Exploring the Effects of Different Drying Methods on Related Differential Metabolites of Pleurotus citrinopileatus Singer Based on Untargeted Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fresh Mushroom and Reagents
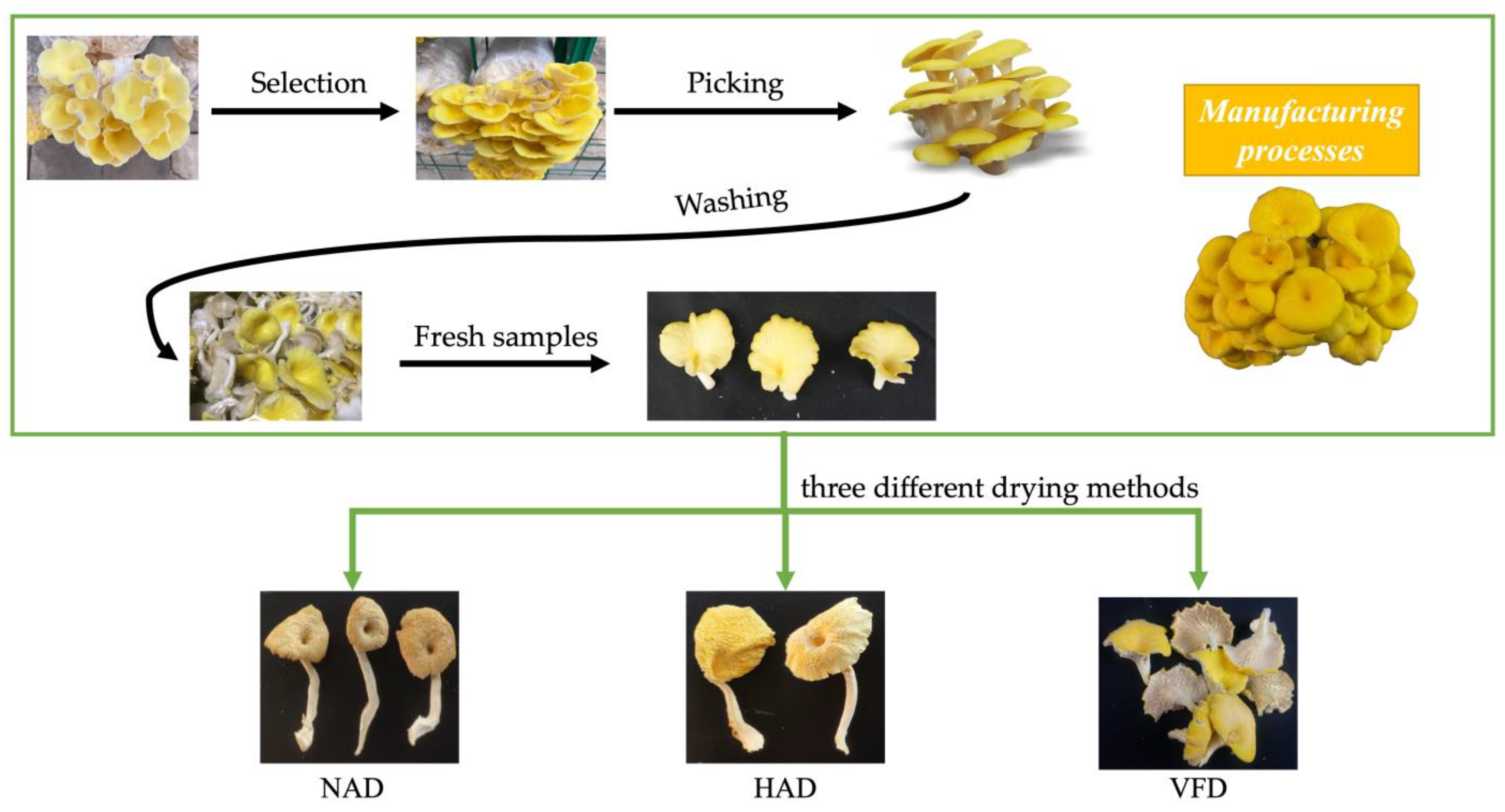
2.2. Mushroom Preparation
- (1)
- Material selection: When the fruit body color of PCS changed to fresh yellow, and this can also be golden yellow, the fruiting bodies free from blemishes and mechanical damage were collected. And mushrooms of uniform size (including the cap and stipe) and maturity were selected as experimental material.
- (2)
- Mushroom picking: The root of PCS was carefully pulled and picked out. Or a knife was used to cut the roots off at slightly above where the root began.
- (3)
- Washing: The picked fruiting bodies were cleaned with running water to remove remaining attached soil, and then the moisture on the surface was wiped with filter papers.
2.3. Drying Process
2.4. Untargeted Metabolomics Analysis by UHPLC-QE-MS
2.4.1. Sample Preparation and Extraction
2.4.2. LC-MS/MS Conditions
2.4.3. Data Processing and Multivariate Analysis
2.5. Statistical Analysis
3. Results
3.1. Overview of the Metabolic Profiles of PCS Collected from Three Different Drying Methods
3.2. Visualize Analysis of Three Drying Methods of PCS
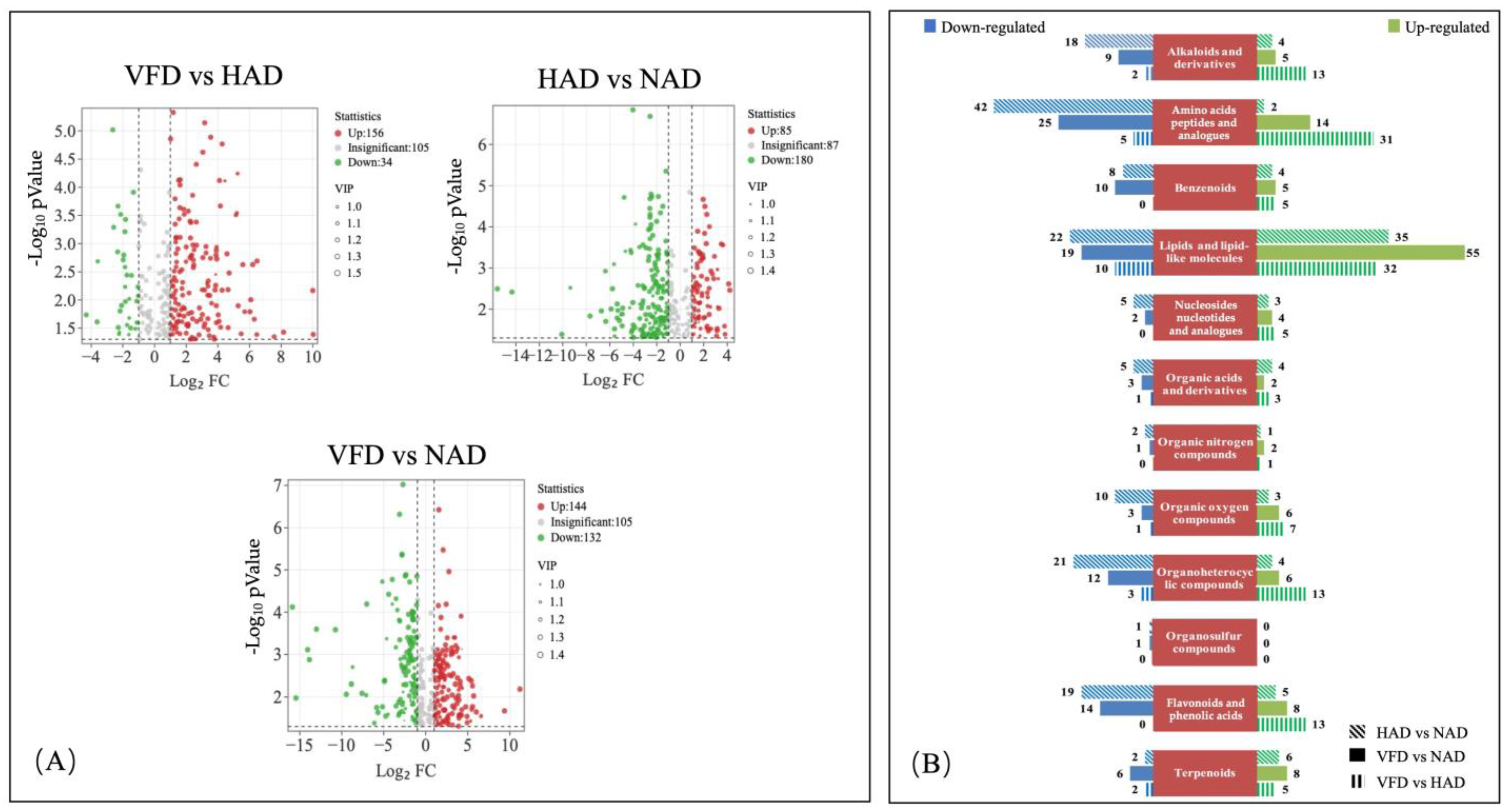
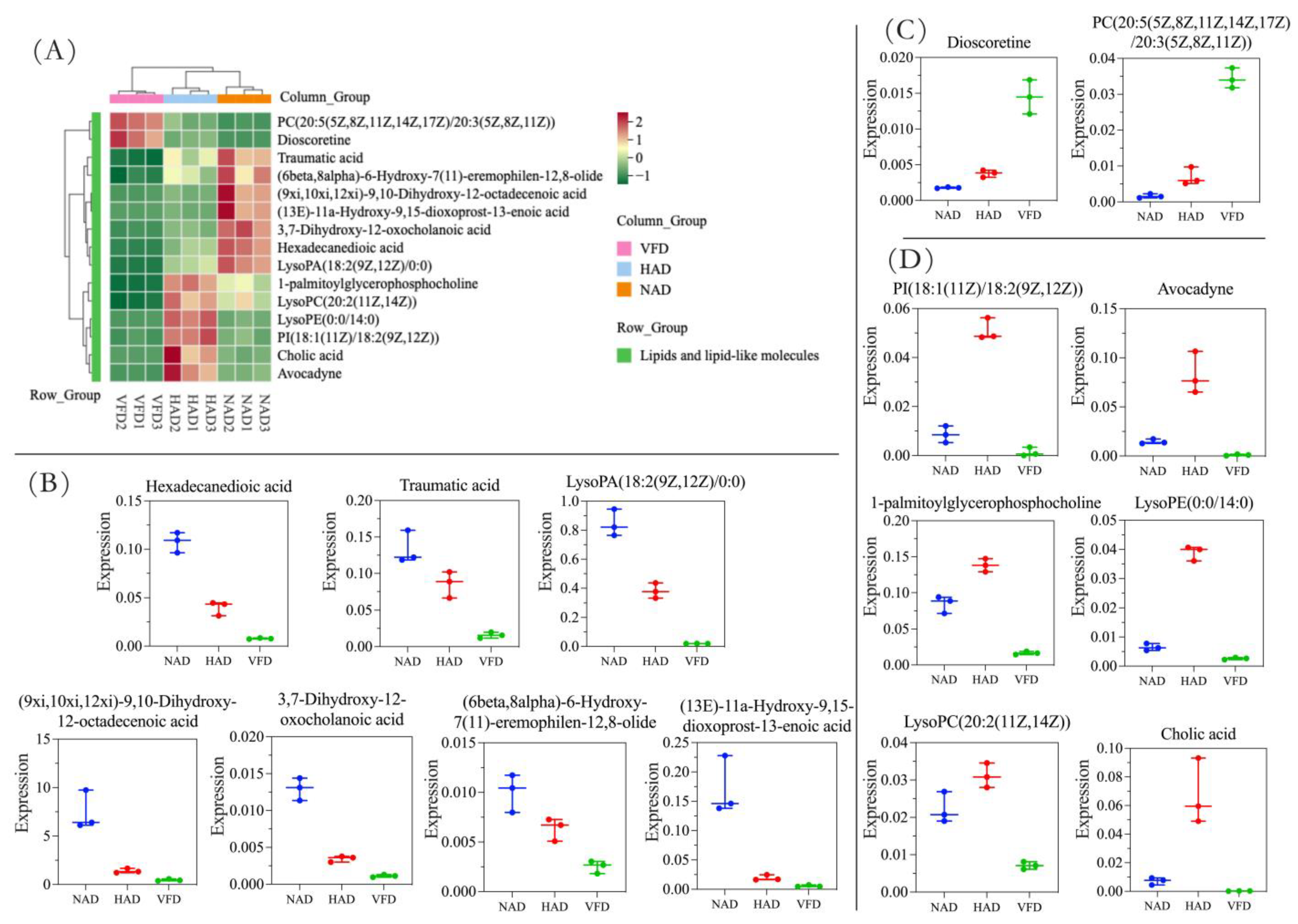
3.3. Differential Metabolite (DM) Analysis of PCS in Three Different Drying Methods
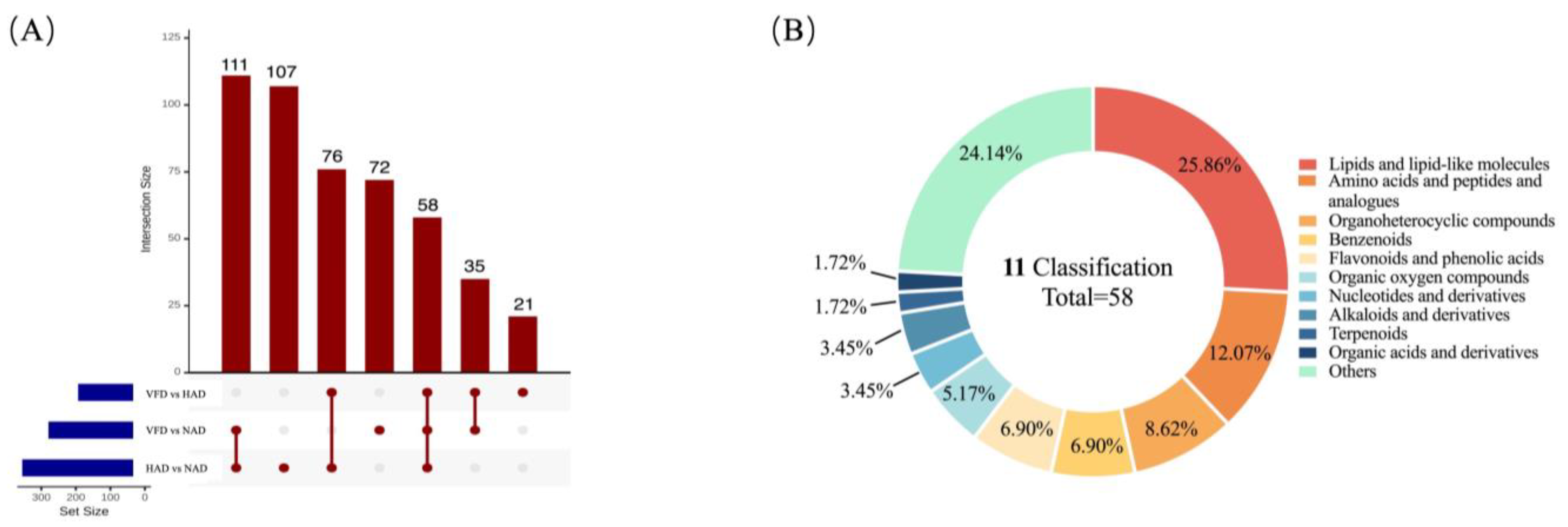
3.3.1. DMs Profiles in Different Drying Treatments of PCS
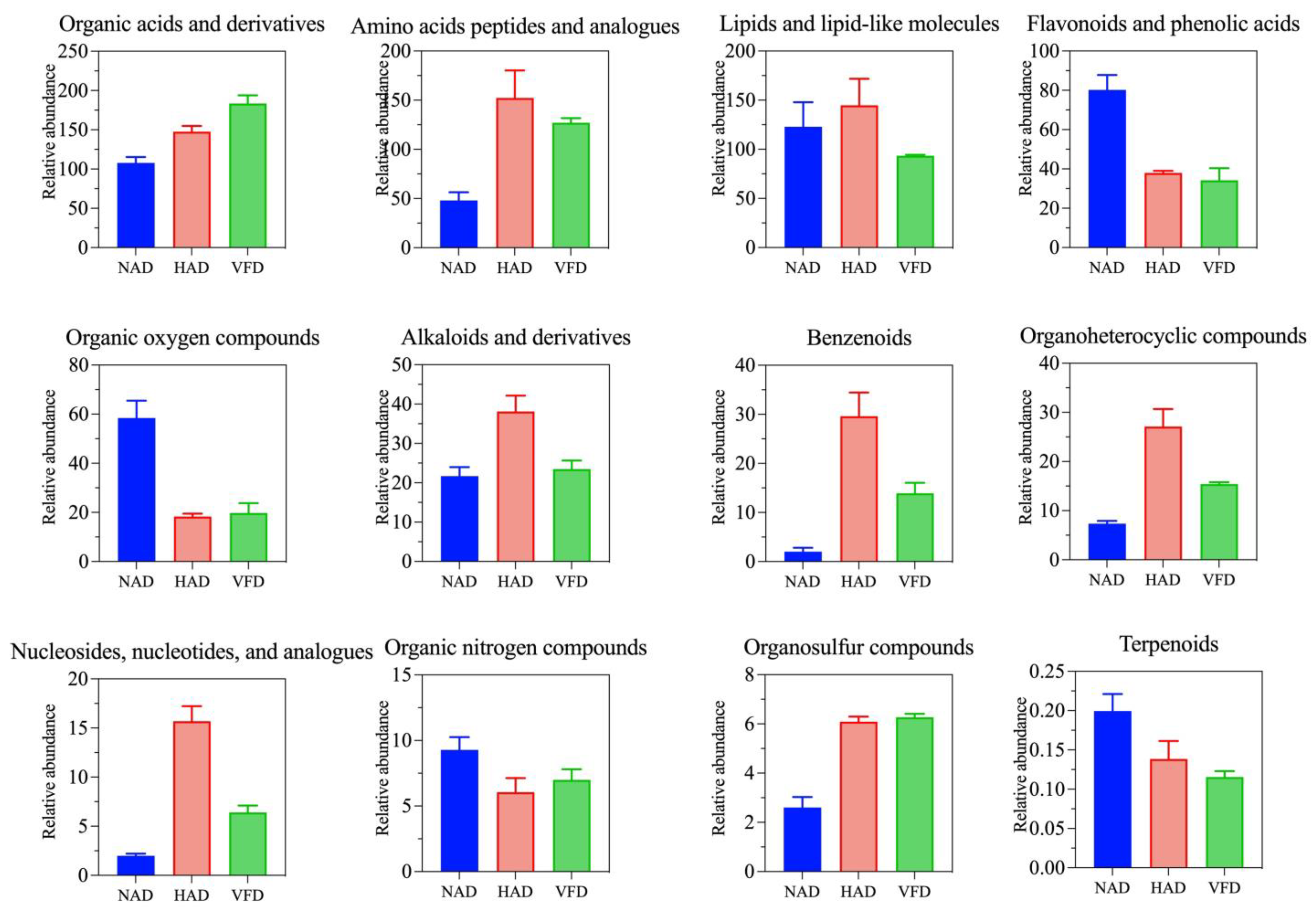
3.3.2. Effects of Different Drying Methods on the DMs of PCS
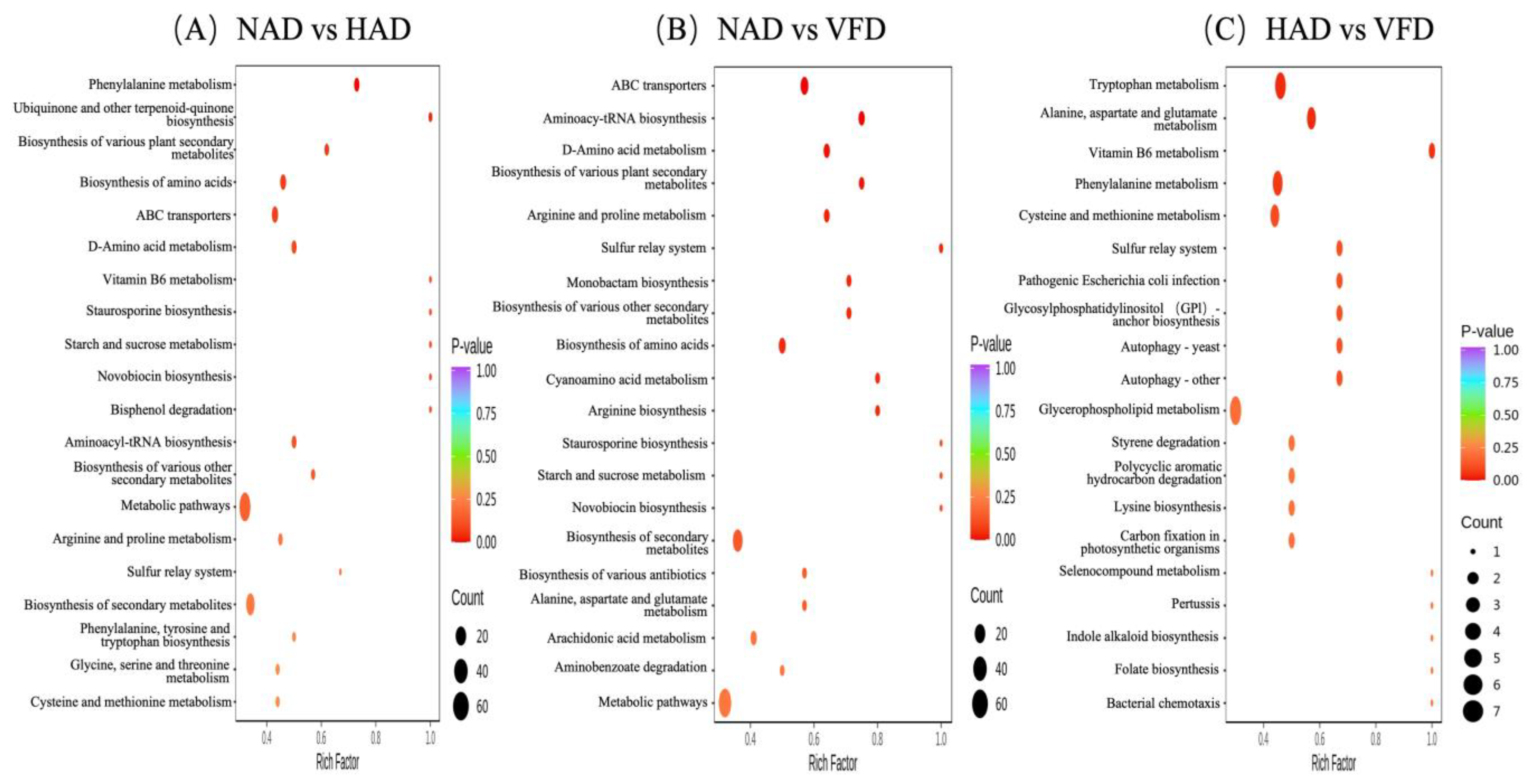
3.4. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tuligur; Li, Y. Resources and Ecological Geographical Distribution of the Edible Fungi of Pleurotus in China. China Edible Mushroom 2021, 5, 8–9. [Google Scholar]
- State Administration of Traditional Chinese Medicine “Chinese Materia Medica” Editorial Board. Chinese Materia Medica; Shanghai Scientific and Technical Publishers: Shanghai, China, 1999. [Google Scholar]
- Wang, Q.; Niu, L.-L.; Liu, H.-P.; Wu, Y.-R.; Li, M.-Y.; Jia, Q. Structural characterization of a novel polysaccharide from Pleurotus citrinopileatus and its antitumor activity on H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef]
- Wu, R.R.; Li, X.; Cao, Y.H.; Peng, X.; Liu, G.F.; Liu, Z.K.; Yang, Z.; Liu, Z.Y.; Wu, Y. China Medicinal Plants of the Ampelopsis grossedentata: A Review of Their Botanical Characteristics, Use, Phytochemistry, Active Pharmacological Components, and Toxicology. Molecules 2023, 28, 7145. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Ezemaduka, A.N.; Luo, N.; Yu, H.; Wang, M. The yields and quality of golden oyster mushroom cultivated on common reed substrates. J. Food Compos. Anal. 2023, 121, 105331. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Sheng, Y.; Liu, J.; Li, H.; Guo, M.; Xu, W.; Luo, Y.; Huang, K.; He, X. Pleurotus Ostreatus Ameliorates Obesity by Modulating the Gut Microbiota in Obese Mice Induced by High-Fat Diet. Nutrients 2022, 14, 1868. [Google Scholar] [CrossRef]
- Minato, K.-I.; Laan, L.C.; van Die, I.; Mizuno, M. Pleurotus citrinopileatus polysaccharide stimulates anti-inflammatory properties during monocyte-to-macrophage differentiation. Int. J. Biol. Macromol. 2019, 122, 705–712. [Google Scholar] [CrossRef]
- Hussein, A.; Ghonimy, A.; Jiang, H.; Qin, G.; El-Ashram, S.; Hussein, S.; El-Razek, I.A.; El-Afifi, T.; Farouk, M.H. LC/MS analysis of mushrooms provided new insights into dietary management of diabetes mellitus in rats. Food Sci. Nutr. 2023, 11, 2321–2335. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and hypolipidemic effect of water extract from Pleurotus citrinopileatus in C57BL/6J mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef]
- Juárez-Hernández, E.O.; Pérez-Zavala, M.L.; Román-Reyes, M.; Barboza-Corona, J.E.; Macías-Sánchez, K.L. Overview of Pleurotus spp., edible fungi with various functional properties. Int. Food Res. J. 2023, 30, 1074–1092. [Google Scholar] [CrossRef]
- Hao, Y.; Sun, H.; Zhang, X.; Wu, L.; Zhu, Z. A novel polysaccharide from Pleurotus citrinopileatus mycelia: Structural characterization, hypoglycemic activity and mechanism. Food Biosci. 2020, 37, 100735. [Google Scholar] [CrossRef]
- Meng, M.; Sun, Y.; Bai, Y.; Xu, J.; Sun, J.; Han, L.; Sun, H.; Han, R. A polysaccharide from Pleurotus citrinopileatus mycelia enhances the immune response in cyclophosphamide-induced immunosuppressed mice via p62/Keap1/Nrf2 signal transduction pathway. Int. J. Biol. Macromol. 2023, 228, 165–177. [Google Scholar] [CrossRef]
- Piskov, S.; Timchenko, L.; Grimm, W.-D.; Rzhepakovsky, I.; Avanesyan, S.; Sizonenko, M.; Kurchenko, V. Effects of Various Drying Methods on Some Physico-Chemical Properties and the Antioxidant Profile and ACE Inhibition Activity of Oyster Mushrooms (Pleurotus ostreatus). Foods 2020, 9, 160. [Google Scholar] [CrossRef]
- Samani, B.H.; Gudarzi, H.; Rostami, S.; Lorigooini, Z.; Esmaeili, Z.; Jamshidi-kia, F. Development and optimization of the new ultrasonic-infrared-vacuum dryer in drying Kelussia odoratissima and its comparison with conventional methods. Ind. Crops Prod. 2018, 123, 46–54. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, C.; Li, X.; Gu, H.; E, H.; Zhang, Y.; Zhou, F.; Zhao, Z.; Fan, T.; Lu, H.; et al. Ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry based untargeted metabolomics to reveal the characteristics of Dictyophora rubrovolvata from different drying methods. Front. Nutr. 2022, 9, 1056598. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, J.; Zhou, L.; Zhao, M.; Liu, J.; Marchioni, E. Characterization and discrimination of volatile organic compounds and lipid profiles of truffles under different treatments by UHPLC-QE Orbitrap/MS/MS and P&T-GC-MS. Food Chem. 2023, 410, 135432. [Google Scholar] [CrossRef]
- Kibar, B. Influence of different drying methods and cold storage treatments on the postharvestquality and nutritional properties of P. ostreatus mushroom. Turk. J. Agric. For. 2021, 45, 565–579. [Google Scholar] [CrossRef]
- He, Z.; Shen, Q.; Wang, L.; Fan, X.; Zhuang, Y. Effects of different drying methods on the physical characteristics and non-volatile taste components of Schizophyllum commune. J. Food Compos. Anal. 2023, 123, 105632. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Wang, X.; Huang, J.; Zhao, D.; Tan, Y.; Zhang, Z.; Zhang, Z.; Zhu, L.; Wu, B.; et al. Anti-Alzheimers molecular mechanism of icariin: Insights from gut microbiota, metabolomics, and network pharmacology. J. Transl. Med. 2023, 21, 277. [Google Scholar] [CrossRef]
- Xing, M.; Zhao, J.; Zhang, J.; Wu, Y.; Khan, R.A.A.; Li, X.; Wang, R.; Li, T.; Liu, T. 6-Pentyl-2H-pyran-2-one from Trichoderma erinaceum Is Fungicidal against Litchi Downy Blight Pathogen Peronophythora litchii and Preservation of Litchi. J. Agric. Food Chem. 2023, 71, 19488–19500. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Zheng, Y.; Song, M.; Zhang, S.; Sun, Y.; Wei, M.; Fan, X. Diosgenin Ameliorates Non-alcoholic Fatty Liver Disease by Modulating the Gut Microbiota and Related Lipid/Amino Acid Metabolism in High Fat Diet-Fed Rats. Front. Pharmacol. 2022, 13, 854790. [Google Scholar] [CrossRef]
- Pang, W.; Wang, D.; Zuo, Z.; Wang, Y.; Sun, W.; Zhang, N.; Zhang, D. Kidney bean fermented broth alleviates hyperlipidemic by regulating serum metabolites and gut microbiota composition. Nutrients 2022, 14, 3202. [Google Scholar] [CrossRef]
- Jin, P.; Liang, Z.; Lu, H.; Pan, J.; Li, P.; Huang, Q.; Guo, Y.; Zhong, J.; Li, F.; Wan, J.; et al. Lipid remodeling reveals the adaptations of a marine diatom to ocean acidification. Front. Microbiol. 2021, 12, 748445. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Xu, Y.; Long, H.; Deng, Y.; Li, S.; Xi, Y.; Li, W.; Cai, H.; Zhang, B.; et al. Emerging LC-MS/MS-based molecular networking strategy facilitates foodomics to assess the function, safety, and quality of foods: Recent trends and future perspectives. Trends Food Sci. Technol. 2023, 139, 104114. [Google Scholar] [CrossRef]
- İnci, Ş.; Kırbağ, S.; Akyüz, M. Growth period, yield, and nutrient contents of Pleurotus citrinopileatus Singer grown on some local agricultural wastes in Turkey. Biomass-Convers. Biorefinery 2023, 13, 15029–15038. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, N.; Li, W.; Zhang, B.; Shi, T.; Xie, M.; Yu, M. Effect of Ultrasonic Induction on the Main Physiological and Biochemical Indicators and γ-Aminobutyric Acid Content of Maize during Germination. Foods 2022, 11, 1358. [Google Scholar] [CrossRef]
- Wang, Z.; Gan, S.; Sun, W.; Chen, Z. Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas. Foods 2022, 11, 1057. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hirata, Y.; Saito, T.; Kumagai, H. Combined Effects of Amino Acids in Garlic and Buna-Shimeji (Hypsizygus marmoreus) on Suppression of CCl4-Induced Hepatic Injury in Rats. Foods 2021, 10, 1491. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragoulakis, V.; Papakonstantinou, E.; Antonaki, M.; Vozikis, A.; Tsatsakis, A.; Buga, A.M.; Mitroi, M.; Calina, D. Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids. Metabolites 2020, 10, 502. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, H.; Du, Z. Effect of drying methods on aroma, taste and antioxidant activity of Dendrobium officinale flower tea: A sensomic and metabolomic study. Food Res. Int. 2024, 187, 114455. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Finnie, J.F.; Van Staden, J. The role of endophytes in secondary metabolites accumulation in medicinal plants under abiotic stress. South Afr. J. Bot. 2020, 134, 126–134. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, Y.; Wei, W.; Yang, J.; Zhong, Q.; Li, Z. Metabolite Analysis of Jerusalem Artichoke (Helianthus tuberosus L.) Seedlings in Response to Polyethylene Glycol-Simulated Drought Stress. Int. J. Mol. Sci. 2021, 22, 3294. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Huang, Y.; Zhou, Q. Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.-J.; Sun, X.-F.; Zhao, L.-L.; Wang, P.-C. Differential physiological, transcriptomic, and metabolomic responses of paspalum wettsteinii under high-temperature stress. Front. Plant Sci. 2022, 13, 865608. [Google Scholar] [CrossRef]
- Gao, X.; Tang, J.; Liu, H.; Liu, L.; Kang, L.; Chen, W. Structure-activity relationship investigation of tertiary amine derivatives of cinnamic acid as acetylcholinesterase and butyrylcholinesterase inhibitors: Compared with that of phenylpropionic acid, sorbic acid and hexanoic acid. J. Enzym. Inhib. Med. Chem. 2018, 33, 519–524. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Fan, F.; Yu, Q.; Zhang, P. A Moss 2-Oxoglutarate/Fe(II)-Dependent Dioxygenases (2-ODD) Gene of Flavonoids Biosynthesis Positively Regulates Plants Abiotic Stress Tolerance. Front. Plant Sci. 2022, 13, 850062. [Google Scholar] [CrossRef]
- Park, Y.S.; Jang, S.; Lee, H.; Kang, S.; Seo, H.; Yeon, S.; Lee, D.; Yun, C.W. Identification of the Antidepressant Function of the Edible Mushroom Pleurotus eryngii. J. Fungi 2021, 7, 190. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, B.; Yang, X.; Huang, Y.; Luo, J.; Zhang, X.; Tan, W.; Song, C.; Ao, Z.; Shen, C.; et al. Metabolomic profiling reveals biomarkers for diverse flesh colors in jelly fungi (Auricularia cornea). Food Chem. 2024, 446, 138906. [Google Scholar] [CrossRef]
- Ohnishi, T.; Balan, S.; Toyoshima, M.; Maekawa, M.; Ohba, H.; Watanabe, A.; Iwayama, Y.; Fujita, Y.; Tan, Y.; Hisano, Y.; et al. Investigation of betaine as a novel psychotherapeutic for schizophrenia. EBioMedicine 2019, 45, 432–446. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, S.; Zhu, G.; Huang, R.; Yin, Y.; Ren, W. Betaine inhibits interleukin-1β production and release: Potential mechanisms. Front. Immunol. 2018, 9, 2670. [Google Scholar] [CrossRef]
- Tan, W.-N.; Nagarajan, K.; Lim, V.; Azizi, J.; Khaw, K.-Y.; Tong, W.-Y.; Leong, C.-R.; Chear, N.J.-Y. Metabolomics analysis and antioxidant potential of endophytic Diaporthe fraxini ED2 grown in different culture media. J. Fungi 2022, 8, 519. [Google Scholar] [CrossRef]
- Xiang, J.; Mlambo, R.; Shaw, I.; Seid, Y.; Shah, H.; He, Y.; Kpegah, J.K.S.K.; Tan, S.; Zhou, W.; He, B. Cryopreservation of bioflavonoid-rich plant sources and bioflavonoid-microcapsules: Emerging technologies for preserving bioactivity and enhancing nutraceutical applications. Front. Nutr. 2023, 10, 1232129. [Google Scholar] [CrossRef]
- Savas, E. The modelling of convective drying variables’ effects on the functional properties of sliced sweet potatoes. Foods 2022, 11, 741. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, X.; Cai, M.; Gu, H.; E, H.; Li, X.; Zhang, Y.; Lu, H.; Zhou, C. Metabolomics Analysis of Morchella sp. From Different Geographical Origins of China Using UPLC-Q-TOF-MS. Front. Nutr. 2022, 9, 865531. [Google Scholar] [CrossRef]
- Szczerbinski, L.; Wojciechowska, G.; Olichwier, A.; Taylor, M.A.; Puchta, U.; Konopka, P.; Paszko, A.; Citko, A.; Goscik, J.; Fiehn, O.; et al. Untargeted Metabolomics Analysis of the Serum Metabolic Signature of Childhood Obesity. Nutrients 2022, 14, 214. [Google Scholar] [CrossRef]
- Wolfs, D.; Lynes, M.D.; Tseng, Y.-H.; Pierce, S.; Bussberg, V.; Darkwah, A.; Tolstikov, V.; Narain, N.R.; Rudolph, M.C.; Kiebish, M.A.; et al. Brown Fat–Activating Lipokine 12,13-diHOME in Human Milk Is Associated with Infant Adiposity. J. Clin. Endocrinol. Metab. 2021, 106, e943–e956. [Google Scholar] [CrossRef]
- Warner, D.; Vatsalya, V.; Zirnheld, K.H.; Warner, J.B.; Hardesty, J.E.; Umhau, J.C.; McClain, C.J.; Maddipati, K.; Kirpich, I.A. Linoleic Acid-Derived Oxylipins Differentiate Early Stage Alcoholic Hepatitis From Mild Alcohol-Associated Liver Injury. Hepatol. Commun. 2021, 5, 947–960. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Carlin, S.; Lotti, C.; Vrhovsek, U.; Mattivi, F. Development of a fully automated method HS-SPME-GC-MS/MS for the determination of odor-active carbonyls in wines: A “Green” approach to improve robustness and productivity in the oenological analytical chemistry. J. Agric. Food Chem. 2023, 72, 1995–2007. [Google Scholar] [CrossRef]
- Ahmed, A.T.; MahmoudianDehkordi, S.; Bhattacharyya, S.; Arnold, M.; Liu, D.; Neavin, D.; Moseley, M.A.; Thompson, J.W.; Williams, L.S.J.; Louie, G.; et al. Acylcarnitine metabolomic profiles inform clinically-defined major depressive phenotypes. J. Affect. Disord. 2020, 264, 90–97. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; McClements, D.J.; Nie, S.; Shen, M.; Li, C.; Huang, Y.; Zhong, Y.; Chen, J.; Zeng, M.; et al. pH and lipid unsaturation impact the formation of acrylamide and 5-hydroxymethylfurfural in model system at frying temperature. Food Res. Int. 2019, 123, 403–413. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Peng, S.; Xu, N.; Shang, X.; Liu, J.; Xu, Z.; Jiang, N.; Dong, H.; Wang, R.; Dong, H. Exploring the Effects of Different Drying Methods on Related Differential Metabolites of Pleurotus citrinopileatus Singer Based on Untargeted Metabolomics. Plants 2024, 13, 1594. https://doi.org/10.3390/plants13121594
Lu H, Peng S, Xu N, Shang X, Liu J, Xu Z, Jiang N, Dong H, Wang R, Dong H. Exploring the Effects of Different Drying Methods on Related Differential Metabolites of Pleurotus citrinopileatus Singer Based on Untargeted Metabolomics. Plants. 2024; 13(12):1594. https://doi.org/10.3390/plants13121594
Chicago/Turabian StyleLu, Huan, Simin Peng, Ning Xu, Xiaodong Shang, Jianyu Liu, Zhen Xu, Ning Jiang, Haoran Dong, Ruijuan Wang, and Hui Dong. 2024. "Exploring the Effects of Different Drying Methods on Related Differential Metabolites of Pleurotus citrinopileatus Singer Based on Untargeted Metabolomics" Plants 13, no. 12: 1594. https://doi.org/10.3390/plants13121594




