Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation
Abstract
:1. Introduction
2. Phytoremediation Types
2.1. Phytoextraction
2.2. Rhizofiltration
2.3. Phytostabilization
2.4. Phytovolatilization
3. Metal Absorption and Tolerance Mechanisms
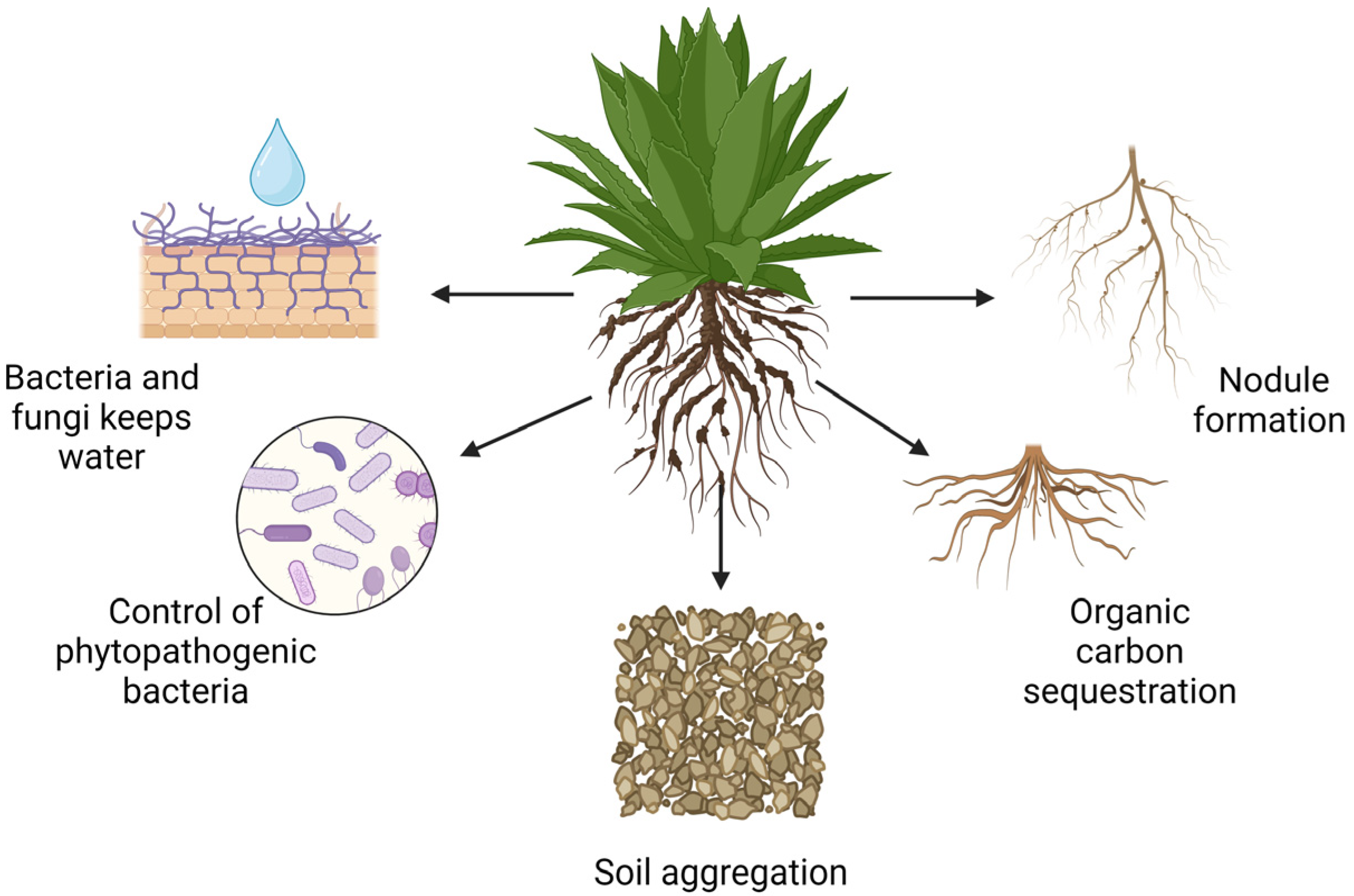
3.1. Rhizosphere Interactions
3.2. Heavy Metal Transporters
3.3. Intracellular Ligands of Heavy Metals
3.4. Phytochelatins
3.5. Detoxification and Tolerance Mechanisms
3.6. Translocation and Accumulation
3.7. Physiological and Biochemical Aspects of Phytoremediation
4. Plant Selection
5. Hyperaccumulating Plants: Mechanisms of Hyperaccumulation
6. Challenges and Limitations of Phytoremediation
6.1. Metal Availability
6.2. Soil Conditions
6.3. Plant Growth and Biomass Productivity
- -
- Plant susceptibility to pests and diseases can diminish plant growth and vitality; therefore, comprehensive pest control strategies are essential for mitigating these threats to the phytoremediation process.
- -
- Plant selection and genetic diversity can significantly influence phytoremediation success. Thus, identifying suitable hyperaccumulators with the necessary genetic traits is critical [8].
6.4. Complex Contamination and Soil Biodiversity
6.5. Advanced Techniques in Phytoremediation
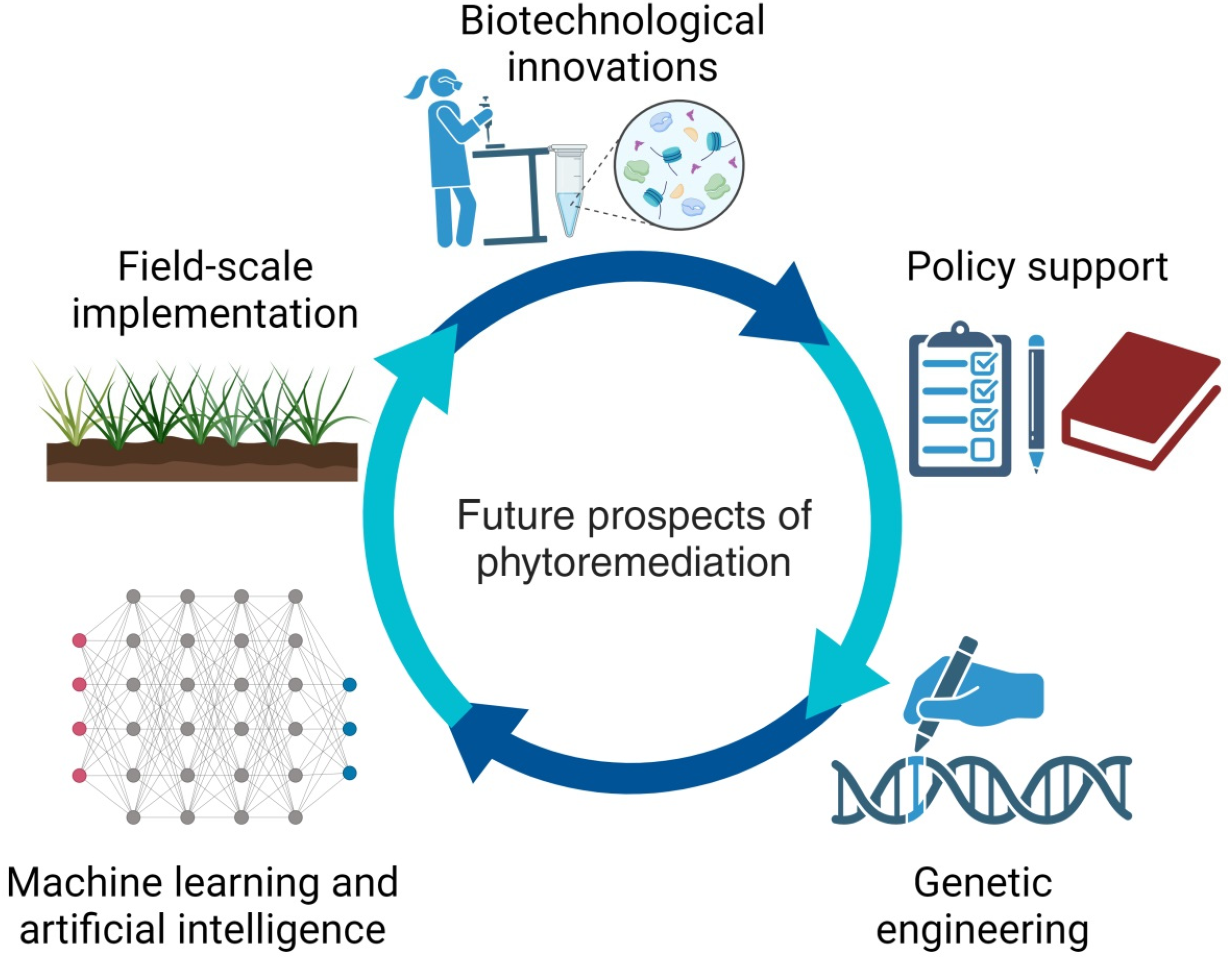
7. Trends and Future Prospects of Phytoremediation
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Aloud, S.S.; Alotaibi, K.D.; Almutairi, K.F.; Albarakah, F.N. Assessment of Heavy Metals Accumulation in Soil and Native Plants in an Industrial Environment, Saudi Arabia. Sustainability 2022, 14, 5993. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Strand, S.E.; Doty, S.L. Phytoremediation of Chlorpyrifos by Populus and Salix. Int. J. Phytoremediation 2012, 14, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Čudić, V.; Stojiljković, D.; Jovović, A. Phytoremediation Potential of Wild Plants Growing on Soil Contaminated with Heavy Metals. Arh. Hig. Rada Toksikol. 2016, 67, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Sow, S. Phytoremediation: An Eco-Friendly Approach towards Clean and Green Future. Pharma J. 2021, 10, 839–850. [Google Scholar] [CrossRef]
- Sabreena, H.; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants 2022, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Montreemuk, J.; Stewart, T.N.; Prapagdee, B. Bacterial-Assisted Phytoremediation of Heavy Metals: Concepts, Current Knowledge, and Future Directions. Environ. Technol. Innov. 2023, 33, 103488. [Google Scholar] [CrossRef]
- Mocek-Płóciniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as an Effective Remedy for Removing Trace Elements from Ecosystems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Bosiacki, M.; Kleiber, T.; Markiewicz, B.; Bosiacki, M.; Kleiber, T.; Markiewicz, B. Continuous and Induced Phytoextraction—Plant-Based Methods to Remove Heavy Metals from Contaminated Soil. In Environmental Risk Assessment of Soil Contamination; IntechOpen: London, UK, 2014; ISBN 978-953-51-1235-8. [Google Scholar]
- Corzo Remigio, A.; Chaney, R.L.; Baker, A.J.M.; Edraki, M.; Erskine, P.D.; Echevarria, G.; Ent, A. Phytoextraction of High Value Elements and Contaminants from Mining and Mineral Wastes: Opportunities and Limitations. Plant Soil. 2020, 449, 11–37. [Google Scholar] [CrossRef]
- Abdullahi, M.S. Chapter 18-Soil Contamination, Remediation and Plants: Prospects and Challenges; Remediation, S., Plants, H., Eds.; Academic Press: San Diego, CA, USA, 2015; ISBN 978-0-12-799937-1. [Google Scholar]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Chapter Four-Phytostabilization: A Green Approach to Contaminant Containment. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 112, pp. 145–204. [Google Scholar]
- Limmer, M.; Burken, J. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Vasudha Priyadharshini, S.; Paramasivan, T.; Dhakal, N.; Naushad, M. Research Updates on Heavy Metal Phytoremediation: Enhancements, Efficient Post-Harvesting Strategies and Economic Opportunities. In Green Materials for Wastewater Treatment; Naushad, E., Mu, L., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 191–222. ISBN 978-3-030-17724-9. [Google Scholar]
- Hussein, H.S.; Ruiz, O.N.; Terry, N.; Daniell, H. Phytoremediation of Mercury and Organomercurials in Chloroplast Transgenic Plants: Enhanced Root Uptake, Translocation to Shoots, and Volatilization. Environ. Sci. Technol. 2007, 41, 8439–8446. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Shen, Z.; Zhu, J.; Jia, X.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J. Field Trials of Phytomining and Phytoremediation: A Critical Review of Influencing Factors and Effects of Additives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2724–2774. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Tiwari, J.; Ankit, S.; Kumar, S.; Korstad, J.; Bauddh, K. Chapter 5-Ecorestoration of Polluted Aquatic Ecosystems through Rhizofiltration. In Phytomanagement of Polluted Sites; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–201. [Google Scholar]
- Odinga, C.; Kumar, A.; Mthembu, M.; Bux, F.; Swalaha, F. Rhizofiltration System Consisting of Phragmites Australis and Kyllinga Nemoralis: Evaluation of Efficient Removal of Metals and Pathogenic Microorganisms. Desalinat. Water Treat. 2019, 169, 120–132. [Google Scholar] [CrossRef]
- Sikhosana, M.L.M.; Botha, A.; Mpenyane-Monyatsi, L.; Coetzee, M.A. Evaluating the Effect of Seasonal Temperature Changes on the Efficiency of a Rhizofiltration System in Nitrogen Removal from Urban Runoff. J. Environ. Manag. 2020, 274, 111192. [Google Scholar] [CrossRef]
- Chirakkara, R.A.; Cameselle, C.; Reddy, K.R. Assessing the Applicability of Phytoremediation of Soils with Mixed Organic and Heavy Metal Contaminants. Rev. Environ. Sci. Biotechnol. 2016, 15, 299–326. [Google Scholar] [CrossRef]
- Benavides, L.C.L.; Pinilla, L.A.C.; Serrezuela, R.R.; Serrezuela, W.F.R. Extraction in laboratory of heavy metals through Rhizofiltration Using the Plant Zea Mays (Maize). Int. J. Appl. Environ. Sci. 2018, 13, 9–26. [Google Scholar]
- Fresno, T.; Moreno-Jiménez, E.; Zornoza, P.; Peñalosa, J.M. Aided Phytostabilisation of As- and Cu-Contaminated Soils Using White Lupin and Combined Iron and Organic Amendments. J. Environ. Manag. 2018, 205, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Lorestani, B.; Yousefi, N.; Cheraghi, M.; Farmany, A. Phytoextraction and Phytostabilization Potential of Plants Grown in the Vicinity of Heavy Metal-Contaminated Soils: A Case Study at an Industrial Town Site. Env. Monit. Assess. 2013, 185, 10217–10223. [Google Scholar] [CrossRef] [PubMed]
- Radziemska, M.; Koda, E.; Bilgin, A.; Vaverková, M.D. Concept of Aided Phytostabilization of Contaminated Soils in Postindustrial Areas. Int. J. Environ. Res. Public Health 2018, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L.; James, C.A.; Moore, A.L.; Vajzovic, A.; Singleton, G.L.; Ma, C.; Khan, Z.; Xin, G.; Kang, J.W.; Park, J.Y.; et al. Enhanced Phytoremediation of Volatile Environmental Pollutants with Transgenic Trees. Proc. Natl. Acad. Sci. USA 2007, 104, 16816–16821. [Google Scholar] [CrossRef]
- Haq, S.; Bhatti, A.A.; Dar, Z.A.; Bhat, S.A. Phytoremediation of Heavy Metals: An Eco-Friendly and Sustainable Approach. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Hakeem, K.R., Bhat, R.A., Qadri, H., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 215–231. ISBN 978-3-030-35691-0. [Google Scholar]
- Liu, C.; Lin, H.; Li, B.; Dong, Y.; Yin, T. Responses of Microbial Communities and Metabolic Activities in the Rhizosphere during Phytoremediation of Cd-Contaminated Soil. Ecotoxicol. Environ. Saf. 2020, 202, 110958. [Google Scholar] [CrossRef]
- Borymski, S.; Cycoń, M.; Beckmann, M.; Mur, L.A.J.; Piotrowska-Seget, Z. Plant Species and Heavy Metals Affect Biodiversity of Microbial Communities Associated With Metal-Tolerant Plants in Metalliferous Soils. Front. Microbiol. 2018, 9, 371452. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere Microbiome Manipulation for Sustainable Crop Production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Ahkami, A.H.; White, R.A., III; Handakumbura, P.P.; Jansson, C. Rhizosphere Engineering: Enhancing Sustainable Plant Ecosystem Productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Competition among Metal Ions for Protein Binding Sites: Determinants of Metal Ion Selectivity in Proteins. Chem. Rev. 2014, 114, 538–556. [Google Scholar] [CrossRef]
- Krämer, U. Conceptualizing Plant Systems Evolution. Curr. Opin. Plant Biol. 2018, 42, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc Absorption in Plants: Uptake, Transport, Translocation and Accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- De Caroli, M.; Furini, A.; DalCorso, G.; Rojas, M.; Di Sansebastiano, G.-P. Endomembrane Reorganization Induced by Heavy Metals. Plants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Cointry, V.; Vert, G. The Bifunctional Transporter-receptor IRT 1 at the Heart of Metal Sensing and Signalling. New Phytol. 2019, 223, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Blindauer, C.A.; Schmid, R. Cytosolic Metal Handling in Plants: Determinants for Zinc Specificity in Metal Transporters and Metallothioneins. Metallomics 2010, 2, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, A.T.; Gaudet, R. Molecular mechanism of Nramp-family transition metal transport. J. Mol. Biol. 2021, 433, 166991. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, X.; Lan, P. Emerging Roles of Protein Phosphorylation in Plant Iron Homeostasis. Trends Plant Sci. 2022, 27, 908–921. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Song, H.; Zhao, J.; Shabala, S.; Tian, S.; Yang, X. A Novel Plasma Membrane-Based NRAMP Transporter Contributes to Cd and Zn Hyperaccumulation in Sedum Alfredii Hance. Environ. Exp. Bot. 2020, 176, 104121. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, H.Q.; Chen, J.; Chang, J.D.; Zhao, F.J. Molecular Mechanisms Underlying the Toxicity and Detoxification of Trace Metals and Metalloids in Plants. J. Integr. Plant Biol. 2023, 65, 570–593. [Google Scholar] [CrossRef]
- Puig, S. Function and Regulation of the Plant COPT Family of High-Affinity Copper Transport Proteins. Adv. Bot. 2014, 2014. [Google Scholar] [CrossRef]
- Morgan, M.T.; Bourassa, D.; Harankhedkar, S.; McCallum, A.M.; Zlatic, S.A.; Calvo, J.S.; Meloni, G.; Faundez, V.; Fahrni, C.J. Ratiometric Two-Photon Microscopy Reveals Attomolar Copper Buffering in Normal and Menkes Mutant Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 12167–12172. [Google Scholar] [CrossRef]
- Socha, A.L.; Guerinot, M.L. Mn-Euvering Manganese: The Role of Transporter Gene Family Members in Manganese Uptake and Mobilization in Plants. Front. Plant Sci. 2014, 5, 79388. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Zhong, Y.; Chen, J.; Qi, X. Deciphering the Functional Roles of Transporter Proteins in Subcellular Metal Transportation of Plants. Planta 2023, 258, 17. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, T.; Chellasamy, G.; Tp, A.K.; Ceasar, S.A.; Yun, K. The Role of Metal Transporters in Phytoremediation: A Closer Look at Arabidopsis. Chemosphere 2023, 310, 136881. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Khan, S.M.; Khalid, N.; Ahmad, Z.; Jehangir, S.; Fatima Rizvi, Z.; Lho, L.H.; Han, H.; Raposo, A. Detoxifying the Heavy Metals: A Multipronged Study of Tolerance Strategies against Heavy Metals Toxicity in Plants. Front. Plant Sci. 2023, 14, 1154571. [Google Scholar] [CrossRef] [PubMed]
- Zlobin, I.E. Current Understanding of Plant Zinc Homeostasis Regulation Mechanisms. Plant Physiol. Biochem. 2021, 162, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive Mechanisms of Heavy Metal Toxicity in Plants, Detoxification, and Remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Phytochelatins: Sulfur-Containing Metal (Loid)-Chelating Ligands in Plants. Int. J. Mol. Sci. 2023, 24, 2430. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Low-Molecular-Weight Ligands in Plants: Role in Metal Homeostasis and Hyperaccumulation. Photosynth. Res. 2021, 150, 51–96. [Google Scholar] [CrossRef]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Singh, B. Metal Tolerance in Plants: Molecular and Physicochemical Interface Determines the “Not so Heavy Effect” of Heavy Metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef] [PubMed]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Front. Microbiol. 2020, 11, 527494. [Google Scholar] [CrossRef]
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.P.; Rea, P.A. AtPCS1, a Phytochelatin Synthase from Arabidopsis: Isolation and in Vitro Reconstitution. Proc. Natl. Acad. Sci. USA 1999, 96, 7110–7115. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S. Phytochelatin Biosynthesis and Function in Heavy-Metal Detoxification. Curr. Opin. Plant Biol. 2000, 3, 211–216. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Morkunas, I.; Woźniak, A.; Mai, V.C.; Rucińska-Sobkowiak, R.; Jeandet, P. The Role of Heavy Metals in Plant Response to Biotic Stress. Molecules 2018, 23, 2320. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.N.V. Phytoremediation of Metal-Polluted Ecosystems: Hype for Commercialization. Rus. J. Plan Phisiol. 2003, 50, 686–701. [Google Scholar] [CrossRef]
- Pang, Y.L.; Quek, Y.Y.; Lim, S.; Shuit, S.H. Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach. Sustainability 2023, 15, 1290. [Google Scholar] [CrossRef]
- Papoyan, A.; Kochian, L.V. Identification of Thlaspi Caerulescens Genes That May Be Involved in Heavy Metal Hyperaccumulation and Tolerance. Characterization of a Novel Heavy Metal Transporting ATPase. Plant Physiol. 2004, 136, 3814–3823. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation Technologies and Their Mechanism for Removal of Heavy Metal from Contaminated Soil: An Approach for a Sustainable Environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Azhdarpoor, A. The Application of Plant Growth Regulators to Improve Phytoremediation of Contaminated Soils: A Review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Shuang, C.; Qing, H.; Song, B. Enhanced Technology of Phytoremediation. E3S Web Conf. 2021, 261, 04034. [Google Scholar] [CrossRef]
- Wei, Z.; Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A Review on Phytoremediation of Contaminants in Air, Water and Soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, M. Phytoremediation of Heavy Metals Principles, Mechanisms, Enhancements with Several Efficiency Enhancer Methods and Perspectives: A Review. Middle East. J. 2020, 9, 186–214. [Google Scholar]
- Seregin, I.V.; Kozhevnikova, A.D.; Schat, H. Nickel Tolerance and Accumulation Capacities in Different Populations of the Hyperaccumulator Noccaea Caerulescens. Russ. J. Plant Physiol. 2022, 69, 70. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Halophytes Present New Opportunities in Phytoremediation of Heavy Metals and Saline Soils. Ind. Eng. Chem. Res. 2011, 50, 656–660. [Google Scholar] [CrossRef]
- Lone, M.I.; He, Z.L.; Stoffella, P.J.; Yang, X.E. Phytoremediation of Heavy Metal Polluted Soils and Water: Progresses and Perspectives. J. Zhejiang Univ. Sci. B 2008, 9, 210–220. [Google Scholar] [CrossRef]
- Oosten, M.J.; Maggio, A. Functional Biology of Halophytes in the Phytoremediation of Heavy Metal Contaminated Soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Ouaini, A.; Yssaad, H.A.; Nouri, T.; Nani, A.; Benouis, S. Influence of Combined Stress by Salinity (NaCl) and Heavy Metals (Pb (NO 3) 2) on the Proline, Chlorophyll and Lead Accumulation in the Tissues of the Atriplex Canescens (Pursh) Nutt. Agric. Sci. Technol. (1313-8820) 2023, 15, 67–75. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.-J. Phytoextraction of Metalsand Metalloids from Contaminated Soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [PubMed]
- Alford, É.R.; Pilon-Smits, E.A.H.; Fakra, S.C.; Paschke, M.W. Selenium Hyperaccumulation by Astragalus (Fabaceae) Does Not Inhibit Root Nodule Symbiosis. Am. J. Bot. 2012, 99, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Mujeeb, A. Halophytes for Phytoremediation of Hazardous Metal(Loid)s: A Terse Review on Metal Tolerance, Bio-Indication and Hyperaccumulation. J. Hazard. Mater. 2022, 424, 127309. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction Potential of Pteris Vittata, L. Co-Planted with Woody Species for As, Cd, Pb and Zn in Contaminated Soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Boorboori, M.R.; Zhang, H.Y. Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium. J. Fungi 2022, 8, 176. [Google Scholar] [CrossRef]
- Ma, Y.; Ankit, T.J.; Bauddh, K. Plant-Mycorrhizal Fungi Interactions in Phytoremediation of Geogenic Contaminated Soils. Front. Microbiol. 2022, 13, 843415. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Laidlaw, W.S.; Doronila, A.; Baker, A.J.M. Erratic Hyperaccumulation of Nickel, with Particular Reference to the Queensland Serpentine Endemic Pimelea Leptospermoides. Aust. J. Bot. 2015, 63, 119–127. [Google Scholar] [CrossRef]
- Peng, J.S.; Guan, Y.H.; Lin, X.J. Comparative Understanding of Metal Hyperaccumulation in Plants: A Mini-Review. Environ. Geochem. Health 2021, 43, 1599–1607. [Google Scholar] [CrossRef]
- Deng, T.-H.-B.; Ent, A.; Tang, Y.-T.; Sterckeman, T.; Echevarria, G.; Morel, J.-L.; Qiu, R.-L. Nickel Hyperaccumulation Mechanisms: A Review on the Current State of Knowledge. Plant Soil. 2018, 423, 1–11. [Google Scholar] [CrossRef]
- Kramer, U. Metal Hyperaccumulation in Plants. Ann. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W.; Bunkowski, M.; Puschenreiter, M.; Horak, O. Rhizosphere characteristics of indigenous growing nickel hyperaccumulator and excluder plants on serpentine soil. Environ. Poll. 2003, 123, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Buono, D.D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric Organic Compounds in the Soil-Microorganism-Plant System: Their Role in Iron Availability. Eur. J. Soil. Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Grennan, A.K. Identification of Genes Involved in Metal Transport in Plants-PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2663745/ (accessed on 25 April 2024).
- Leitenmaier, B.; Ktipper, H. Compartmentation and Complexation of Metals in Hyperaccumulator Plants. Front. M. Plant Sci. 2013, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Cosio, C.; Santis, L.; Frey, B.; Diallo, S.; Keller, C. Distribution of Cadmium in Leaves of Thlaspi Caerulescens. J. Exp. Bot. 2005, 56, 565–575. [Google Scholar] [CrossRef]
- Mortel, J.E.; Almar, J.E.; Villanueva, L.; Schat, H.; Kwekkeboom, J.; Coughlan, S. Large Expression Differences in Genes for Iron and Zinc Homeostasis, Stress Response, and Lignin Biosynthesis Distinguish Roots of Arabidopsis Thaliana and the Related Metal Hyperaccumulator Thlaspi Caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef]
- Clemens, S. Casting a Wide Cross-Species Transcriptomics Net: Convergent Evolution of Nickel Hyperaccumulation. New Phytol. 2021, 229, 653–655. [Google Scholar] [CrossRef]
- Takahashi The Role of Heavy-Metal ATPases, HMAs, in Zinc and Cadmium Transport in Rice. Plant Signal. Behav. 2012, 7, 1605–1607. [CrossRef] [PubMed]
- Nosek, M.; Kaczmarczyk, A.; Jędrzejczyk, R.J.; Supel, P.; Kaszycki, P.; Miszalski, Z. Expression of Genes Involved in Heavy Metal Trafficking in Plants Exposed to Salinity Stress and Elevated Cd Concentrations. Plants 2020, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Gustin, J.L.; Loureiro, M.E.; Run, D.; Na, G.; Tikhonova, M.; Salt, D.E. MTP1-Dependent Zn Sequestration into Shoot Vacuoles Suggests Dual Roles in Zn Tolerance and Accumulation in Zn Hyperaccumulating Plants. Plant J. 2009, 57, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.F.; Burken, J.G.; Maier, R.M.; Newman, L.A.; Rock, S.; Schnoor, J.L.; Suk, W.A. Phytotechnologies–Preventing Exposures, Improving Public Health. Int. J. Phytoremed. 2013, 15, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, S.; Liu, S.; Peng, J.; Zhang, H.; Zhao, Q.; Zheng, L.; Chen, Y.; Shen, Z.; Xu, X.; et al. Enhancing the Phytoremediation of Heavy Metals by Combining Hyperaccumulator and Heavy Metal-Resistant Plant Growth-Promoting Bacteria. Front. Plant Sci. 2022, 13, 912350. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Faz, A.; Zornoza, R.; Martinez-Martinez, S.; Acosta, J.A. Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(Loid)s and Rare Earth Elements. Plants 2023, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Guidi Nissim, W.; Castiglione, S.; Guarino, F.; Pastore, M.C.; Labra, M. Beyond Cleansing: Ecosystem Services Related to Phytoremediation. Plants 2023, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Song, Y.; Sui, J.; Hua, X. Advances in the Involvement of Metals and Metalloids in Plant Defense Response to External Stress. Plants 2024, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Skuza, L.; Szućko-Kociuba, I.; Filip, E.; Bożek, I. Natural Molecular Mechanisms of Plant Hyperaccumulation and Hypertolerance towards Heavy Metals. Int. J. Mol. Sci. 2022, 23, 9335. [Google Scholar] [CrossRef]
- Hasan, M.M.; Uddin, M.N.; Ara-Sharmeen, I.; Alharby, F.; Alzahrani, Y.; Hakeem, K.R.; Zhang, L. Assisting Phytoremediation of Heavy Metals Using Chemical Amendments. Plants 2019, 8, 295. [Google Scholar] [CrossRef]
- Rangel, T.S.; Santana, N.A.; Jacques, R.J.S.; Ramos, R.F.; Scheid, D.L.; Koppe, E.; Tabaldi, L.A.; De Oliveira Silveira, A. Organic Fertilization and Mycorrhization Increase Copper Phytoremediation by Canavalia Ensiformis in a Sandy Soil. Environ. Sci. Pollut. Res. 2023, 30, 68271–68289. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Qi, S.-S.; Gul, F.; Manan, S.; Rono, J.K.; Naz, M.; Shi, X.-N.; Zhang, H.; Dai, Z.-C.; Du, D.-L. A Green Approach Used for Heavy Metals ‘Phytoremediation’ Via Invasive Plant Species to Mitigate Environmental Pollution: A Review. Plants 2023, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Rosen, B.P. Perspectives for Genetic Engineering for the Phytoremediation of Arsenic-Contaminated Environments: From Imagination to Reality? Curr. Opin. Biotechnol. 2009, 20, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wu, Z.; Glick, B.R.; He, S.; Huang, C.; Wu, L. Co-Occurrence Patterns of Microbial Communities Affected by Inoculants of Plant Growth-Promoting Bacteria during Phytoremediation of Heavy Metal-Contaminated Soils. Ecotoxicol. Environ. Saf. 2019, 183, 109504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Chen, Y.; Li, Y.-Y.; Ding, C.-Y.; Li, B.-L.; Han, H.; Chen, Z.-J. Plant Growth-Promoting Bacteria Improve the Cd Phytoremediation Efficiency of Soils Contaminated with PE–Cd Complex Pollution by Influencing the Rhizosphere Microbiome of Sorghum. J. Hazard. Mater. 2024, 469, 134085. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Islam, N.F.; Prasad, R.; Prasad, M.N.V.; Ma, L.Q.; Rinklebe, J. Enhancing phytoremediation of hazardous metal(loid)s using genome engineering CRISPR-Cas9 technology. J. Hazard. Mater. 2021, 414, 125493. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, P.; Ellison, E.; Polley, B.; Bollina, V.; Kulkarni, M.; Ghanbarnia, K.; Song, H.; Gao, C.; Voytas, D.F.; Kagale, S. Targeted mutagenesis in wheat microspores using CRISPR/Cas9. Sci. Rep. 2018, 8, 6502. [Google Scholar] [CrossRef] [PubMed]
- Basharat, Z.; Novo, L.A.B.; Yasmin, A. Genome Editing Weds CRISPR: What Is in It for Phytoremediation? Plants 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Garbisu, C.; Alkorta, I. Phytoextraction: A Cost-Effective Plant-Based Technology for the Removal of Metals from the Environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Girdhar, M.; Sharma, N.R.; Rehman, H.; Kumar, A.; Mohan, A. Comparative Assessment for Hyperaccumulatory and Phytoremediation Capability of Three Wild Weeds. Epub 2014, 4, 579–589. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Aghajani Delavar, M. Techno-Economic Analysis of Phytoremediation: A Strategic Rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef] [PubMed]
- Bartucca, M.L.; Cerri, M.; Forni, C. Phytoremediation of Pollutants: Applicability and Future Perspective. Plants 2023, 12, 2462. [Google Scholar] [CrossRef] [PubMed]


| Methods | Advantages | Limitations | Mechanism | Contaminants | Refs. |
|---|---|---|---|---|---|
| Phytoextraction | Cost-effective and eco-friendly. Suitable for both large- and small-scale remediation projects. A sustainable way to reduce HM concentrations in soil. | Limited to sites contaminated with specific HMs. Requires careful selection of appropriate accumulator plants. May take several decades to achieve desired results. | Hyperaccumulation in harvestable plant tissues | Elements: Pb, Zn, Au, Co, Cr, Ni, Hg, Mo, Ag, and Cd Radionuclides: Pb, Sr, U, and Cs | [12] |
| Rhizofiltration | Efficient for remediation of contaminated water. Suitable for various water bodies, including ponds, rivers, and constructed wetlands. Lower operating costs. | Limited to water bodies with suitable vegetation. May require careful monitoring to prevent plant overgrowth. Efficiency depends on water flow rates and environmental factors. | Rhizosphere accumulation through precipitation, sorption | Inorganic: Cr, Cd, Cu, and Ni | [13] |
| Phytostabilization | Reduced risk of contaminant migration. Can be combined with other phytoremediation techniques. Lower maintenance requirements. | Contaminant specific. Efficiency depends on plant selection and soil conditions. May take a longer time to achieve desired results. | Sorption, precipitation, and chelation | Inorganic: Cu, As, Cr, Zn, Cd, and Pb | [15] |
| Phytovolatilization | Effective for airborne contaminants. Suitable for in/outdoor remediation projects. Aids to reduce the overall contamination level of the environment. | Limited to contaminants that can be transformed into gas form. May require careful selection of suitable plants. The release of gases into the atmosphere may raise air quality concerns. | Pollutant eradication | Organic: phenols, munitions herbicides, chlorinated solvents | [16] |
| STRENGTHS (S) | OPPORTUNITIES (O) |
|
|
| WEAKNESSES (W) | THREATS (T) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhakypbek, Y.; Kossalbayev, B.D.; Belkozhayev, A.M.; Murat, T.; Tursbekov, S.; Abdalimov, E.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation. Plants 2024, 13, 1534. https://doi.org/10.3390/plants13111534
Zhakypbek Y, Kossalbayev BD, Belkozhayev AM, Murat T, Tursbekov S, Abdalimov E, Pashkovskiy P, Kreslavski V, Kuznetsov V, Allakhverdiev SI. Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation. Plants. 2024; 13(11):1534. https://doi.org/10.3390/plants13111534
Chicago/Turabian StyleZhakypbek, Yryszhan, Bekzhan D. Kossalbayev, Ayaz M. Belkozhayev, Toktar Murat, Serik Tursbekov, Elaman Abdalimov, Pavel Pashkovskiy, Vladimir Kreslavski, Vladimir Kuznetsov, and Suleyman I. Allakhverdiev. 2024. "Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation" Plants 13, no. 11: 1534. https://doi.org/10.3390/plants13111534
APA StyleZhakypbek, Y., Kossalbayev, B. D., Belkozhayev, A. M., Murat, T., Tursbekov, S., Abdalimov, E., Pashkovskiy, P., Kreslavski, V., Kuznetsov, V., & Allakhverdiev, S. I. (2024). Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation. Plants, 13(11), 1534. https://doi.org/10.3390/plants13111534









