistan: Characteristics, distribution and potential economic usages. In Sabkha Ecosystems; Springer: Berlin/Heidelberg, Germany, 2006; pp. 129–153. [Google Scholar]
- Oueslati, S.; Ksouri, R.; Pichette, A.; Lavoie, S.; Girard-Lalancette, K.; Mshvildadze, V.; Abdelly, C.; Legault, J. A new flavonol glycoside from the medicinal halophyte Suaeda fruticosa. Nat. Prod. Res. 2014, 28, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Ansari, R.; Gul, B.; Khan, M.A. Potential of halophytes as source of edible oil. J. Arid Environ. 2007, 68, 315–321. [Google Scholar] [CrossRef]
- Freitag, H.; Golub, V.; Yuritsyna, N. Halophytic plant communities in the northern Caspian lowlands: 1, annual halophytic communities. Phytocoenologia 2000, 31, 63–108. [Google Scholar] [CrossRef]
- Abideen, Z.; Qasim, M.; Rizvi, R.F.; Gul, B.; Ansari, R.; Khan, M.A. Oilseed halophytes: A potential source of biodiesel using saline degraded lands. Biofuels 2015, 6, 241–248. [Google Scholar] [CrossRef]
- Towhidi, A.; Saberifar, T.; Dirandeh, E. Nutritive value of some herbage for dromedary camels in the central arid zone of Iran. Trop. Anim. Health Prod. 2011, 43, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ansari, R.; Ali, H.; Gul, B.; Nielsen, B.L. Panicum turgidum, a potentially sustainable cattle feed alternative to maize for saline areas. Agric. Ecosyst. Environ. 2009, 129, 542–546. [Google Scholar] [CrossRef]
- Bareen, F.-e.; Tahira, S.A. Metal accumulation potential of wild plants in tannery effluent contaminated soil of Kasur, Pakistan: Field trials for toxic metal cleanup using Suaeda fruticosa. J. Hazard. Mater. 2011, 186, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and biochemical responses of Suaeda fruticosa to cadmium and copper stresses: Growth, nutrient uptake, antioxidant enzymes, phytochelatin, and glutathione levels. Environ. Sci. Pollut. Res. 2015, 22, 13058–13069. [Google Scholar] [CrossRef]
- Chopra, I.; Hacker, K. Inhibition of K88-mediated adhesion of Escherichia coli to mammalian receptors by antibiotics that affect bacterial protein synthesis. J. Antimicrob. Chemother. 1986, 18, 441–451. [Google Scholar] [CrossRef]
- Bennani-Kabchi, N.; El Bouayadi, F.; Kehel, L.; Fdhil, H.; Marquie, G. Effect of Suaeda fruticosa aqueous extract in the hypercholesterolaemic and insulin-resistant sand rat. Therapie 1999, 54, 725–730. [Google Scholar]
- Benwahhoud, M.; Jouad, H.; Eddouks, M.; Lyoussi, B. Hypoglycemic effect of Suaeda fruticosa in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2001, 76, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.A.; Albinhassan, T.H.; Al-Ghazzawi, A.M.; Mohaya, A.; Shati, A.A.; Ayoub, H.J.; Abdallah, Q.M. Anticancer property of hexane extract of Suaeda fruticose plant leaves against different cancer cell lines. Trop. J. Pharm. Res. 2020, 19, 129–136. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J. Arid Environ. 2000, 45, 73–84. [Google Scholar] [CrossRef]
- Sleimi, N.; Abdelly, C. Salt-tolerance strategy of two halophyte species: Spartina alterniflora and Suaeda fruticosa. In Cash Crop Halophytes: Recent Studies; Springer: Berlin/Heidelberg, Germany, 2003; pp. 79–85. [Google Scholar]
- Hameed, A.; Hussain, T.; Gulzar, S.; Aziz, I.; Gul, B.; Khan, M.A. Salt tolerance of a cash crop halophyte Suaeda fruticosa: Biochemical responses to salt and exogenous chemical treatments. Acta Physiol. Plant. 2012, 34, 2331–2340. [Google Scholar] [CrossRef]
- Wungrampha, S.; Joshi, R.; Rathore, R.S.; Singla-Pareek, S.L.; Pareek, A. CO2 uptake and chlorophyll a fluorescence of Suaeda fruticosa grown under diurnal rhythm and after transfer to continuous dark. Photosynth. Res. 2019, 142, 211–227. [Google Scholar] [CrossRef]
- Yadav, R.; Srivastava, P. Clustering, Pathway enrichment, and protein-protein interaction analysis of gene expression in neurodevelopmental disorders. Adv. Pharmacol. Sci. 2018, 2018, 3632159. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; John Wiley and Sons, Inc.: New York, NY, USA, 1972. [Google Scholar]
- Koyro, H.-W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Cuin, T.A.; Tian, Y.; Betts, S.A.; Chalmandrier, R.; Shabala, S. Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct. Plant Biol. 2009, 36, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.; Offer, C.; Hansen, U. Induction of CO2-gas exchange and electron transport: Comparison of dynamic and steady-state responses in Fagus sylvatica leaves. Trees-Struct. Funct. 2003, 17, 153–163. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, O.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Polle, A.; Otter, T.; Seifert, F. Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol. 1994, 106, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Abei, H. Measurement of H2O2 in vitro. In Methods in Enzymology; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 121–124. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Zaharieva, T.; Yamashita, K.; Matsumoto, H. Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant Cell Physiol. 1999, 40, 273–280. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Beyer Jr, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Guri, A. Variation in glutathione and ascorbic acid content among selected cultivars of Phaseolus vulgaris prior to and after exposure to ozone. Can. J. Plant Sci. 1983, 63, 733–737. [Google Scholar] [CrossRef]
- Luwe, M.W.; Takahama, U.; Heber, U. Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves. Plant Physiol. 1993, 101, 969–976. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Cambrollé, J.; Luque, T.; Figueroa, M.E.; Davy, A.J. Carry-over of differential salt tolerance in plants grown from dimorphic seeds of Suaeda splendens. Ann. Bot. 2008, 102, 103–112. [Google Scholar] [CrossRef]
- Meychik, N.R.; Nikolaeva, Y.I.; Yermakov, I.P. Physiological response of halophyte (Suaeda altissima (L.) Pall.) and glycophyte (Spinacia oleracea L.) to salinity. Am. J. Plant Sci. 2013, 4, 427–435. [Google Scholar] [CrossRef]
- Storey, R.; Jones, R.G.W. Responses of Atriplex spongiosa and Suaeda monoica to salinity. Plant Physiol. 1979, 63, 156–162. [Google Scholar] [CrossRef]
- Liu, X.; Duan, D.; Li, W.; Tadano, T.; Khan, M.A. A comparative study on responses of growth and solute composition in halophytes Suaeda salsa and Limonium bicolor to salinity. In Ecophysiology of High Salinity Tolerant Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 135–143. [Google Scholar]
- Short, D.C.; Colmer, T.D. Salt tolerance in the halophyte Halosarcia pergranulata subsp. pergranulata. Ann. Bot. 1999, 83, 207–213. [Google Scholar] [CrossRef]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Munns, R. Plant adaptations to salt and water stress: Differences and commonalities. Adv. Bot. Res. 2011, 57, 1–32. [Google Scholar]
- Flowers, T.J.; Glenn, E.P.; Volkov, V. Could vesicular transport of Na+ and Cl− be a feature of salt tolerance in halophytes? Ann. Bot. 2019, 123, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.K.A.; Islam, R.; Le Van, D.; Rahman, M.M.; Yu, R.M.K.; MacFarlane, G.R. Accumulation and partitioning of metals and metalloids in the halophytic saltmarsh grass, saltwater couch, Sporobolus virginicus. Sci. Total Environ. 2020, 713, 136576. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Sümer, A.; Hanstein, S.; Yan, F.; Schubert, S. In vitro effect of different Na+/K+ ratios on plasma membrane H+-ATPase activity in maize and sugar beet shoot. Plant Physiol. Biochem. 2011, 49, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Grof, C.; Brownell, P. The effect of sodium nutrition on the pool sizes of intermediates of the C photosynthetic pathway. Funct. Plant Biol. 1988, 15, 749–760. [Google Scholar] [CrossRef]
- Brownell, P.; Crossland, C. The requirement for sodium as a micronutrient by species having the C4 dicarboxylic photosynthetic pathway. Plant Physiol. 1972, 49, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Safeena, M. Effect of Mycorrhiza Inoculum and Inorganic Fertilizers (N and P) Photosynthetic Parameters of Amaranthus tricolor; Faculty of Applied Science, Sri Lanka. Oluvil, Sri Lanka. 2019. Available online: http://ir.lib.seu.ac.lk/handle/123456789/3963 (accessed on 27 May 2024).
- Li, J.; Wu, W.H.; Wang, Y. Potassium channel AKT1 is involved in the auxin-mediated root growth inhibition in Arabidopsis response to low K+ stress. J. Integr. Plant Biol. 2017, 59, 895–909. [Google Scholar] [CrossRef]
- Assaha, D.V.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Swarbreck, S.M.; Colaço, R.; Davies, J.M. Plant calcium-permeable channels. Plant Physiol. 2013, 163, 514–522. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.-g.; Liu, Z.-h.; Yuan, Y.-j.; Guo, L.-l.; Mao, D.-d.; Tian, L.-f.; Chen, L.-b.; Luan, S.; Li, D.-p. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009, 19, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, M.; Farhat, N.; Msilini, N.; Rajhi, H.; Smaoui, A.; Abdelly, C.; Lachaâl, M.; Karray-Bouraoui, N. Physiological responses of Carthamus tinctorius to CaCl2 salinity under Mg-sufficient and Mg-deficient conditions. Flora 2018, 246, 96–101. [Google Scholar] [CrossRef]
- Kader, M.A.; Lindberg, S. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal. Behav. 2010, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, M.; Rejeb, K.B.; Debez, A.; Messedi, D.; Abdelly, C. Photosynthetic activity and leaf antioxidative responses of Atriplex portulacoides subjected to extreme salinity. Acta Physiol. Plant. 2012, 34, 1679–1688. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Wharmby, C.; Castillo, J.M.; Mateos-Naranjo, E.; Luque, C.J.; De Cires, A.; Luque, T.; Davy, A.J.; Enrique Figueroa, M. Growth and photosynthetic responses to salinity in an extreme halophyte, Sarcocornia fruticosa. Physiol. Plant. 2006, 128, 116–124. [Google Scholar] [CrossRef]
- Rangani, J.; Parida, A.K.; Panda, A.; Kumari, A. Coordinated changes in antioxidative enzymes protect the photosynthetic machinery from salinity induced oxidative damage and confer salt tolerance in an extreme halophyte Salvadora persica L. Front. Plant Sci. 2016, 7, 50. [Google Scholar] [CrossRef]
- Sankari, M.; Hridya, H.; Sneha, P.; Doss, C.G.P.; Christopher, J.G.; Mathew, J.; Zayed, H.; Ramamoorthy, S. Implication of salt stress induces changes in pigment production, antioxidant enzyme activity, and qRT-PCR expression of genes involved in the biosynthetic pathway of Bixa orellana L. Funct. Integr. Genom. 2019, 19, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Uzilday, B.; Ozgur, R.; Sekmen, A.H.; Yildiztugay, E.; Turkan, I. Changes in the alternative electron sinks and antioxidant defence in chloroplasts of the extreme halophyte Eutrema parvulum (Thellungiella parvula) under salinity. Ann. Bot. 2015, 115, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Gulzar, S.; Hameed, A.; Edwards, G.; Khan, M.; Gul, B. Maintenance of photosynthesis and the antioxidant defence systems have key roles for survival of Halopeplis perfoliata (Amaranthaceae) in a saline environment. Plant Biol. 2019, 21, 1167–1175. [Google Scholar] [CrossRef]
- Song, J.; Shi, G.; Gao, B.; Fan, H.; Wang, B. Waterlogging and salinity effects on two Suaeda salsa populations. Physiol. Plant. 2011, 141, 343–351. [Google Scholar] [CrossRef]
- English, J.P.; Colmer, T.D. Salinity and waterlogging tolerances in three stem-succulent halophytes (Tecticornia species) from the margins of ephemeral salt lakes. Plant Soil 2011, 348, 379–396. [Google Scholar] [CrossRef]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The Salt Overly Sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- White, P.J.; Davenport, R.J. The voltage-independent cation channel in the plasma membrane of wheat roots is permeable to divalent cations and may be involved in cytosolic Ca2+ homeostasis. Plant Physiol. 2002, 130, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Spalding, E.P.; Harper, J.F. The ins and outs of cellular Ca2+ transport. Curr. Opin. Plant Biol. 2011, 14, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Plieth, C. Calcium: Just another regulator in the machinery of life? Ann. Bot. 2005, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Hussain, T.; Li, J.; Feng, X.; Asrar, H.; Gul, B.; Liu, X. Salinity induced alterations in photosynthetic and oxidative regulation are ameliorated as a function of salt secretion. J. Plant Res. 2021, 134, 779–796. [Google Scholar] [CrossRef]
- Cherian, S.; Reddy, M. Micropropagation of the halophyte Suaeda nudiflora Moq. through axillary bud culture. Indian J. Plant Physiol. 2002, 7, 40–43. [Google Scholar]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. The role of antioxidant responses on the tolerance range of extreme halophyte Salsola crassa grown under toxic salt concentrations. Ecotoxicol. Environ. Saf. 2014, 110, 21–30. [Google Scholar] [CrossRef]


| Salinity (NaCl; mmol L−1) | |||
|---|---|---|---|
| 0 | 300 | 900 | |
| Plant Biomass | |||
| lDM (g) | 8.64 ± 1.0 b | 12.63 ± 0.8 a | 10.04 ± 0.9 ab |
| sDM (g) | 10.10 ± 1.4 b | 14.24 ± 0.8 a | 4.02 ± 0.6 c |
| rDM (g) | 2.16 ± 0.3 a | 1.97 ± 0.2 a | 2.47 ± 0.5 a |
| Plant Height | |||
| rL(cm) | 24.87 ± 2.5 a | 27.37 ± 1.3 a | 28.40 ± 0.9 a |
| sL (cm) | 60.40 ± 1.8 a | 58.63 ± 4.1 a | 33.47 ± 5.0 b |
| Leaf Water Relations | |||
| RWC (%) | 86.31 ± 1.20 a | 84.89 ± 1.30 a | 90.28 ± 1.15 a |
| OP (MPa) | −2.11 ± 0.10 c | −3.38 ± 0.10 b | −5.19 ± 0.04 a |
| XPP (MPa) | −1.31 ± 0.07 c | −2.42 ± 0.08 b | −4.02 ± 0.02 a |
| Salinity (NaCl; mmol L−1) | |||
|---|---|---|---|
| 0 | 300 | 900 | |
| Leaf Gas-exchange | |||
| Rd | 2.83 ± 0.1 b | 2.25 ± 0.1 b | 5.02 ± 0.2 a |
| Photo (A) | 11.21 ± 0.1 a | 13.71 ± 0.1 a | 8.32 ± 0.3 b |
| Cond (gs) | 0.03 ± 0.0 a | 0.04 ± 0.0 a | 0.03 ± 0.0 a |
| Ci | 213 ± 3.8 a | 213 ± 7.0 a | 189 ± 2.6 b |
| E | 1.72 ± 0.0 ab | 2.12 ± 0.0 b | 1.30 ± 0.0 a |
| WUE | 6.52 ± 0.0 a | 6.48 ± 0.1 a | 6.41 ± 0.2 a |
| Ic | 298 ± 2.4 b | 211 ± 3.7 c | 311 ± 1.4 a |
| Is | 1960 ± 18.7 a | 1576 ± 9.9 b | 1455 ± 15.3 c |
| Leaf Photochemistry (Induction Curve) | |||
| Fv/Fm | 0.70 ± 0.03 a | 0.73 ± 0.01 a | 0.74 ± 0.00 a |
| Y(II) | 0.11 ± 0.03 a | 0.06 ± 0.01 b | 0.08 ± 0.01 ab |
| Y(NPQ) | 0.44 ± 0.04 a | 0.45 ± 0.03 a | 0.47 ± 0.03 a |
| Y(NO) | 0.46 ± 0.05 a | 0.49 ± 0.03 a | 0.45 ± 0.02 a |
| NPQ | 0.99 ± 0.17 a | 0.93 ± 0.12 a | 1.05 ± 0.13 a |
| qN | 0.70 ± 0.03 a | 0.67 ± 0.03 a | 0.64 ± 0.03 a |
| qP | 0.25 ± 0.07 a | 0.14 ± 0.01 b | 0.14 ± 0.01 b |
| qL | 0.17 ± 0.05 a | 0.08 ± 0.00 b | 0.06 ± 0.01 b |
| ETR | 26 ± 2.91 a | 24 ± 1.76 a | 30 ± 4.18 a |
| Photosynthetic Pigments | |||
| Chl a | 96 ± 7.1 a | 52 ± 7.1 b | 49 ± 10 b |
| Chl b | 44 ± 10.0 a | 19 ± 4.0 b | 18 ± 5 b |
| Total | 141 ± 17.1 a | 72 ± 11.0 b | 67 ± 14 b |
| CAR | 25 ± 0.8 a | 14 ± 2.1 b | 12 ± 2 b |
| Chl a/b | 2.29 ± 0.3 a | 2.82 ± 0.3 a | 3.00 ± 0 a |
| Chl/CAR | 5.47 ± 0.5 a | 5.12 ± 0.3 a | 5.23 ± 0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, B.; Hameed, A.; Ahmed, M.Z.; Hussain, T.; Rasool, S.G.; Nielsen, B.L. Thriving under Salinity: Growth, Ecophysiology and Proteomic Insights into the Tolerance Mechanisms of Obligate Halophyte Suaeda fruticosa. Plants 2024, 13, 1529. https://doi.org/10.3390/plants13111529
Gul B, Hameed A, Ahmed MZ, Hussain T, Rasool SG, Nielsen BL. Thriving under Salinity: Growth, Ecophysiology and Proteomic Insights into the Tolerance Mechanisms of Obligate Halophyte Suaeda fruticosa. Plants. 2024; 13(11):1529. https://doi.org/10.3390/plants13111529
Chicago/Turabian StyleGul, Bilquees, Abdul Hameed, Muhammad Zaheer Ahmed, Tabassum Hussain, Sarwat Ghulam Rasool, and Brent L. Nielsen. 2024. "Thriving under Salinity: Growth, Ecophysiology and Proteomic Insights into the Tolerance Mechanisms of Obligate Halophyte Suaeda fruticosa" Plants 13, no. 11: 1529. https://doi.org/10.3390/plants13111529










