Transgenic Soybean for Production of Thermostable α-Amylase
Abstract
1. Introduction
2. Results
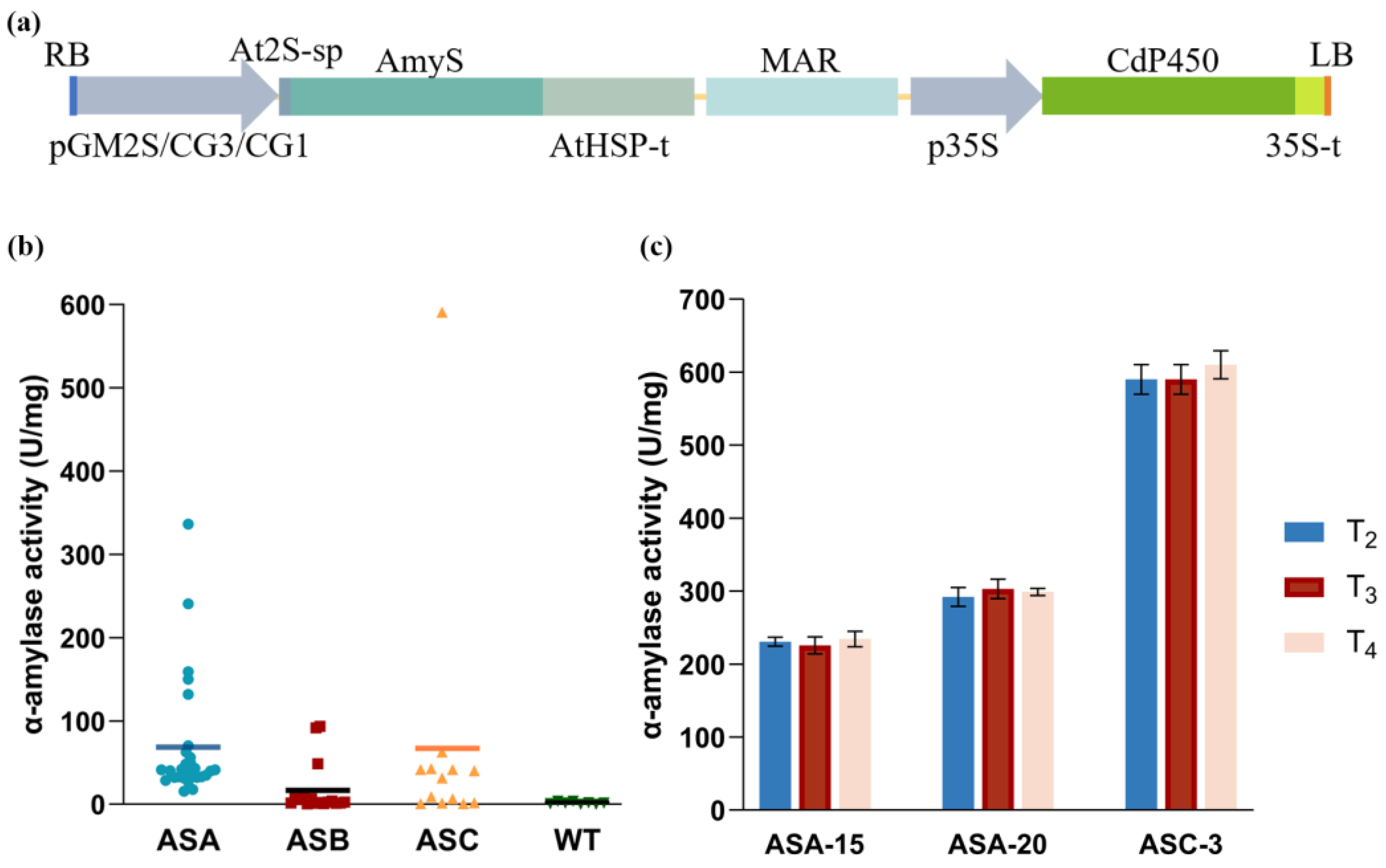
2.1. Generation of Transgenic Soybean Plants
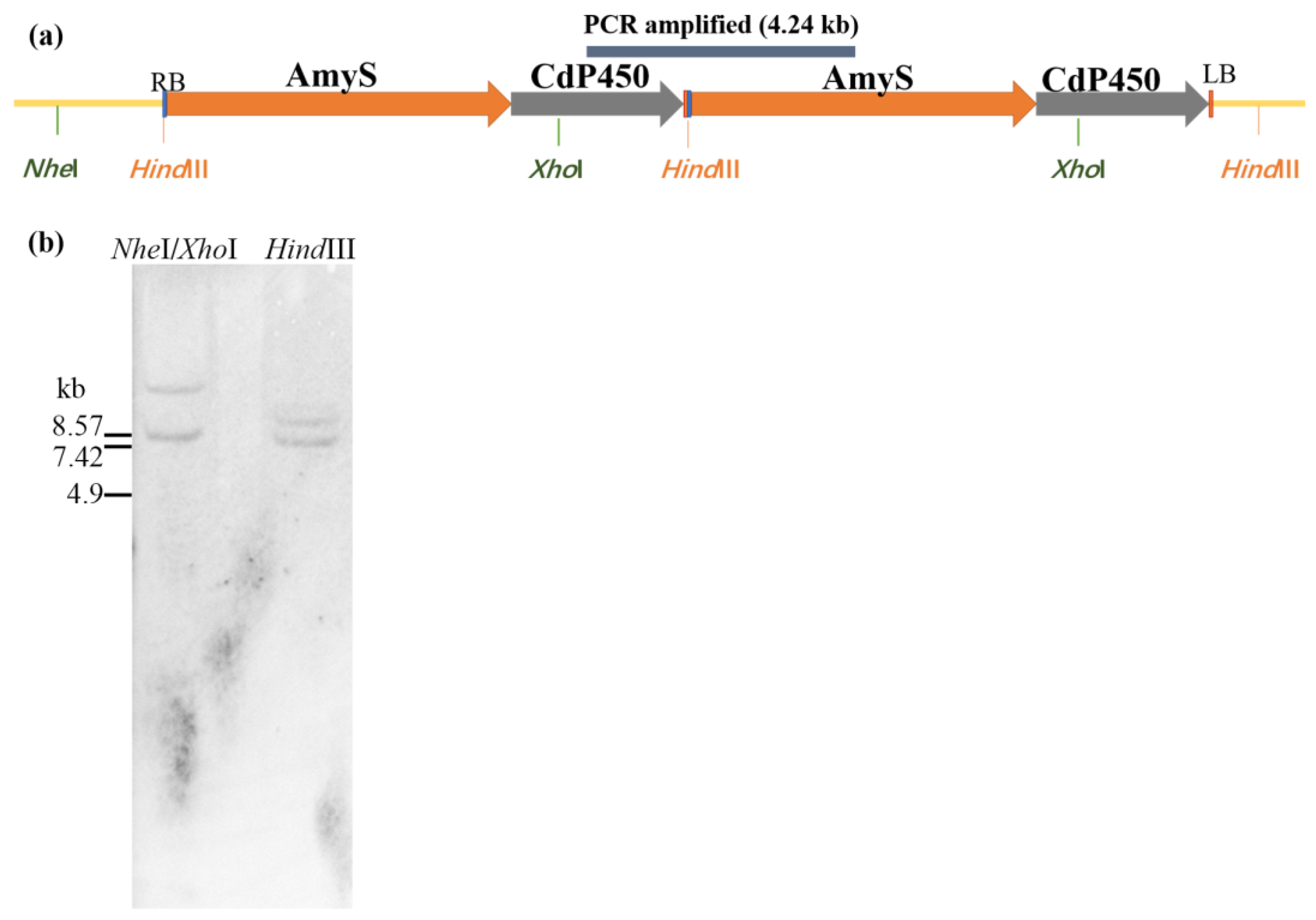
2.2. Characterization of the T-DNA Insertion of ASC-3
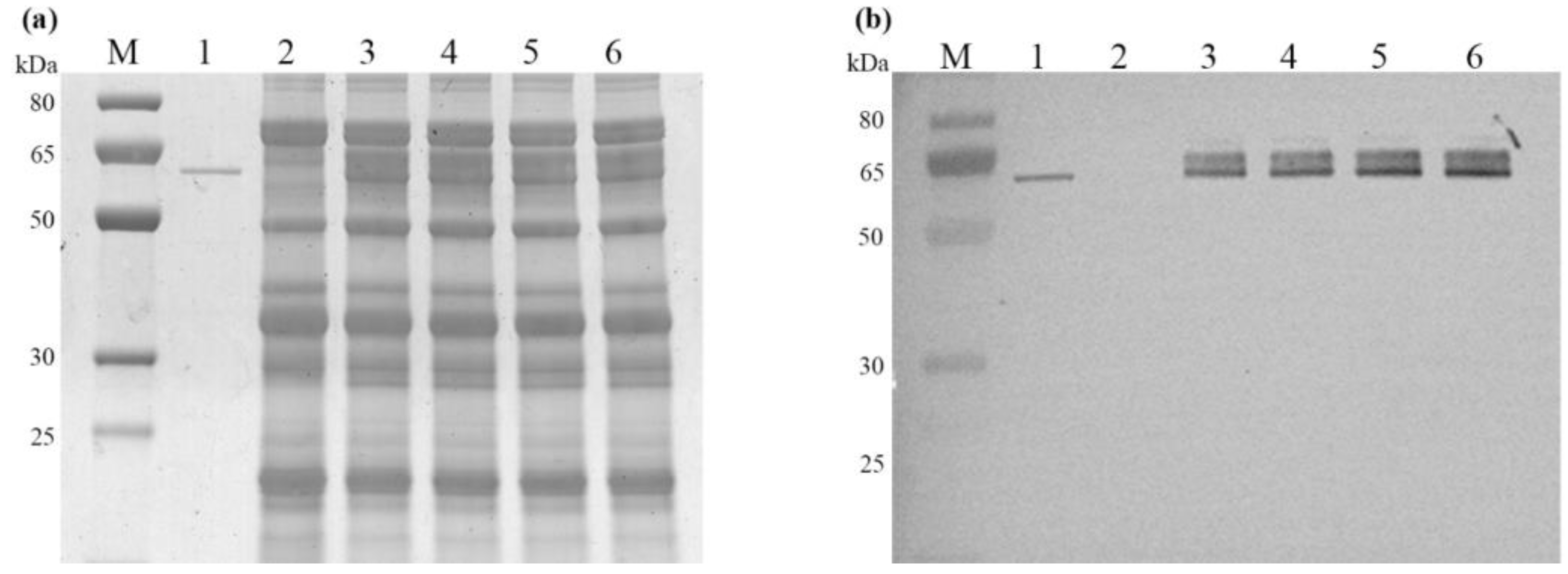
2.3. Characterization of Recombinant AmyS
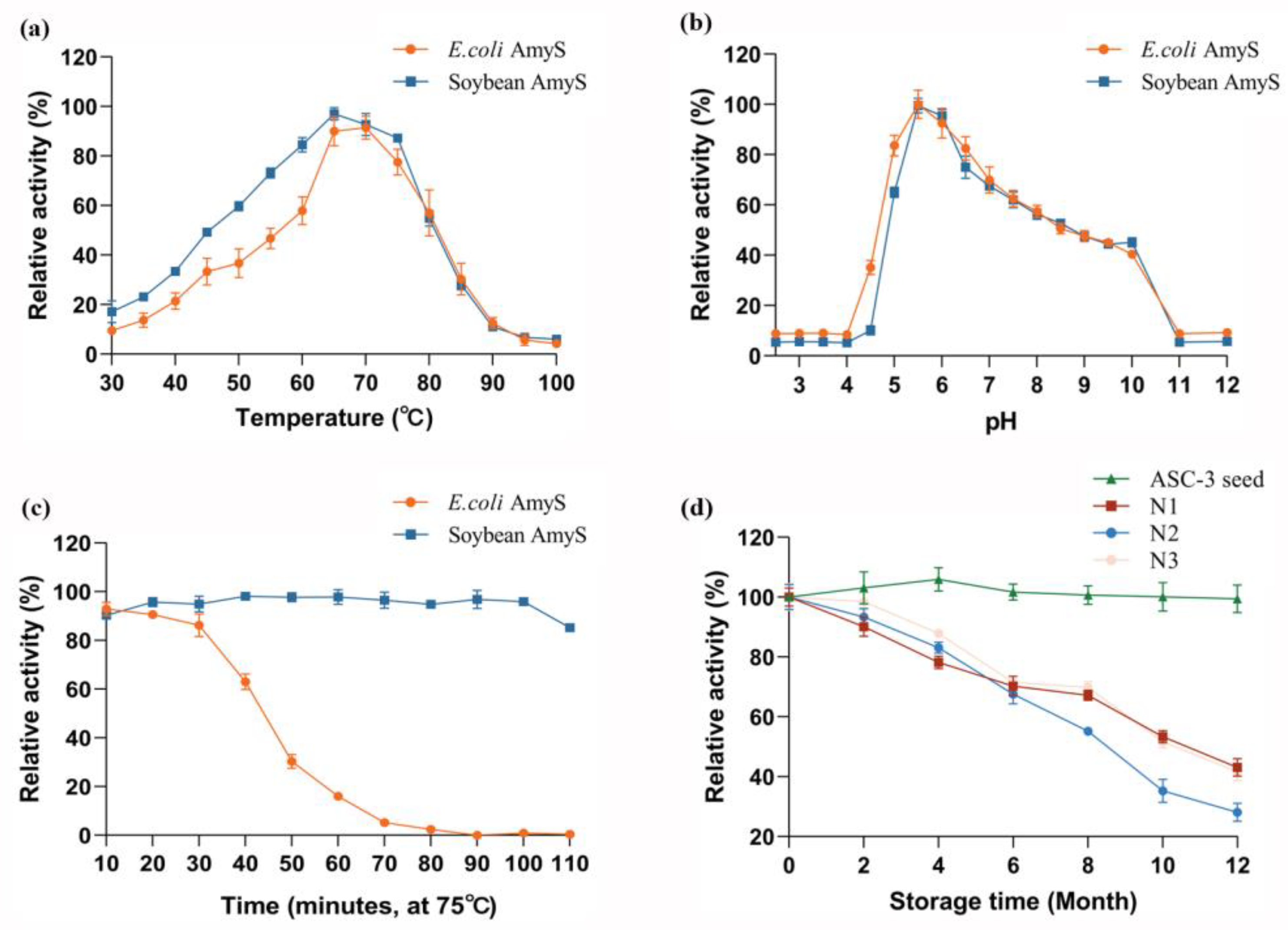
2.4. Enzymatic Properties of Recombinant AmyS
2.5. Accumulation and Tissue-Specific Expression of AmyS in Transgenic Soybean
2.6. Agronomic Traits and Yield Performance of Transgenic Plants
3. Discussion
3.1. Soybean Is a Suitable Bioreactor for Amylase Mass Production
3.2. ASC-3 Is a Cost-Effective Amylase Production Line
3.3. Glycosylated Amylase May Be More Thermostable
4. Materials and Methods
4.1. Cloning of Seed-Specific Promoters from Soybean
4.2. Construction of the T-DNA Vectors for Soybean Transformation
4.3. Soybean Transformation
4.4. Determination of α-Amylase Activity
4.5. Identification of T-DNA Flanking Sequences by hiTAIL-PCR
4.6. Southern Analysis
4.7. Expression and Purification of AmyS Protein from E. coli
4.8. Purification of the α-Amylase in Transgenic Soybean Seeds
4.9. Analysis of AmyS Proteins
4.9.1. SDS-PAGE
4.9.2. Western Blot
4.9.3. Characteristic of Soybean AmyS Protein
4.9.4. Starch Substrate Gel
4.10. Analysis of the Enzyme Properties
4.11. Analysis Agronomic Traits and Yield Performance of Transgenic Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paul, J.S.; Gupta, N.; Beliya, E.; Tiwari, S.; Jadhav, S.K. Aspects and recent trends in microbial α-Amylase: A Review. Appl. Biochem. Biotechnol. 2021, 193, 2649–2698. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ali, S.; Hassan, A.; Tahir, H.M.; Mumtaz, S.; Mumtaz, S. Biosynthesis and industrial applications of α-amylase: A review. Arch. Microbiol. 2021, 203, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.E. From green plants to industrial enzymes. Enzym. Microb. Technol. 2002, 30, 279–283. [Google Scholar] [CrossRef]
- Ashok, P.P.; Dasgupta, D.; Ray, A.; Suman, S.K. Challenges and prospects of microbial α-amylases for industrial application: A review. World J. Microbiol. Biotechnol. 2024, 40, 44. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.E.; Borchert, T.V. Protein engineering of bacterial α-amylases. Biochim. Et Biophys. Acta-Protein Struct. Mol. Enzymol. 2000, 1543, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Torabizadeh, H.; Montazeri, E. Nano co-immobilization of α-amylase and maltogenic amylase by nanomagnetic combi-cross-linked enzyme aggregates method for maltose production from corn starch. Carbohydr. Res. 2020, 488, 107904. [Google Scholar] [CrossRef]
- Dey, T.B.; Kumar, A.; Banerjee, R.; Chandna, P.; Kuhad, R.C. Improvement of microbial α-amylase stability: Strategic approaches. Process Biochem. 2016, 51, 1380–1390. [Google Scholar] [CrossRef]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2022, 7, 372–388. [Google Scholar] [CrossRef]
- Hood, E.E.; Love, R.; Lane, J.; Bray, J.; Clough, R.; Pappu, K.; Drees, C.; Hood, K.R.; Yoon, S.; Ahmad, A.; et al. Subcellular targeting is a key condition for high-level accumulation of cellulase protein in transgenic maize seed. Plant Biotechnol. J. 2007, 5, 709–719. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.F.; Zhang, X.W.; Fang, J.; Shen, Z.C.; Lin, C.Y. Transgenic rice as bioreactor for production of the Candida antarctica lipase B. Plant Biotechnol. J. 2014, 12, 963–970. [Google Scholar] [CrossRef]
- Cunha, N.B.; Murad, A.M.; Cipriano, T.M.; Araújo, A.C.G.; Aragao, F.J.L.; Leite, A.; Vianna, G.R.; McPhee, T.R.; Souza, G.; Waters, M.J.; et al. Expression of functional recombinant human growth hormone in transgenic soybean seeds. Transgenic Res. 2011, 20, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M. Commercialization of whole-plant systems for biomanufacturing of protein products: Evolution and prospects. Plant Biotechnol. J. 2010, 8, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.; Hudson, L.C.; Lambirth, K.C.; Luth, D.; Wang, K.; Bost, K.L.; Piller, K.J. Recombinant expression of homodimeric 660 kDa human thyroglobulin in soybean seeds: An alternative source of human thyroglobulin. Plant Cell Rep. 2011, 30, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Very-large-scale production of antibodies in plants: The biologization of manufacturing. Biotechnol. Adv. 2017, 35, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Merecz-Sadowska, A.; Picot, L.; Karaconji, I.B.; Wieczfinska, J.; Sliwinski, T.; Sitarek, P. Genetic manipulation and bioreactor culture of plants as a tool for industry and its applications. Molecules 2022, 27, 795. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; van Dolleweerd, C.; Drake, P.M.W.; Reljic, R.; Thangaraj, H.; Barbi, T.; Stylianou, E.; Pepponi, I.; Both, L.; Hehle, V.; et al. Molecular pharming Future targets and aspirations. Hum. Vaccines 2011, 7, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Czihal, A.; Conrad, B.; Buchner, P.; Brevis, R.; Farouk, A.A.; Manteuffel, R.; Adler, K.; Wobus, U.; Hofemeister, J.; Baumlein, H. Gene farming in plants: Expression of a heatstable Bacillus amylase in transgenic legume seeds. J. Plant Physiol. 1999, 155, 183–189. [Google Scholar] [CrossRef]
- Boehm, J.D.; Nguyen, V.; Tashiro, R.M.; Anderson, D.; Shi, C.; Wu, X.G.; Woodrow, L.; Yu, K.F.; Cui, Y.H.; Li, Z.L. Genetic mapping and validation of the loci controlling 7S α’ and 11S A-type storage protein subunits in soybean Glycine max (L.) Merr. Theor. Appl. Genet. 2018, 131, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Amandeep, S.; Megha, M.; Dhiraj, K.; Ashok, K.D.; Imtaiyaz Hassan, M. Structural and functional analysis of various globulin proteins from soy seed. Crit. Rev. Food Sci. Nutr. 2015, 55, 1491–1502. [Google Scholar]
- Schuler, M.A.; Ladin, B.F.; Pollaco, J.C.; Freyer, G.; Beachy, R.N. Structural sequences are conserved in the genes-coding for the alpha, alpha’-subunits and beta-subunits of the soybean. Nucleic Acids Res. 1982, 10, 8245–8261. [Google Scholar] [CrossRef]
- Laguia-Becher, M.; Zaldua, Z.; Xu, W.J.; Marconi, P.L.; Velander, W.; Alvarez, M.A. Co-expressing Turnip Crinkle Virus-coat protein with the serine protease α-thrombin precursor (pFIIa) in Nicotiana benthamiana Domin. Vitr. Cell. Dev. Biol. Plant 2019, 55, 88–98. [Google Scholar] [CrossRef]
- Piron, R.; De Koker, S.; De Paepe, A.; Goossens, J.; Grooten, J.; Nauwynck, H.; Depicker, A. Boosting in planta production of antigens derived from the porcine reproductive and Respiratory Syndrome Virus (PRRSV) and subsequent evaluation of their immunogenicity. PLoS ONE 2014, 9, e91386. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.A.; Jiang, Y.N.; Yao, L.M.; Wu, T.L. Improved production of the human granulocyte-macrophage colony stimulating factor in transgenic Arabidopsis thaliana seeds using a dual sorting signal peptide. Plant Mol. Biol. Rep. 2009, 27, 462–468. [Google Scholar] [CrossRef]
- Ruiz, Y.; Ramos, P.L.; Soto, J.; Rodriguez, M.; Carlos, N.; Reyes, A.; Callard, D.; Sanchez, Y.; Pujol, M.; Fuentes, A. The M4 insulator, the TM2 matrix attachment region, and the double copy of the heavy chain gene contribute to the enhanced accumulation of the PHB-01 antibody in tobacco plants. Transgenic Res. 2020, 29, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yu, X.X.; Sun, Y.Z.; Zhang, Q.; Zhang, X.W.; Tang, M.Z.; Lin, C.Y.; Shen, Z.C. Expression of a cytochrome P450 gene from Bermuda Grass Cynodon dactylon in soybean confers tolerance to multiple herbicides. Plants 2022, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Chen, Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 2007, 43, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-M.; Yeh, F.-S.; Huang, L.-F.; Tseng, T.-H.; Chung, M.-C.; Wang, C.-S.; Lur, H.-S.; Shaw, J.-F.; Yu, S.-M. Expression of a bi-functional and thermostable amylopullulanase in transgenic rice seeds leads to autohydrolysis and altered composition of starch. Mol. Breed. 2005, 15, 125–143. [Google Scholar] [CrossRef]
- Lin, K.-H.; Fu, H.; Chan, C.-H.; Lo, H.-F.; Shih, M.-C.; Chang, Y.-M.; Chen, L.-F.O. Generation and analyses of the transgenic potatoes expressing heterologous thermostable β-amylase. Plant Sci. 2008, 174, 649–657. [Google Scholar] [CrossRef]
- Santa-Maria, M.C.; Yencho, C.G.; Haigler, C.H.; Thompson, W.F.; Kelly, R.M.; Sosinski, B. Starch self-processing in transgenic sweet potato roots expressing a hyperthermophilic α-Amylase. Biotechnol. Prog. 2011, 27, 351–359. [Google Scholar] [CrossRef]
- Austin, S.; Bingham, E.T.; Mathews, D.E.; Shahan, M.N.; Will, J.; Burgess, R.R. Production and field performance of transgenic Alfalfa (Medicago sativa L.) expressing α-amylase and manganese-dependent lignin peroxidase. Euphytica 1995, 85, 381–393. [Google Scholar] [CrossRef]
- Pen, J.; Molendijk, L.; Quax, W.J.; Sijmons, P.C.; Vanooyen, A.J.J.; Vandenelzen, P.J.M.; Rietveld, K.; Hoekema, A. Production of active Bacillus licheniformis alpha-amylase in tobacco and its application in starch liquefaction. Bio/Technology 1992, 10, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Wolt, J.D.; Karaman, S. Estimated environmental loads of α-amylase from transgenic high-amylase maize. Biomass Bioenergy 2007, 31, 831–835. [Google Scholar] [CrossRef]
- Xu, X.L.; Fang, J.; Wang, W.; Guo, J.L.; Chen, P.N.; Cheng, J.A.; Shen, Z.C. Expression of a bacterial α-amylase gene in transgenic rice seeds. Transgenic Res. 2008, 17, 645–650. [Google Scholar] [CrossRef]
- Lanahan, M.B.; Basu, S.S.; Batie, C.J.; Chen, W.; Craig, J.; Kinkema, M. Self Processing Plants and Plant Parts. U.S. Patent US07855322, 21 December 2010. [Google Scholar]
- Fischer, R.; Buyel, J.F. Molecular farming—The slope of enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef]
- Liu, H.; Timko, M.P. Improving protein quantity and quality-the next level of plant molecular farming. Int. J. Mol. Sci. 2022, 23, 1326. [Google Scholar] [CrossRef] [PubMed]
- Rozov, S.M.; Permyakova, N.V.; Deineko, E.V. Main strategies of plant expression system glycoengineering for producing humanized recombinant pharmaceutical proteins. Biochemistry 2018, 83, 215–232. [Google Scholar] [CrossRef]
- Srivastava, R.A.K. Studies on stabilization of amylase by covalent coupling to soluble polysaccharides. Enzym. Microb. Technol. 1991, 13, 164–170. [Google Scholar] [CrossRef]
- Hoshida, H.; Fujita, T.; Cha-aim, K.; Akada, R. N-glycosylation deficiency enhanced heterologous production of a Bacillus licheniformis thermostable alpha-amylase in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97, 5473–5482. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Yuan, X.; He, N.S.; Zhuang, T.Z.; Wu, P.; Zhang, G.M. Expression of Bacillus licheniformis α-amylase in Pichia pastoris without antibiotics-resistant gene and effects of glycosylation on the enzymic thermostability. 3 Biotech 2019, 9, 427. [Google Scholar] [CrossRef]
- Cunha, N.B.; Araujo, A.C.G.; Leite, A.; Murad, A.M.; Vianna, G.R.; Rech, E.L. Correct targeting of proinsulin in protein storage vacuoles of transgenic soybean seeds. Genet. Mol. Res. 2010, 9, 1163–1170. [Google Scholar] [CrossRef]
- Paz, M.M.; Martinez, J.C.; Kalvig, A.B.; Fonger, T.M.; Wang, K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]


Source | Specific Activity (U/mg) | Kinetic | |
|---|---|---|---|
| Km (mg/mL) | Vmax (mL/min) | ||
| E. coli AmyS | 17,300 | 28.71 | 4.26 |
| Purified soybean AmyS | 15,057 | 25.57 | 3.70 |
| Lines | Title 2 | Title 3 |
|---|---|---|
| Emergence (%) | 99.71 | 99.98 |
| Plant height (cm) | 99.75 ± 10.04 | 103.00 ± 14.03 |
| Nodes | 13.19 ± 1.72 | 15.17 ± 1.72 * |
| Branches | 5.17 ± 0.41 | 5.16 ± 0.41 |
| Pods of per plant | 96.50 ± 39.84 | 115.17 ± 23.27 |
| Seeds of per plant | 203.71 ± 72.66 | 275.50 ± 69.30 |
| Kernel weight of per plant (g) | 53.80 ± 17.91 | 56.78 ± 24.54 |
| Hundred kernel weight (g) | 25.23 ± 0.90 | 24.20 ± 0.55 |
| Days to maturity (day) | 92.0 | 92.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Jiang, Y.; Li, J.; Zheng, T.; Lin, C.; Shen, Z. Transgenic Soybean for Production of Thermostable α-Amylase. Plants 2024, 13, 1539. https://doi.org/10.3390/plants13111539
Cao Z, Jiang Y, Li J, Zheng T, Lin C, Shen Z. Transgenic Soybean for Production of Thermostable α-Amylase. Plants. 2024; 13(11):1539. https://doi.org/10.3390/plants13111539
Chicago/Turabian StyleCao, Zhenyan, Ye Jiang, Jiajie Li, Ting Zheng, Chaoyang Lin, and Zhicheng Shen. 2024. "Transgenic Soybean for Production of Thermostable α-Amylase" Plants 13, no. 11: 1539. https://doi.org/10.3390/plants13111539
APA StyleCao, Z., Jiang, Y., Li, J., Zheng, T., Lin, C., & Shen, Z. (2024). Transgenic Soybean for Production of Thermostable α-Amylase. Plants, 13(11), 1539. https://doi.org/10.3390/plants13111539









