Physiological and Cellular Ultrastructural Responses of Isatis indigotica Fort. under Salt Stress
Abstract
1. Introduction
2. Results
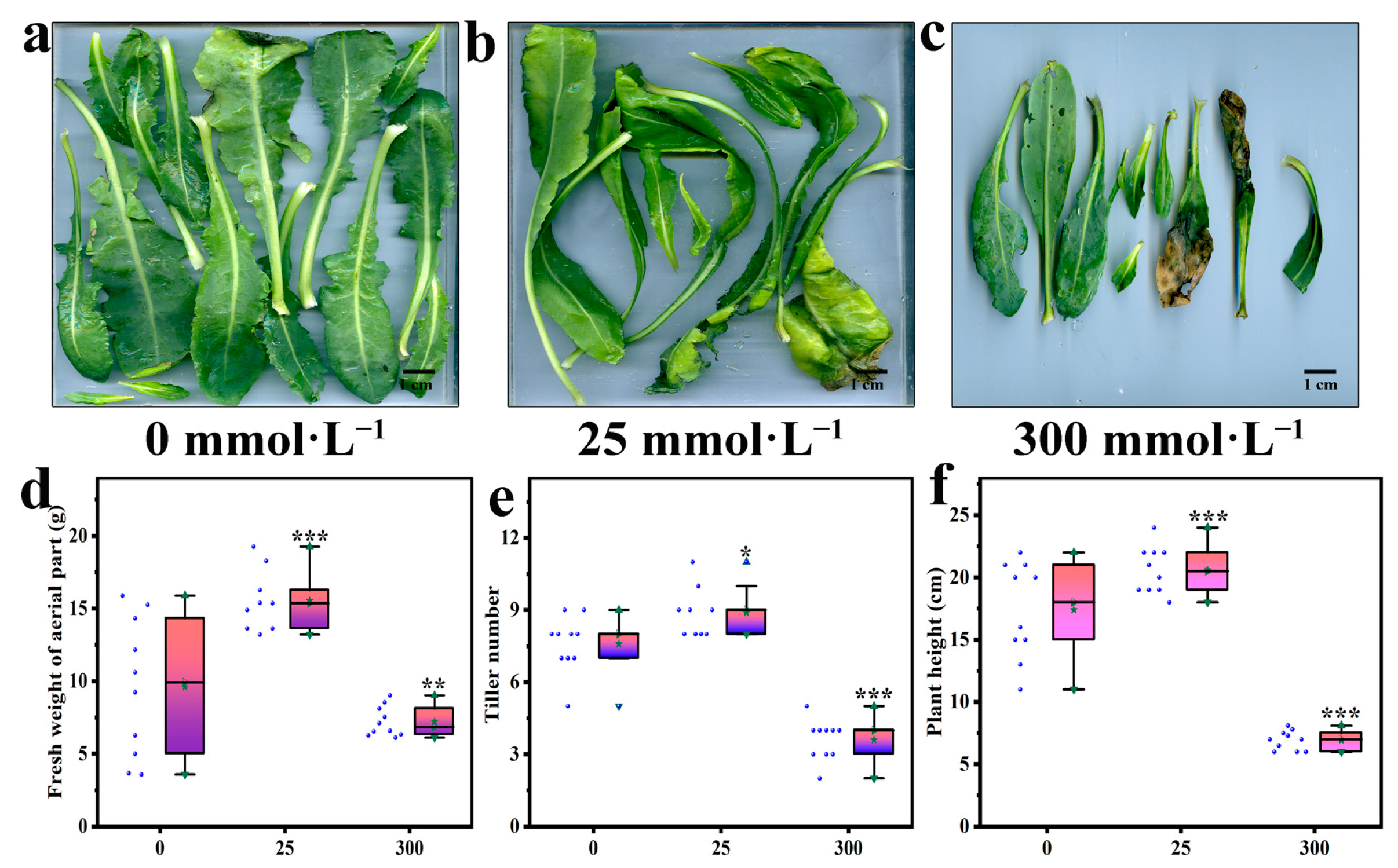
2.1. Effects of Salt Stress on the Growth of Aerial Part
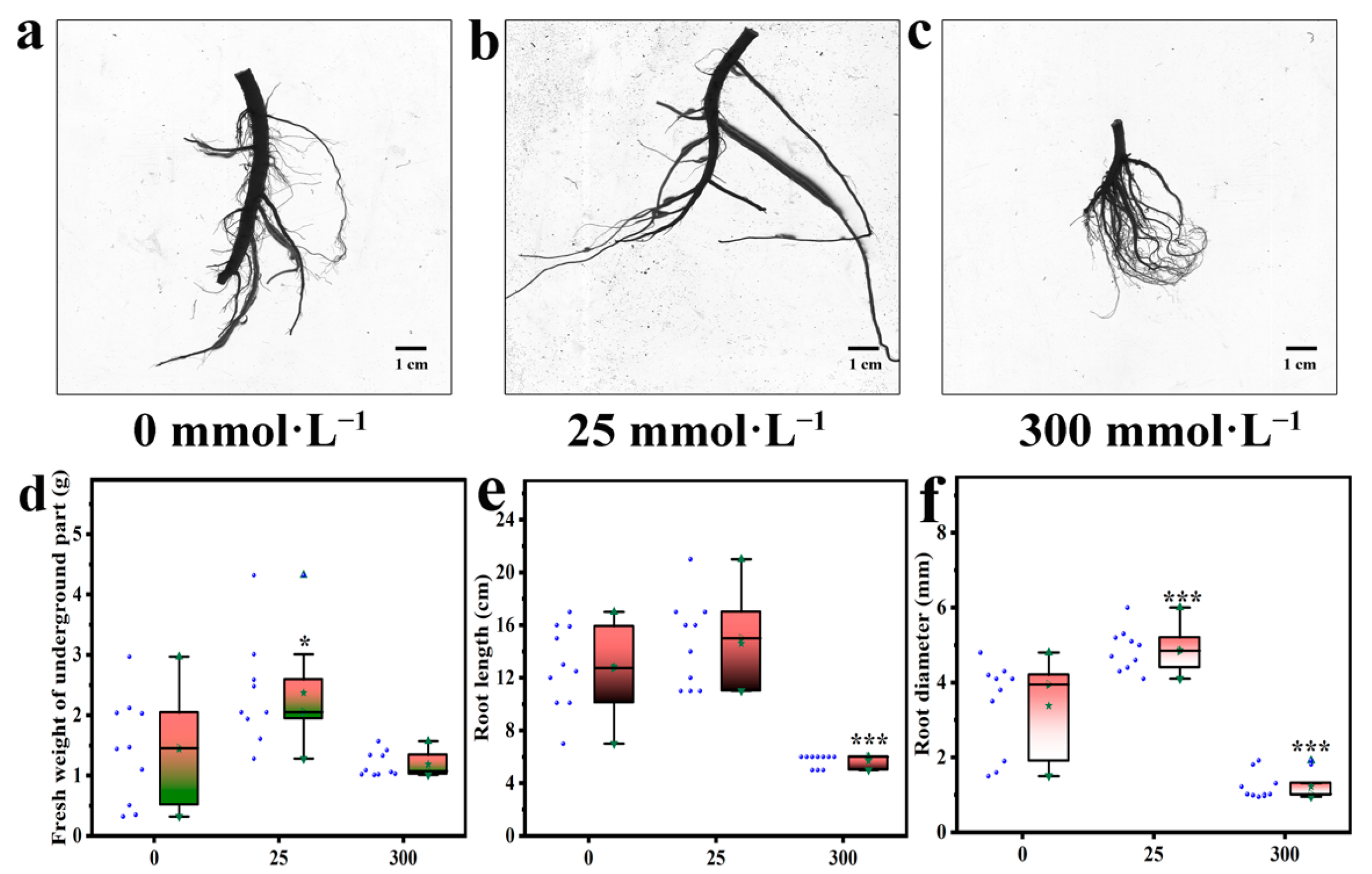
2.2. Effects of Salt Stress on Underground Part
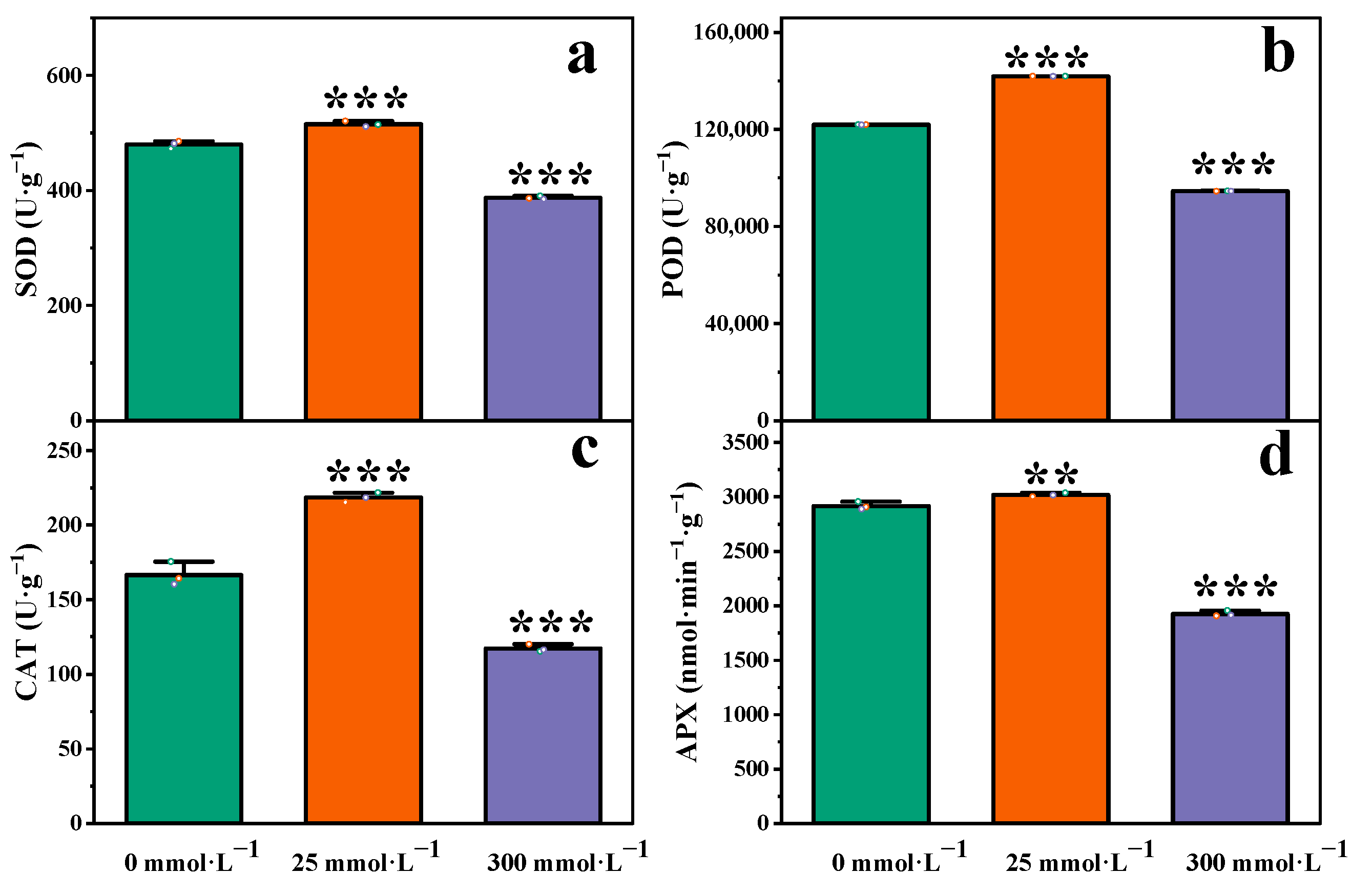
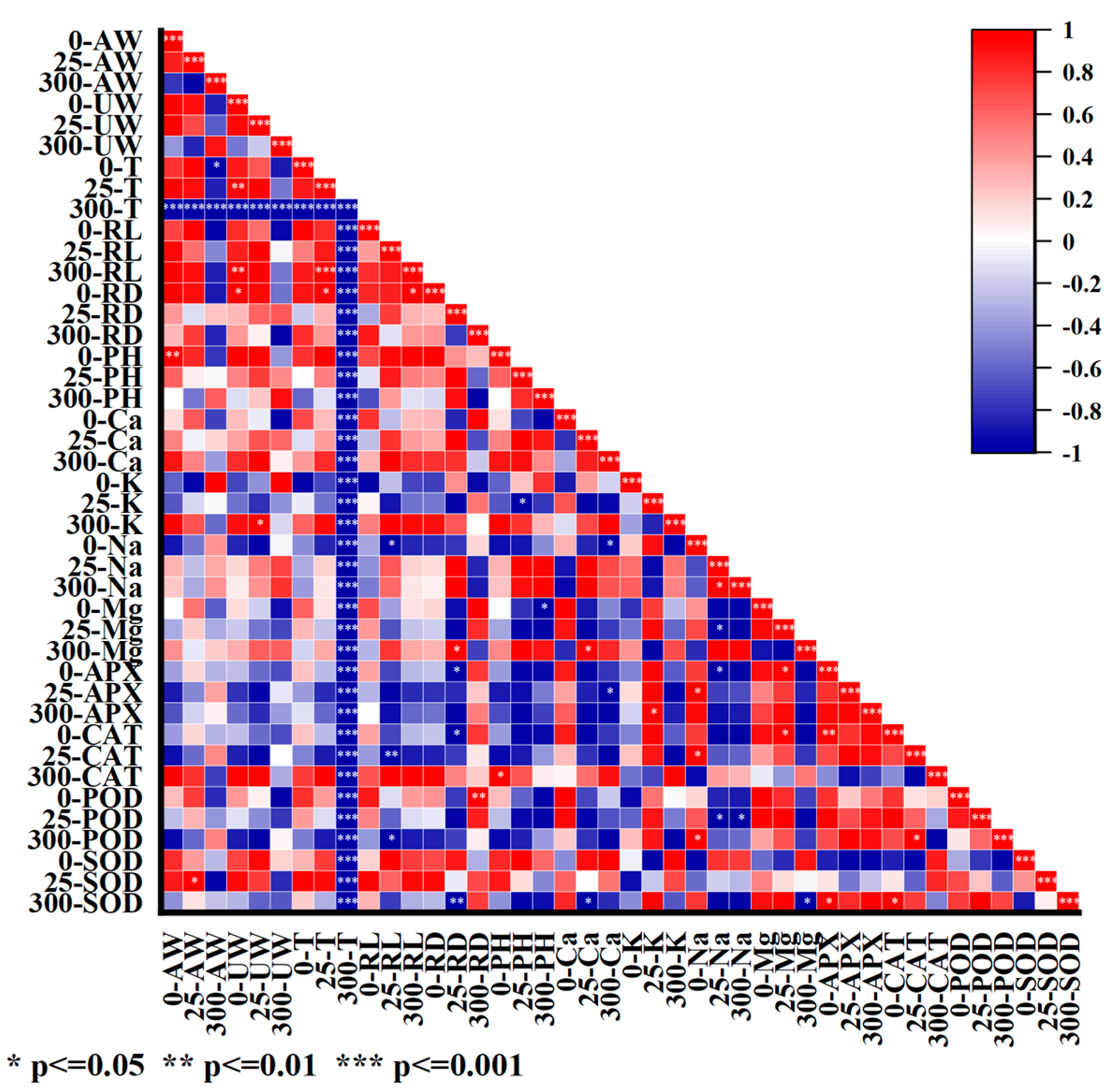
2.3. Effects of Salt Stress on Antioxidant System in Roots of I. indigotica
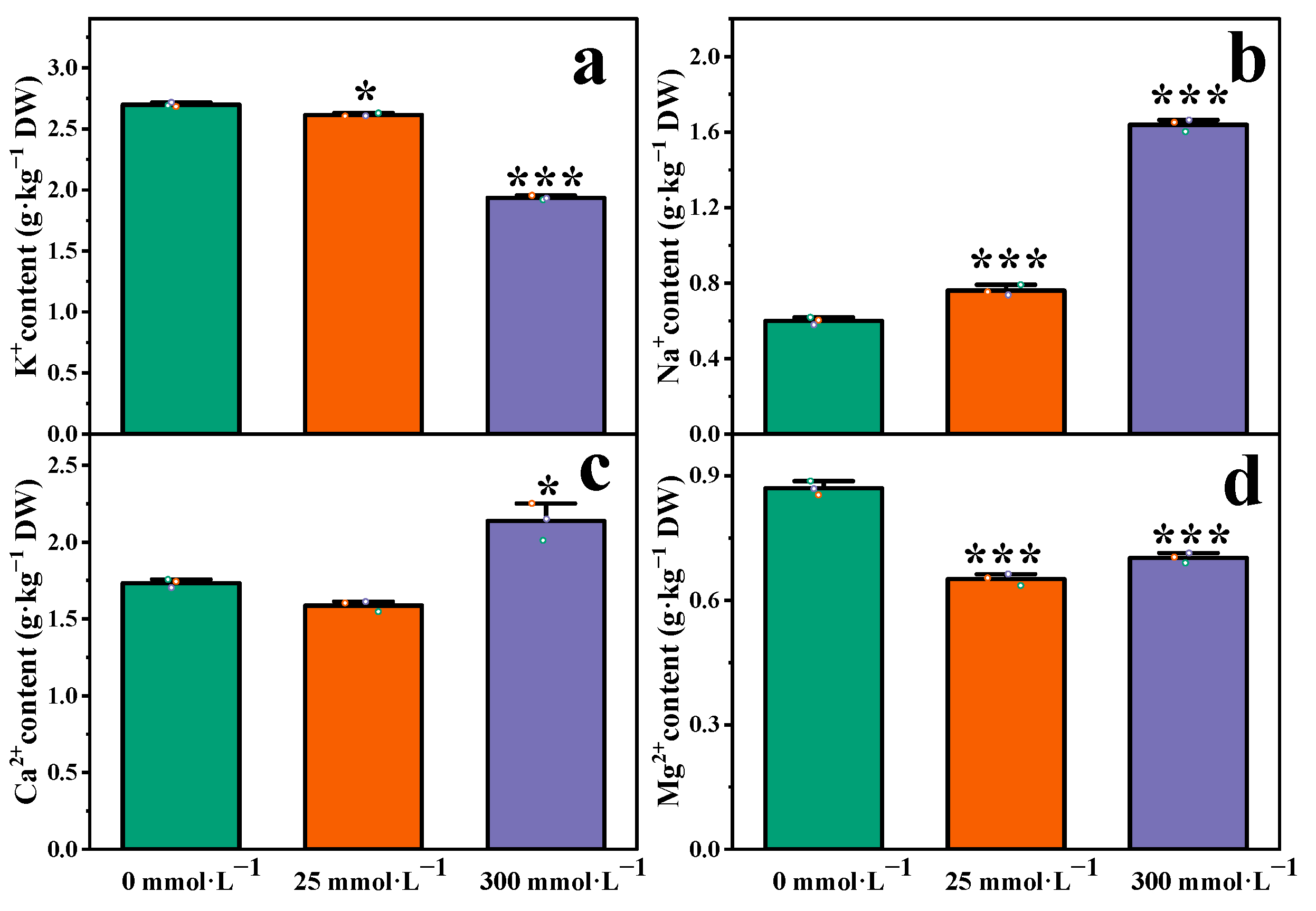
2.4. Effect of Salt Stress on Ion Content in Roots of I. indigotica
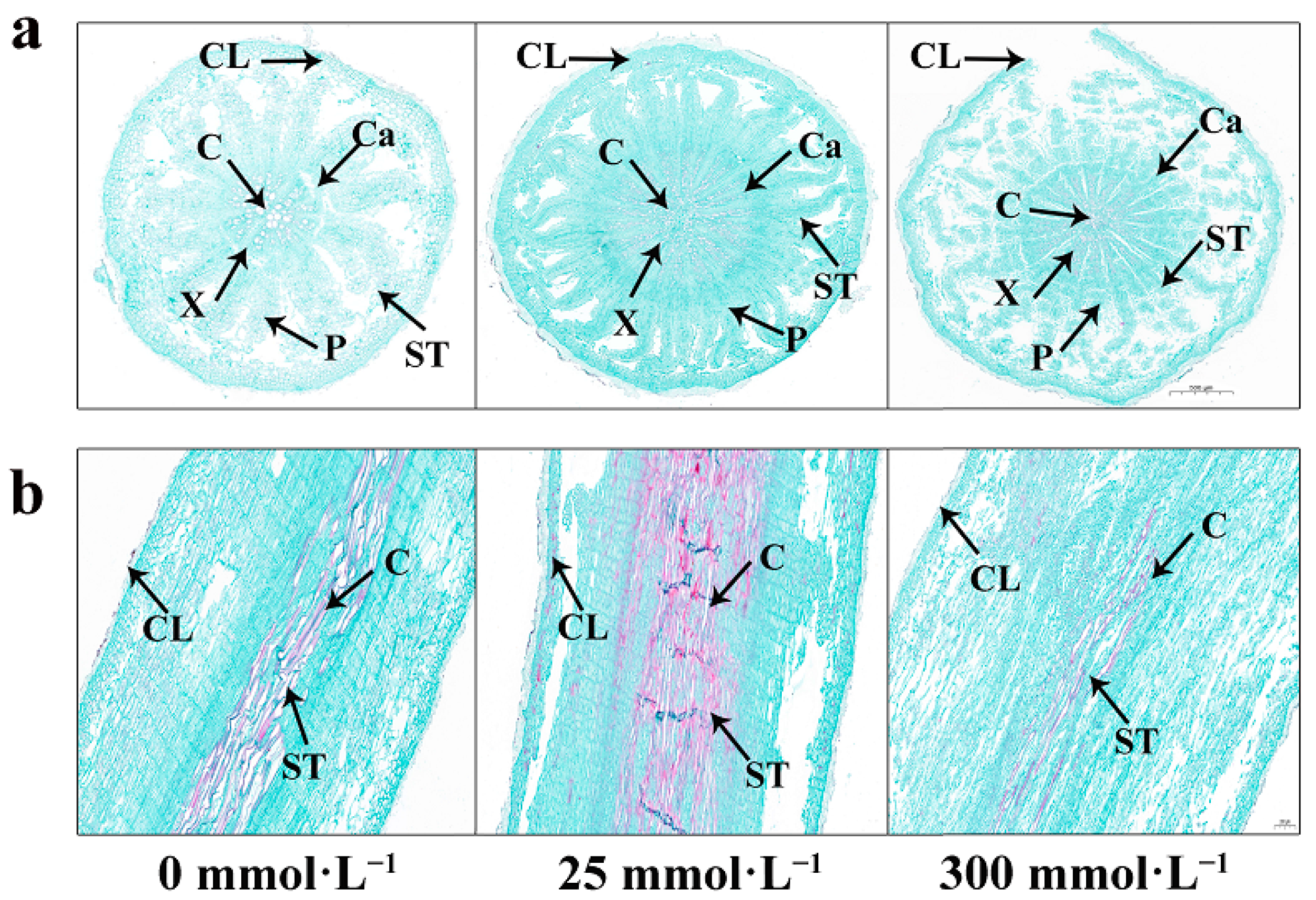
2.5. Effects of Salt Stress on the Microstructure in Roots of I. indigotica
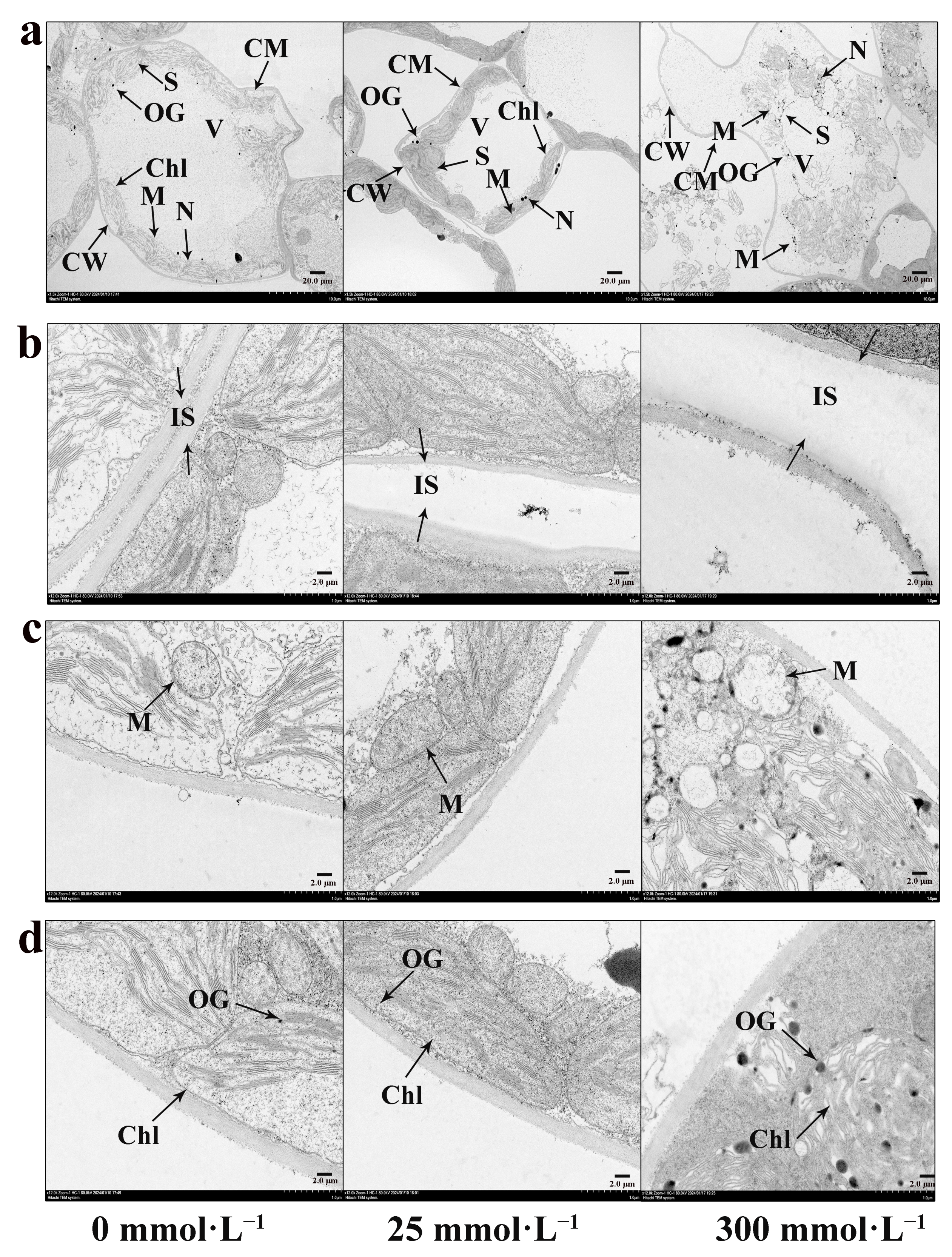
2.6. Effects of Salt Stress on Ultrastructure of Mesophyll Cells of I. indigotica
2.6.1. Effect of Salt Stress on the Intercellular Space of I. indigotica
2.6.2. Effect of Salt Stress on the Mitochondrial Ultrastructure of I. indigotica
2.6.3. Effect of Salt Stress on the Chloroplast Ultrastructure of I. indigotica
2.7. Effects of Salt Stress on the Ultrastructure of Mesophyll Cells of I. indigotica
3. Discussion
4. Materials and Methods
4.1. Effect of Salt Stress on Ion Content
4.2. Measurement of Aerial and Underground Part Growth Indices
4.3. Determination of the Activities of Antioxidant Enzymes
4.4. Extraction and Quantification of Ion Contents
4.5. Extraction and Quantification of Ion Contents
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Chang, L.; Li, G.; Li, Y. Advances and Future Research in Ecological Stoichiometry under Saline-Alkali Stress. Envrion. Sci. Pollut. Res. 2023, 30, 5475–5486. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. How Plants Perceive Salt. Nature 2019, 572, 318–320. [Google Scholar] [CrossRef]
- Cao, Y.; Song, H.; Zhang, L. New Insight into Plant Saline-Alkali Tolerance Mechanisms and Application to Breeding. Int. J. Mol. Sci. 2022, 23, 16048. [Google Scholar] [CrossRef]
- Sarkar, S.; Guha, A.; Narayanan, T.N.; Mondal, J. Zwitterionic Osmolytes Revive Surface Charges under Salt Stress via Dual Mechanisms. J. Phys. Chem. Lett. 2022, 13, 5660–5668. [Google Scholar] [CrossRef]
- Guo, H.; Huang, Z.; Li, M.; Hou, Z. Growth, Ionic Homeostasis, and Physiological Responses of Cotton under Different Salt and Alkali Stresses. Sci. Rep. 2020, 10, 21844. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Yuan, F.; Guo, J.; Zhang, Y.; Sui, N.; Wang, B. AtSIZ1 Improves Salt Tolerance by Maintaining Ionic Homeostasis and Osmotic Balance in Arabidopsis. Plant Sci. 2019, 285, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fricke, W. Salt Stress—Regulation of Root Water Uptake in a Whole-Plant and Diurnal Context. Int. J. Mol. Sci. 2023, 24, 8070. [Google Scholar] [CrossRef]
- Rashid, M.; Noreen, S.; Shah, K.H.; Gaafar, A.R.Z.; Waqar, R. Morphological and Biochemical Variations Caused by Salinity Stress in Some Varieties of Pennisetum glaucum L. J. King Saud Univ.-Sci. 2024, 36, 102994. [Google Scholar] [CrossRef]
- Silva BR, S.; Batista, B.L.; Lobato, A.S. Anatomical Changes in Stem and Root of Soybean Plants Submitted to Salt Stress. Plant Biol. 2020, 23, 57–65. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Zhao, F.; Li, J.; Zhang, X.; Kong, X.; Wu, H.; Zhang, Z. Plant Salinity Stress Response and Nano-Enabled Plant Salt Tolerance. Front. Plant Sci. 2022, 13, 843994. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rajput, V.D.; Sharma, R.; Ghazaryan, K.; Minkina, T. Salinity Stress and Nanoparticles: Insights into Antioxidative Enzymatic Resistance, Signaling, and Defense Mechanisms. Environ. Res. 2023, 235, 116585. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hu, Z.; Zhang, H.; Min, W.; Hou, Z. Comparative Effects of Salt and Alkali Stress on Antioxidant System in Cotton (Gossypium hirsutum L.) Leaves. Open Chem. 2019, 17, 1352–1360. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhang, S.; Du, S.; Wang, G.; Zhang, J.; Jiang, J. Analysis of the Antioxidant Mechanism of Tamarix Ramosissima Roots under NaCl Stress Based on Physiology, Transcriptomic and Metabolomic. Antioxidants 2022, 11, 2362. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Han, Y.; Cheng, Z.; Lv, Q.; Pompelli, M.F.; Pereira, J.D.; Araújo, W.L. Long Exposure to Salt Stress in Jatropha Curcas Leads to Stronger Damage to the Chloroplast Ultrastructure and Its Functionality Than the Stomatal Function. Forests 2023, 14, 1868. [Google Scholar] [CrossRef]
- Shu, S.; Yuan, L.-Y.; Guo, S.-R.; Sun, J.; Yuan, Y.-H. Effects of Exogenous Spermine on Chlorophyll Fluorescence, Antioxidant System and Ultrastructure of Chloroplasts in Cucumis sativus L. under Salt Stress. Plant Physiol. Biochem. PPB 2013, 63, 209–216. [Google Scholar]
- Bejaoui, F.; Salas, J.J.; Nouairi, I.; Smaoui, A.; Abdelly, C.; Martínez-Force, E.; Youssef, N.B. Changes in Chloroplast Lipid Contents and Chloroplast Ultrastructure in Sulla carnosa and Sulla coronaria Leaves under Salt Stress. J. Plant Physiol. 2016, 198, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lan, H.-Y.; Peng, W.; Rahman, K.; Liu, Q.-C.; Luan, X.; Zhang, H. Isatis indigotica: A Review of Phytochemistry, Pharmacological Activities and Clinical Applications. J. Pharm. Pharmacol. 2021, 73, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Gao, D.; Jeong, W.S.; Kim, C.T.; Cho, C.W.; Kim, H.M.; Kang, J.S. Anti-Wrinkle Effect of Isatis indigotica Leaf Extract: Evaluation of Antioxidant, Anti-Inflammation, and Clinical Activity. Antioxidants 2021, 10, 1339. [Google Scholar] [CrossRef]
- Chen, C.; Fan, N.; Xu, C.; Shao, S.; Shi, G.; Zhou, Y.; Wei, Y.; Wu, L.; Wang, B.; Shi, J.; et al. A Synthetic Derivative of Bioactive Constituents from Isatis indigotica Ameliorates Hypersensitivity and Arthritis by Inhibiting JAK2-STAT3 Pathway in Mice. Int. Immunopharmacol. 2023, 124, 110884. [Google Scholar] [CrossRef]
- Tang, X.-Q.; Wang, Y.; Lv, T.-T.; Xiao, Y.-H. Role of 5-Aminolevulinic Acid on Growth, Photosynthetic Parameters and Antioxidant Enzyme Activity in NaCl-Stressed Isatis indigotica Fort. Russ. J. Plant Physiol. 2017, 64, 198–206. [Google Scholar] [CrossRef]
- Tang, X.-Q.; Tan, H.-W.; Shi, S.-L.; Wang, L.-L.; Wang, F.-Q.; Wang, K.-C.; Yang, J. Characterization of Transcriptome Expression: The Response of Isatis indigotica to Salt Stress. Russ. J. Plant Physiol. 2020, 67, 1135–1143. [Google Scholar] [CrossRef]
- Grisafi, F.; Lorusso, R.; Rocchetta, L.; Tombesi, S. Dark Respiration in Leaves, Stems, and Fruits of Corylus avellana. Sci. Hortic. 2024, 327, 112794. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, W.; Kong, S.; Li, L.; Lin, S. Overview of Molecular Mechanisms of Plant Leaf Development: A Systematic Review. Front. Plant Sci. 2023, 14, 1293424. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant Salt-Tolerance Mechanism: A Review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tang, J.; Zhang, J. Effects of Salt Stress on the Morphology, Growth and Physiological Parameters of Juglansmicrocarpa microcarpa L. Seedlings. Plants 2022, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ahmad, N.; Zhao, S.; Zhao, C.; Zhong, W.; Wang, X.; Li, G. Effect of Salt Stress on Growth and Physiological Properties of Asparagus Seedlings. Plants 2022, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Feng, J.; Wang, L.; Liu, Y.; He, D.; Sun, Y.; Luo, Y.; Jin, C.; Zhang, Y. OsMDH12: A Peroxisomal Malate Dehydrogenase Regulating Tiller Number and Salt Tolerance in Rice. Plants 2023, 12, 3558. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Testerink, C. Root Dynamic Growth Strategies in Response to Salinity. Plant Cell Environ. 2022, 45, 695–704. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, W.; Tian, D.; Cheng, B.; Yang, T. Salinity Distribution Pattern and Its Induced Adaptability of Tomato Roots. Sci. Hortic. 2023, 313, 111905. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Calero Hurtado, A.; Olivera Viciedo, D.; Habermann, E.; De Mello Prado, R.; De Oliveira, R.; Ajila, G.; Tenesaca, L.F.L.; Rodríguez, J.C.; Gratão, P.L. Regulatory Role of Silicon on Growth, Potassium Uptake, Ionic Homeostasis, Proline Accumulation, and Antioxidant Capacity of Soybean Plants Under Salt Stress. J. Plant Growth Regul. 2023, 42, 4528–4540. [Google Scholar] [CrossRef]
- Kuppusamy, A.; Alagarswamy, S.; Karuppusami, K.M.; Maduraimuthu, D.; Natesan, S.; Ramalingam, K.; Muniyappan, U.; Subramanian, M.; Kanagarajan, S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants 2023, 12, 2535. [Google Scholar] [CrossRef]
- Abu-Ria, M.; Shukry, W.; Abo-Hamed, S.; Albaqami, M.; Almuqadam, L.; Ibraheem, F. Humic Acid Modulates Ionic Homeostasis, Osmolytes Content, and Antioxidant Defense to Improve Salt Tolerance in Rice. Plants 2023, 12, 1834. [Google Scholar] [CrossRef] [PubMed]
- Didi, D.A.; Su, S.; Sam, F.E.; Tiika, R.J.; Zhang, X. Effect of Plant Growth Regulators on Osmotic Regulatory Substances and Antioxidant Enzyme Activity of Nitraria tangutorum. Plants 2022, 11, 2559. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, W.G.E.; Atia, M.M.; Ali, A.Z.; Abbas, E.E.A.; Salim, B.M.A.; Marey, S.A.; Hatamleh, A.A.; Elnahal, A.S.M. Assessing the Effectiveness of Eco-Friendly Management Approaches for Controlling Wheat Yellow Rust and Their Impact on Antioxidant Enzymes. Plants 2023, 12, 2954. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Huang, J.; Qi, T.; Fu, Q.; Meng, A.; Fu, Y. Effects of Plant Regulators on the Seed Germination and Antioxidant Enzyme Activity of Cotton under Compound Salt Stress. Plants 2023, 12, 4112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, M.; Han, Z. Effects of NaCl Stress on the Growth, Physiological Characteristics and Anatomical Structures of Populus Talassica × Populus Euphratica Seedlings. Plants 2022, 11, 3025. [Google Scholar] [CrossRef] [PubMed]
- Theerawitaya, C.; Samphumphuang, T.; Tisarum, R.; Siangliw, M.; Cha-um, S.; Takabe, T.; Toojinda, T. Expression Level of Na+ Homeostasis-Related Genes and Salt-Tolerant Abilities in Backcross Introgression Lines of Rice Crop under Salt Stress at Reproductive Stage. Protoplasma 2020, 257, 1595–1606. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the Grape (Vitis vinifera L.) Berry: Transport and Function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Ran, X.; Huang, X.; Wang, X.; Liang, H.; Wang, Y.; Li, J.; Huo, Z.; Liu, B.; Ma, C. Ion Absorption, Distribution and Salt Tolerance Threshold of Three Willow Species under Salt Stress. Front. Plant Sci. 2022, 13, 969896. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Yang, X.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous Spermidine Enhances the Photosynthesis and Ultrastructure of Lettuce Seedlings under High-Temperature Stress. Sci. Hortic. 2022, 291, 110570. [Google Scholar] [CrossRef]
- Xin, L.; Tang, M.; Zhang, L.; Huang, W.; Wang, X.; Gao, Y. Effects of Saline-Fresh Water Rotation Irrigation on Photosynthetic Characteristics and Leaf Ultrastructure of Tomato Plants in a Greenhouse. Agric. Water Manag. 2024, 292, 108671. [Google Scholar] [CrossRef]
- Manaa, A.; Goussi, R.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Salinity Tolerance of Quinoa (Chenopodium Quinoa Willd) as Assessed by Chloroplast Ultrastructure and Photosynthetic Performance. Environ. Exp. Bot. 2019, 162, 103–114. [Google Scholar] [CrossRef]
- Liang, B.B.; Wang, W.J.; Fan, X.X.; Kurakov, A.V.; Liu, Y.F.; Song, F.Q.; Chang, W. Arbuscular Mycorrhizal Fungi Can Ameliorate Salt Stress in Elaeagnus angustifolia by Improving Leaf Photosynthetic Function and Ultrastructure. Plant Biol. J. 2021, 23, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Culicov, O.; Stegarescu, A.; Soran, M.-L.; Lung, I.; Opriș, O.; Ciorîță, A.; Nekhoroshkov, P. The Effect of Copper Salts on Bioactive Compounds and Ultrastructure of Wheat Plants. Molecules 2022, 27, 4835. [Google Scholar] [CrossRef]
- Wang, W.-B.; Cao, L.; Chen, W.; Lu, C.; Hu, Z.; Leng, P. Quercus Dentata Responds to Na2CO3 Stress with Salt Crystal Deposits: Ultrastructure, and Physiological–Biochemical Parameters of Leaves. Trees 2023, 37, 1001–1011. [Google Scholar] [CrossRef]
- Bai, J.-P.; Gao, H.-J.; Yang, H.-Y.; Lou, Y.; Zhang, J.-L.; Wang, D.; Zhang, J.-L. Comparison of Ultrastructural and Physiological Changes of Potato (Solanum tuberosum L.) Plantlets Subjected to Salt and Modeling Drought Stresses. Acta Physiol. Plant 2016, 38, 182. [Google Scholar] [CrossRef]
- El-Dakak, R.A.; Badr, R.H.; Zeineldein, M.H.; Swedan, E.A.; Batrawy, O.E.; Hassaballah, A.F.; Hassan, I.A. Effect of Chilling and Salinity Stress on Photosynthetic Performance and Ultrastructure of Chloroplast in Faba Beans (Vicia faba L.) Leaves. Rend. Fis. Acc. Lincei 2023, 34, 447–456. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Y.; Hu, Y.; Jia, X.; Zhao, T.; Cheng, L.; Wang, Y. Tolerance of Two Apple Rootstocks to Short-Term Salt Stress: Focus on Chlorophyll Degradation, Photosynthesis, Hormone and Leaf Ultrastructures. Acta Physiol. Plant 2019, 41, 87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Jia, X.; Tian, B.; Zhang, F.; Zhao, J.; Xie, X.; Shan, C.; Wang, H.; Guo, X.; Han, J. Physiological and Cellular Ultrastructural Responses of Isatis indigotica Fort. under Salt Stress. Plants 2024, 13, 1593. https://doi.org/10.3390/plants13121593
Wu S, Jia X, Tian B, Zhang F, Zhao J, Xie X, Shan C, Wang H, Guo X, Han J. Physiological and Cellular Ultrastructural Responses of Isatis indigotica Fort. under Salt Stress. Plants. 2024; 13(12):1593. https://doi.org/10.3390/plants13121593
Chicago/Turabian StyleWu, Shuang, Xiuwen Jia, Beijing Tian, Feng Zhang, Jingying Zhao, Xinjing Xie, Chenggang Shan, Huimei Wang, Xiaorui Guo, and Jinlong Han. 2024. "Physiological and Cellular Ultrastructural Responses of Isatis indigotica Fort. under Salt Stress" Plants 13, no. 12: 1593. https://doi.org/10.3390/plants13121593
APA StyleWu, S., Jia, X., Tian, B., Zhang, F., Zhao, J., Xie, X., Shan, C., Wang, H., Guo, X., & Han, J. (2024). Physiological and Cellular Ultrastructural Responses of Isatis indigotica Fort. under Salt Stress. Plants, 13(12), 1593. https://doi.org/10.3390/plants13121593





