Resilience of Canola to Plasmodiophora brassicae (Clubroot) Pathotype 3H under Different Resistance Genes and Initial Inoculum Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Canola Plants
2.2. The Pathogen Inoculum
2.3. Experiments for Clubroot Resistance Durability
2.4. Disease Assessment
2.5. Quantification of P. brassicae Inoculum in Soil
2.6. Data Analysis
3. Results
3.1. Resistance Performance of Canola Varieties
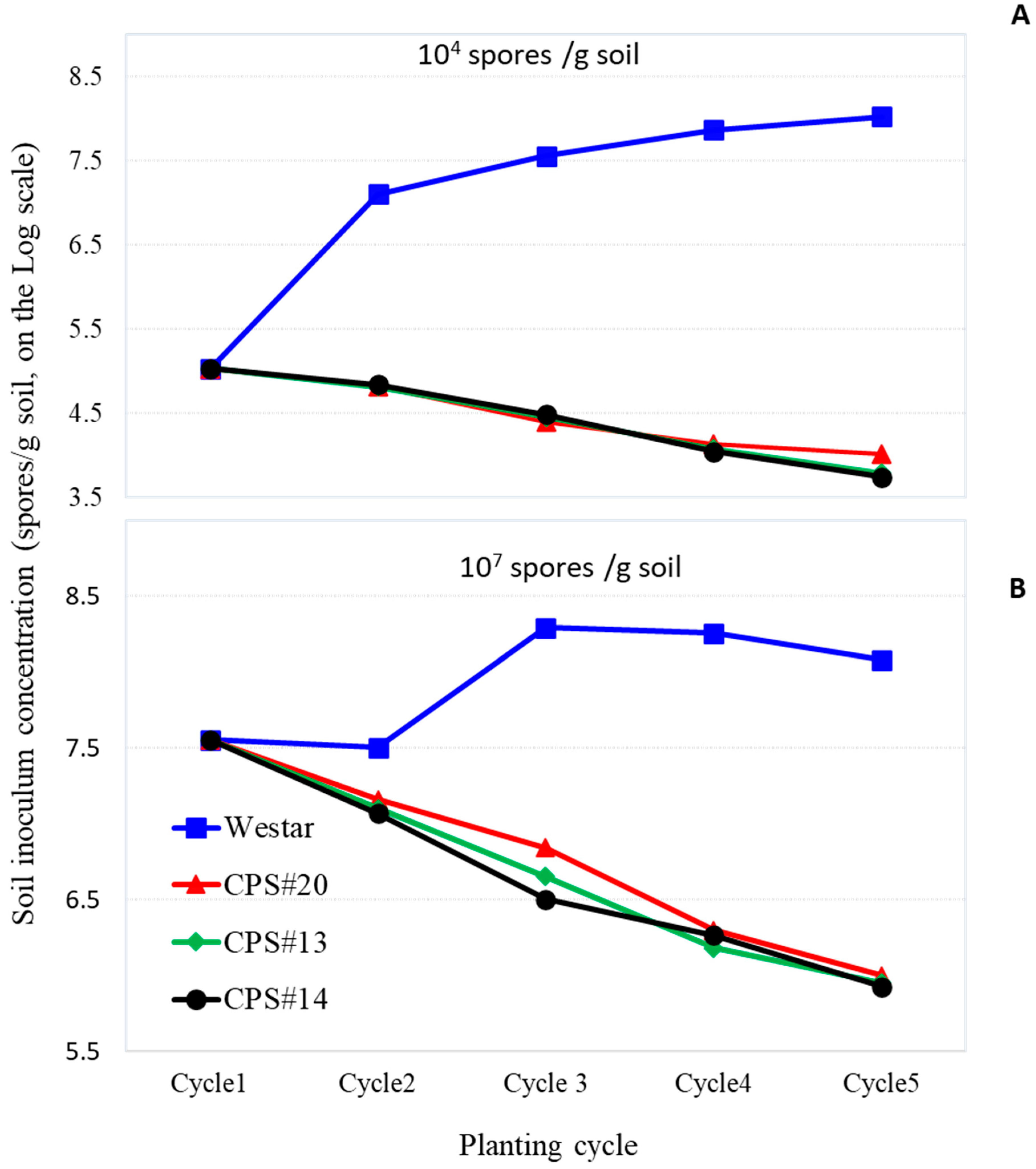
3.2. Effect on Soil Inoculum Buildup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rempel, C.B.; Hutton, S.N.; Jurke, C.J. Clubroot and the importance of canola in Canada. Can. J. Plant Pathol. 2014, 36, 19–26. [Google Scholar] [CrossRef]
- Dixon, G.R. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Kageyama, K.; Asano, T. Life cycle of Plasmodiophora brassicae. J Plant Growth Regul. 2009, 28, 203–211. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Feindel, D. Emergence of new virulence phenotypes of Plasmodiophora brassicae on canola (Brassica napus) in Alberta, Canada. Eur. J. Plant Pathol. 2016, 145, 517–529. [Google Scholar] [CrossRef]
- Wallenhammar, A.-C. Monitoring and control of Plasmodiophora brassicae in spring oilseed brassica crops. Acta Hort. 1999, 867. [Google Scholar] [CrossRef]
- Peng, G.; Pageau, D.; Strelkov, S.E.; Gossen, B.D.; Hwang, S.E.; Lahlali, R. A > 2-year rotation reduces Plasmodiophora brassicae resting spores in soil and the impact of clubroot on canola. Eur. J. Agron. 2015, 70, 78–84. [Google Scholar] [CrossRef]
- Drury, S.; Sedaghatkish, A.; Gossen, B.D.; McDonald, M.R. Perennial grasses and common field crops reduce the concentration of resting spores of Plasmodiophora brassicae in soil. Plant Pathol. 2022, 71, 1793–1800. [Google Scholar] [CrossRef]
- Suwabe, K.; Tsukazaki, H.; Iketani, H.; Hatakeyama, K.; Fujimura, M.; Nunome, T.; Fukuoka, H.; Matsumoto, S.; Hirai, M. Identification of two loci for resistance to clubroot (Plasmodiophora brassicae Woronin) in Brassica rapa L. Theor. Appl. Genet. 2003, 107, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Harada, T.; Kubo, N.; Tsukada, M.; Suwabe, K.; Matsumoto, S. A novel locus for clubroot resistance in Brassica rapa and its linkage markers. Theor. Appl. Genet. 2004, 108, 639–643. [Google Scholar] [CrossRef]
- Piao, Z.; Deng, Y.; Choi, S.; Park, Y.; Lim, Y.J.T. SCAR and CAPS mapping of CRb, a gene conferring resistance to Plasmodiophora brassicae in Chinese cabbage (Brassica rapa ssp. pekinensis). Theor. Appl. Genet. 2004, 108, 1458–1465. [Google Scholar] [CrossRef]
- Saito, M.; Kubo, N.; Matsumoto, S.; Suwabe, K.; Tsukada, M.; Hirai, M. Fine mapping of the clubroot resistance gene Crr3 in Brassica rapa. Theor. Appl. Genet. 2006, 114, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Suwabe, K.; Tsukazaki, H.; Iketani, H.; Hatakeyama, K.; Fujimura, M.; Kondo, M.; Nunome, T.; Fukuoka, H.; Hirai, M.; Matsumoto, S. Simple Sequence Repeat-based comparative genomics between Brassica rapa and Arabidopsis thaliana: The genetic origin of clubroot resistance. Genetics 2006, 173, 309–319. [Google Scholar]
- Sakamoto, K.; Saito, A.; Hayashida, N.; Taguchi, G.; Matsumoto, E. Mapping of isolate-specific QTL for clubroot resistance in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Theor. Appl. Genet 2008, 117, 759–767. [Google Scholar]
- Matsumoto, E.; Ueno, H.; Aruga, D.; Sakamoto, K.; Hayashida, N. Accumulation of three clubroot resistance genes through marker-assisted selection in Chinese cabbage (Brassica rapa ssp. pekinensis). J. Jpn. Soc. Hortic. Sci. 2012, 81, 184–190. [Google Scholar] [CrossRef]
- Chu, M.; Song, T.; Falk, K.C.; Zhang, X.; Liu, X.; Chang, A.; Yu, F.; Peng, G. Fine mapping of Rcr1 and analyses of its effect on transcriptome patterns during infection by Plasmodiophora brassicae. BMC Genomics. 2014, 15, 1166. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.M.; Dakouri, A.; Zhang, Y.; Chen, Q.; Peng, G.; Strelkov, S.E.; Gossen, B.D.; Yu, F. Two clubroot-resistance genes, Rcr3 and Rcr9, mapped in Brassica rapa using bulk segregant RNA sequencing. Int. J. Mol. Sci. 2020, 21, 5033. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, Z.; Yu, F. A CRISPR/Cas9-based vector system enables the fast breeding of selection-marker-free canola with Rcr1-rendered clubroot resistance. J. Exp. Bot. 2024, 75, 1347–1363. [Google Scholar] [CrossRef] [PubMed]
- Fredua-Agyeman, R.; Rahman, H. Mapping of clubroot disease resistance in spring Brassica napus canola introgressed from European winter canola cv. ‘Mendel’. Euphytica 2016, 211, 201–213. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Fredua-Agyeman, R.; Harding, M.; Peng, G.; Gossen, B.D.; Mcdonald, M.R.; Feindel, D. Virulence and pathotype classification of Plasmodiophora brassicae populations collected from clubroot resistant canola (Brassica napus) in Canada. Can. J. Plant Pathol. 2018, 40, 284–298. [Google Scholar] [CrossRef]
- Askarian, H.; Akhavan, A.; Manolii, V.P.; Cao, T.; Hwang, S.F.; Strelkov, S.E. Virulence Spectrum of Single-Spore and Field Isolates of Plasmodiophora brassicae Able to Overcome Resistance in Canola (Brassica napus). Plant Dis. 2020, 105, 43–52. [Google Scholar] [CrossRef]
- LeBoldus, J.; Manolii, V.; Turkington, T.; Strelkov, S. Adaptation to Brassica host genotypes by a single-spore isolate and population of Plasmodiophora brassicae (clubroot). Plant Dis. 2012, 96, 833–838. [Google Scholar] [CrossRef]
- Joshi, R.K.; Nayak, S. Gene pyramiding-A broad spectrum technique for developing durable stress resistance in crops. Biotechnol. Mol. Biol. Rev. 2010, 5, 51–60. [Google Scholar]
- Hirani, A.H.; Li, G. Chapter 1: Understanding the genetics of clubroot resistance for effectively controlling this disease in Brassica species. In Plants for the Future; El-Shemy, H., Ed.; IntechOpen Limited: London, UK, 2015; ISBN 978-953-51-5409-9. [Google Scholar] [CrossRef]
- Hasan, M.J.; Rahman, H. Genetics and molecular mapping of resistance to Plasmodiophora brassicae pathotypes 2, 3, 5, 6, and 8 in rutabaga (Brassica napus var. napobrassica). Genome 2016, 59, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Tonu, N.N.; Wen, R.; Song, T.; Guo, X.; Murphy, L.A.; Gossen, B.D.; Peng, G. Canola with stacked genes shows moderate resistance and resilience against a field population of Plasmodiophora brassicae (clubroot) pathotype X. Plants 2023, 12, 726. [Google Scholar] [CrossRef]
- Kuginuki, Y.; Yoshikawa, H.; Hirai, M. Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinensis) . Eur. J. Plant Pathol. 1999, 105, 327–332. [Google Scholar] [CrossRef]
- Wen, R.; Lee, J.; Chu, M.; Tonu, N.; Dumonceaux, T.; Gossen, B.D.; Yu, F.; Peng, G. Quantification of Plasmodiophora brassicae resting spores in soils using droplet digital PCR (ddPCR). Plant Dis. 2019, 104, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-K.; Sanchez, A.; Angeles, E.; Singh, S.; Domingo, J.; Huang, N.; Khush, G. Are the dominant and recessive plant disease resistance genes similar?: A case study of rice R genes and Xanthomonas oryzae pv. oryzae races. Genetics 2001, 159, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatkish, A.; Gossen, B.D.; Yu, F.; Torkamaneh, D.; McDonald, M.R. Whole-genome DNA similarity and population structure of Plasmodiophora brassicae strains from Canada. BMC Genomics 2019, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fredua-Agyeman, R.; Strelkov, S.E.; Hwang, S.-F. Suppression of Canola (Brassica napus) resistance by virulent isolates of Plasmodiophora brassicae (clubroot) during primary infection. Plant Dis. 2019, 104, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yang, Y.; Mishra, V.; Zhou, Q.; Zuzak, K.; Feindel, D.; Feng, J. Most Plasmodiophora brassicae populations in single canola root galls from Alberta fields are mixtures of multiple strains. Plant Dis. 2019, 104, 116–120. [Google Scholar] [CrossRef]
- Al-Daoud, F.; Gossen, B.D.; Mcdonald, M.R. Maturation of resting spores of Plasmodiophora brassicae continues after host cell death. Plant Pathol. 2019, 69, 310–319. [Google Scholar] [CrossRef]
- Ernst, T.W.; Kher, S.; Stanton, D.; Rennie, D.C.; Hwang, S.F.; Strelkov, S.E. Plasmodiophora brassicae resting spore dynamics in clubroot resistant canola (Brassica napus) cropping systems. Plant Pathol. 2018, 68, 399–408. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Ahmed, H.U.; Zhou, Q.X.; Fu, H.; Fredua-Agyeman, R.; Strelkov, S.E.; Gossen, B.D.; Peng, G. Influence of resistant cultivars and crop intervals on clubroot of canola. Can. J. Plant Sci. 2019, 99, 862–872. [Google Scholar] [CrossRef]
- Dalton, J. Effect of Plasmodiophora brassicae Resting Spore Concentration and Crop Rotation on Growth of Clubroot-Resistant Crops. Ph.D. Dissertation, University of Guelph, Guelph, ON, Canada, 2016. [Google Scholar]
- Faggian, R.; Strelkov, S.E. Detection and measurement of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 282–288. [Google Scholar] [CrossRef]
- Mundt, C. Probability of mutation to multiple virulence and durability of resistance gene pyramids. Phytopathology 1990, 80, 221–223. [Google Scholar] [CrossRef]

| Variety | Internal Coding by the Breeding Company | CR Genes Involved | # CR Genes |
|---|---|---|---|
| CPS#20 | SC15-NB3-01 | Crr1rutb/Crr1rutb | ONE |
| CPS#13 | PS-FCA 15-3978 | Rcr1/Rcr1 | ONE |
| CPS#14 | PS-ARK 14-3562 | Rcr1/Crr1rutb | TWO |
| Westar | N/A + | None | ZERO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, R.; Song, T.; Tonu, N.N.; Franke, C.; Peng, G. Resilience of Canola to Plasmodiophora brassicae (Clubroot) Pathotype 3H under Different Resistance Genes and Initial Inoculum Levels. Plants 2024, 13, 1540. https://doi.org/10.3390/plants13111540
Wen R, Song T, Tonu NN, Franke C, Peng G. Resilience of Canola to Plasmodiophora brassicae (Clubroot) Pathotype 3H under Different Resistance Genes and Initial Inoculum Levels. Plants. 2024; 13(11):1540. https://doi.org/10.3390/plants13111540
Chicago/Turabian StyleWen, Rui, Tao Song, Nazmoon Naher Tonu, Coreen Franke, and Gary Peng. 2024. "Resilience of Canola to Plasmodiophora brassicae (Clubroot) Pathotype 3H under Different Resistance Genes and Initial Inoculum Levels" Plants 13, no. 11: 1540. https://doi.org/10.3390/plants13111540
APA StyleWen, R., Song, T., Tonu, N. N., Franke, C., & Peng, G. (2024). Resilience of Canola to Plasmodiophora brassicae (Clubroot) Pathotype 3H under Different Resistance Genes and Initial Inoculum Levels. Plants, 13(11), 1540. https://doi.org/10.3390/plants13111540








