Abstract
Botrytis cinerea is considered the second most important fungal plant pathogen, and can cause serious disease, especially on tomato. The TPK1b gene encodes a receptor-like kinase that can positively regulate plant resistance to B. cinerea. Here, we identified a tomato WRKY transcription factor SlWRKY3 that binds to the W-box on the TPK1b promoter. It can negatively regulate TPK1b transcription, then regulate downstream signaling pathways, and ultimately negatively regulate tomato resistance to B. cinerea. SlWRKY3 interference can enhance resistance to B. cinerea, and SlWRKY3 overexpression leads to susceptibility to B. cinerea. Additionally, we found that B. cinerea can significantly, and rapidly, induce the upregulation of SlWRKY3 expression. In SlWRKY3 transgenic plants, the TPK1b expression level was negatively correlated with SlWRKY3 expression. Compared with the control, the expression of the SA pathway marker gene PR1 was downregulated in W3-OE plants and upregulated in W3-Ri plants when inoculated with B. cinerea for 48 h. Moreover, SlWRKY3 positively regulated ROS production. Overall, SlWRKY3 can inhibit TPK1b transcription in tomato, and negatively regulate resistance to B. cinerea by modulating the downstream SA and ROS pathways.
1. Introduction
Botrytis cinerea is a necrotrophic pathogen, which invades through the stomata, wounds, and other plant parts; triggers enzymolysis and releases phytotoxins; decomposes and eliminates host cells; and self-replicates and reproduces after successfully entering host cells. B. cinerea can invade the stems, leaves, flowers, fruits, and other organs of more than 200 plant species, seriously threatening global fruit and vegetable yield and quality. In production, chemicals are the main control method, but they not only affect food quality, but also potentially harm the environment. Therefore, it is particularly important to determine the B. cinerea resistance mechanism and cultivate resistant varieties. B. cinerea infects plants and produces an immune response in a process of mutual competition and coevolution [1].
Plants experience biological stress caused by pathogen infection, but can recognize stress-generated signals and trigger their natural immune system, resulting in a defense response [1]. The plant innate immunity concept originated from animal studies, and was systematically outlined in 2002 [2]. In short, PTI (PAMP-triggered immunity) and ETI (Effector-triggered immunity) coexist in plants to respond to pathogen infection. The immune signals produced by a pathogen infection can be transmitted by complex biomolecular networks in plants, thus regulating immune response at all levels. For example, MAPK cascades (mitogen-activated protein kinases) are indispensable components of plant immune signal transduction [3]. Transcription factors such as WRKY, bZIPs, and MYB can act as target MARK proteins, regulate downstream gene expression, and transmit immune signals [4]. Hormone signal transduction is the most extensive component of plant immune signal transduction, and changes in auxin (IAA), ethylene (ET), salicylic acid (SA), and jasmonic acid (JA) hormone levels can play a direct role in immune response [5,6,7,8]. Strigolactones (SLs) are a class of relatively new plant hormones that play a pivotal role in maintaining the structural integrity of plants. They are instrumental in regulating lateral branching and modulating the plant’s response to a variety of abiotic stresses [9]. Pathogenic fungus triggers the defense response of plants. In response, the plant initiates intricate networks of signaling pathways, culminating in the induction of hypersensitive responses (HRs) and/or the acquisition of systemic acquired resistance (SAR). The HR response is accompanied by the activation of programmed cell death (PCD), which is typically characterized by oxidative burst. This burst results in the accumulation of reactive oxygen species (ROS) such as superoxide anions, hydrogen peroxide, and hydroxyl radicals. These reactive oxygen species serve as signaling molecules, produced by a variety of enzymatic and non-enzymatic processes, and are integral to the defense mechanisms that are triggered during the plant–pathogen interaction. While low levels of ROS act as pivotal signal transducers, excessive ROS can exert a potent oxidative force, leading to detrimental effects on the plant [10].
Receptor kinases (RKs) and receptor-like protein kinases (RLKs) recognize various pathogens early and play a key role in defense and symbiosis. Plant RLKs are a kind of plant protein kinase, which can transfer phosphate groups from donors to substrate protein receptors, realize substrate protein trans-phosphorylation, and complete protein activity regulation and signal amplification and transmission [11]. Plant cytoplasmic receptor-like kinases (RLCK) are an RLK subfamily, and can be widely involved in the transduction of related signals such as defense, growth and development, and hormones [12]. BIK1 is a typical RLCK in Arabidopsis thaliana that can interact with complexes formed by two RLKS, FLS2 and BAK1, and participate in necrotic pathogen-induced immune and ethylene-mediated defense responses [13]. It can also participate in Ca2+ signal transduction, reactive oxygen species production, and other immune pathways [14,15]. Additionally, it can also participate in the regulation of hormones such as ET, JA, and SA [14,16,17]. The tomato TPK1b gene encodes a functional cytoplasmic receptor-like kinase with phosphorylation activity, which is independent of JA synthesis and response, and mediates the regulation of plant defense response to necrotic fungi and herbivorous insects through ET [18]. TPK1b-RNAi transgenic lines reduced resistance to B. cinerea and insects, confirming that they play an important role in tomato defense response. Moreover, TPK1b in tomato and BIK1 in Arabidopsis may perform similar biological functions [18,19]. Additionally, the cerato-platanin family protein BcSpl1 and glycoprotein BcGs1 isolated from B. cinerea can upregulate TPK1b expression in plants [20,21]. However, the TPK1b regulation mechanism in immune response is still unclear, and deserves further study.
Plant transcription factors can regulate the expression of stress-related genes at the transcription level and then regulate the plant immune response. The WRKY transcription factor is a plant-specific transcription factor, and belongs to one of the largest transcription factor families in plants. Numerous WRKY transcription factors occur in >20 plants, including rice, soybean, and Arabidopsis thaliana [22], and 81 WRKY transcription factors have been identified in tomato [23]. The names of the WRKY transcription factors reflect that this protein family contains a conserved domain consisting of 60 amino acids, a domain N-terminal that contains seven absolutely conserved amino acids with the WRKYGQK sequence, and a C-terminal with a special zinc finger structure [24]. Based on the number of domains and zinc finger structure characteristics, WRKY transcription factors can be divided into categories I, II, and III [25]. WRKY transcription factors can bind to the specific cis-acting element W-box (TTGACC/T) in its target gene promoter, thus regulating target gene transcription. WRKY transcription factors play an important role in plant immune response. In pepper, CaWRKY1 silencing leads to a decrease in Xanthomonas leaf growth, while HR response caused by tobacco mosaic virus and Pseudomonas in CaWRKY1 overexpression plants is more rapid [26]. The mutant wrky27-1 in Arabidopsis displayed a phenotype that delayed the development of bacterial wilt disease [27]. WRKY11 and WRKY17 could negatively regulate plant resistance to Pseudomonas syringae [28], while WRKY3 and WRKY4 could regulate Arabidopsis resistance to necrotic pathogens, and WRKY4 could also regulate plant resistance to biotrophic pathogens [29]. Additionally, OsWRKY53 can control the scale of early defense responses [30]. HvWRKY1 plays an important role in the resistance of barley to leaf stripe disease [31]. Tobacco WRKY8 can regulate plant sensitivity to pathogens such as Phytophthora infestans, and SlWRKY1 and SlWRKY33 can regulate plant resistance to B. cinerea [32,33,34]. SlWRKY3 is an important regulator of tomato salt tolerance [35] and can be used as a positive regulator of the tomato–Phytophthora interaction, enhancing tomato resistance to Phytophthora [36].
Previous research has identified TPK1b as a positive regulator of tomato resistance against B. cinerea [18]. In our current investigation, we have ascertained that SlWRKY3 can bind to the W-box element on the TPK1b promoter, as evidenced by our screening of a yeast one-hybrid library and subsequent experimental validation. While TPK1b’s role in tomato resistance is well documented, the specific contribution of SlWRKY3 to the defense mechanisms against B. cinerea has been less explored. To elucidate the potential involvement of SlWRKY3 in modulating tomato resistance to B. cinerea via TPK1b, we conducted inoculation experiments with SlWRKY3 transgenic plants. Our results revealed a surprising negative regulatory role of SlWRKY3 in tomato resistance to B. cinerea. Furthermore, we observed that TPK1b expression was diminished in SlWRKY3 overexpression (OE) plants and increased in SlWRKY3 RNA interference (Ri) plants. The outcomes of this study suggest that SlWRKY3 exerts a negative regulatory effect on tomato resistance to B. cinerea by directly suppressing the transcriptional activity of TPK1b. This revelation not only deepens our comprehension of the intricate molecular mechanisms at play in tomato resistance but also offers novel insights that could inform the development of strategies aimed at bolstering crop resistance to B. cinerea.
2. Results
2.1. Analysis of the TPK1b Gene Promoter and Screening Library for Yeast One Hybridization
To find the upstream regulatory factors, we selected a TPK1b promoter fragment (−1 to −3000 bp) and predicted the cis-acting elements on it using the PlantCARE database (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 8 May 2019)). They were mainly related to light response, followed by hormone-related elements such as abscisic acid, auxin, and JA, and also included some elements related to other life activities such as anaerobic induction, defense response, and circadian rhythm regulation (Figure 1). Simultaneously, we found six W-box elements that can be combined by WRKY transcription factors (Figure 1B). Subsequently, the yeast one-hybrid screening system was used to screen the TPK1b promoter fragment (−1 to −3000 bp), and five transcription factors were identified (Table 1), including two bHLH transcription factors, two ERF transcription factors, and one WRKY transcription factor.
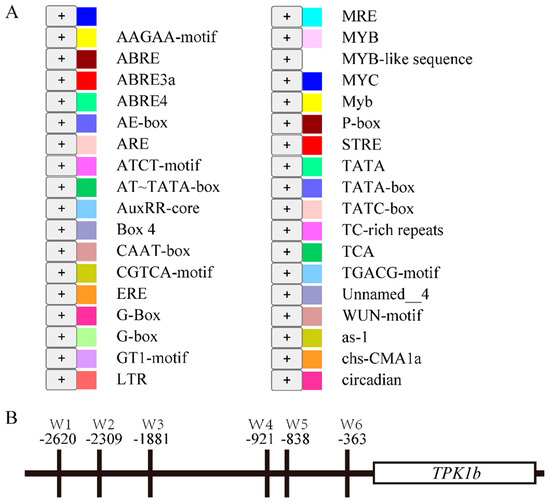
Figure 1.
Cis-acting elements on the TPK1b promoter: (A) Prediction of cis-acting elements on the TPK1b promoter based on the PlantCARE database. (B) Distribution of W-box elements in the TPK1b promoter.

Table 1.
TPK1b promoter yeast one-hybrid screens.
2.2. SlWRKY3 Negatively Regulates Tomato Resistance to B. cinerea
To explore whether the five transcription factors obtained from the TPK1b promoter yeast one-hybrid screening experiment are involved in tomato resistance to B. cinerea, we conducted tomato genetic transformation on these five transcription factors. However, only SlWRKY3 transgenic plants had significant changes in resistance to B. cinerea. Therefore, we focused on the effect of SlWRKY3 on resistance to B. cinerea and its relationship with TPK1b. Firstly, we detected the relative expression level of SlWRKY3 transgenic plants, and found that it increased significantly in overexpression lines (W3-OE), while it decreased significantly in interference lines (W3-Ri) (Figure 2A,B). Through inoculating detached leaves with B. cinerea spores, we found that W3-OE plant lesion areas were significantly larger, while W3-Ri plant lesion areas were significantly smaller than the WT (Figure 2C,D). The results showed that W3-OE plant resistance to B. cinerea decreased compared with the control, while W3-Ri plants had higher B. cinerea resistance. Moreover, they indicate that SlWRKY3 could negatively regulate tomato plant resistance to B. cinerea.
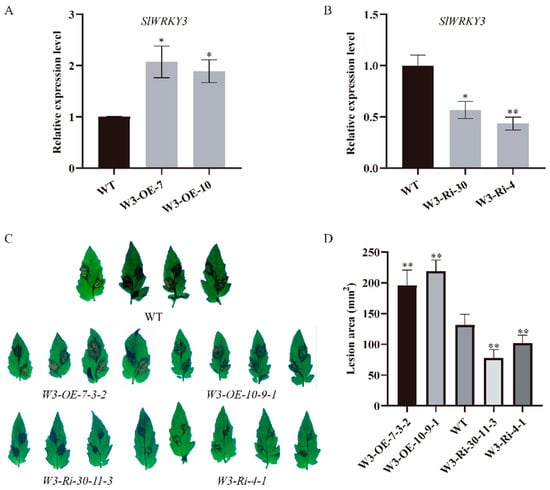
Figure 2.
SlWRKY3 negatively regulates tomato resistance to B. cinerea: (A,B): SlWRKY3 transgenic line SlWRKY3 expression level detection, (A): SlWRKY3 overexpression lines, (B): SlWRKY3 interference lines; (C): phenotype of SlWRKY3 transgenic lines inoculated with B. cinerea; (D): the lesion area of SlWRKY3 transgenic line inoculated with B. cinerea. (Dunnett-t test p * < 0.05, p ** < 0.01.)
2.3. SlWRKY3 Is an Evolutionarily Conserved Type I WRKY Transcription Factor Highly Expressed in Flowers and Leaves
WRKY transcription factors can be classified into three categories according to the number of WRKY functional domains and the zinc finger motif type. By analyzing the SlWRKY3 amino acid sequence, we found that it contains two typical WRKY functional domains with WRKYGQK sequences, and two C2H2 zinc finger motifs (Figure 3A), which belong to type I WRKY transcription factors. Furthermore, there is also a nuclear localization signal between the two WRKY functional domains. We compared it with the other three type I WRKY transcription factors and found that they have high sequence homology (Figure 3B). According to the phylogenetic tree analysis, SlWRKY3 is closely related to type I WRKY transcription factors in tomato and other species, indicating that its evolutionary relationship is conservative (Figure 3C). AtWRKY3 and AtWRKY4, which are closely related to SlWRKY3, have been reported to regulate plant defense response to biological stress. SlWRKY3 expression in tomato roots, stems, leaves, and flowers, and young, green-ripe, and red-ripe fruits was detected. Furthermore, SlWRKY3’s relative expression was very low in roots and stems, but high in flowers, leaves, and red-ripe fruits (Figure 3D).
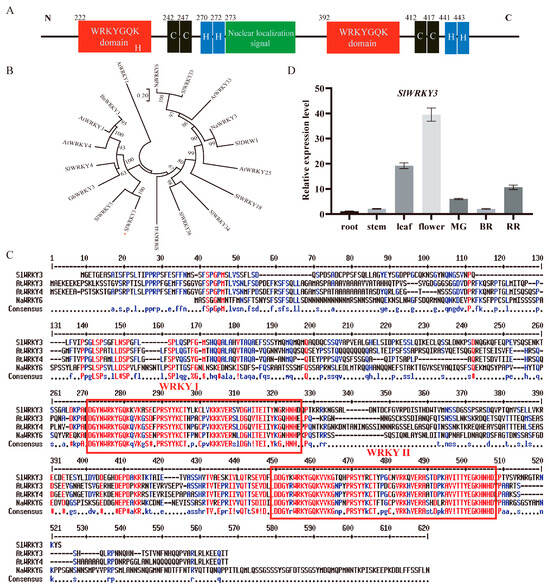
Figure 3.
SlWRKY3 sequence analysis and tissue expression: (A): SlWRKY3 sequence structure; (B): phylogenetic tree of some type I WRKY transcription factors (Sl: tomato; Bn: Rape; Na: wild tobacco; At: Arabidopsis thaliana; GH: upland cotton); (C): amino acid sequence alignment of several type I WRKY transcription factors; (D): SlWRKY3 tissue expression profile. MG: green ripening stage; BR: discoloration period; RR: red ripening stage.
2.4. SlWRKY3 Can Bind to Two W-Boxes on TPK1b Promoter
The yeast one-hybrid assay indicated that SlWRKY3 could interact with the TPK1b promoter, but the SlWRKY3 binding site on the TPK1b promoter was unclear. It has been reported that the WRKY transcription factor regulates target gene transcription expression by binding with W-box, a cis-acting element on the target gene promoter. To determine the binding site, we found six W-boxes on the TPK1b promoter (−1 to −3000 bp)-positive and -negative DNA strands. Based on the location of these six W-boxes, we divided the promoter into four fragments, a, b, c, and d (Figure 4A, where a, b, and c each contain one W-box, and d contains three W-boxes), and carried out yeast one-hybrid experiments with SlWRKY3, respectively. On SD/-Ura-Leu of 50 ng·mL−1 aureobasidin A (AbA), we observed that the TPK1b-b and TPK1b-c groups’ yeast growth rate was significantly faster than blank control pGADT7, while there was no significant difference between the TPK1b-a and TPK1b-d promoter groups and the control. The results indicate that SlWRKY3 could bind to the W-box TPK1b promoter’s b and c parts, and then regulate TPK1b transcription.
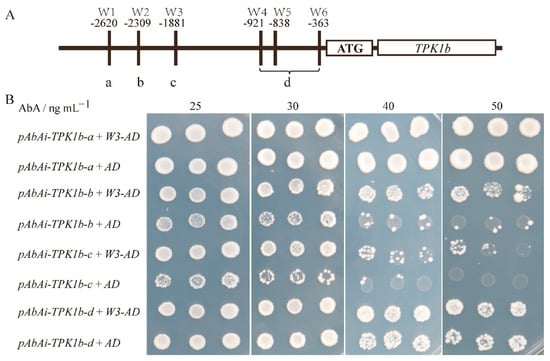
Figure 4.
SlWRKY3 and TPK1b promoter yeast one-hybrid experiment: (A): TPK1b promoter segmentation based on W-box position; (B): SlWRKY3 and TPK1b promoter yeast one hybridization. The yeast strain Y1HGold was transformed with the bait vector pAbAi-TPK1b and the prey vector SlWRKY3-AD (W3-AD) and plated on SD/-Leu-Ura medium containing different concentrations of aureobasidin A (AbA). The empty pGADT7 vector (AD) was used as a negative control.
2.5. SlWRKY3 and TPK1b Expression Was Negatively Correlated and SlWRKY3 Could Be Significantly Induced by B. cinerea
To clarify the regulatory relationship between SlWRKY3 and TPK1b, their relative expression levels in the leaves of SlWRKY3 transgenic lines were detected by RT-qPCR. TPK1b expression levels in W3-OE lines decreased significantly compared with WT, while in W3-Ri lines they increased significantly (Figure 5A,B), indicating that the TPK1b expression level was regulated by SlWRKY3 in tomato plants, and negatively correlated with it.
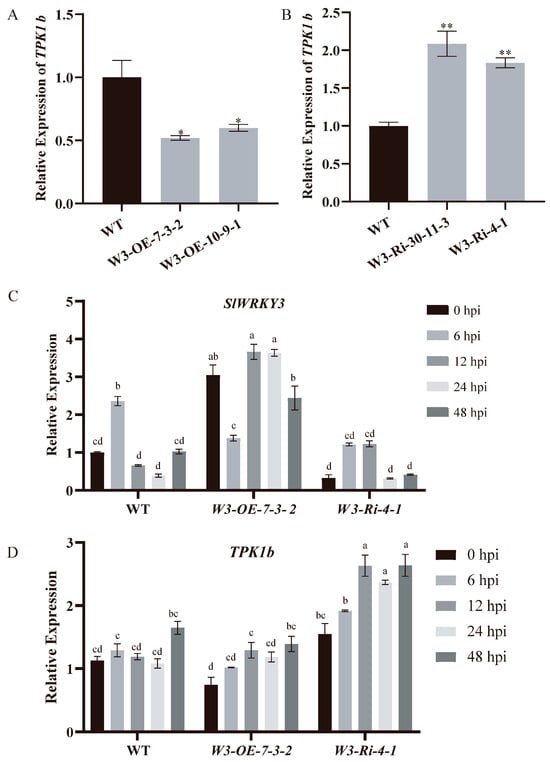
Figure 5.
SlWRKY3 and TPK1b expression levels: (A,B): Relationship between expression levels of SlWRKY3 and TPK1b in SlWRKY3 transgenic lines. (Dunnett-t test p * < 0.05, p ** < 0.01.) (C,D): Changes in expression in SlWRKY3 and TPK1b in SlWRKY3 transgenic lines inoculated with B. cinerea. The relative gene expression levels at 0, 6, 12, 24, and 48 h post inoculation (hpi) of B. cinerea were detected by real-time fluorescent quantitative PCR technology. Statistically significant differences were determined using a One-Way ANOVA. Different letters indicate statistically significant differences.
Both SlWRKY3 and TPK1b can change tomato resistance to B. cinerea, so we investigated whether their expression relationship changed due to B. cinerea infection. W3-OE, W3-Ri, and WT of the same age and size were sprayed with B. cinerea spores, and SlWRKY3 and TPK1b expression levels were detected in their leaves at 0, 6, 12, 24, and 48 h after inoculation (Figure 5C,D). In the WT and the W3-Ri line, the relative expression of SlWRKY3 exhibited a rapid increase at 6 h post inoculation with B. cinerea. In contrast, the relative expression of SlWRKY3 was observed to be repressed at 6 h post inoculation, followed by a significant increase in the W3-OE line. This pattern suggests that SlWRKY3 acts as an early-response gene to B. cinerea. Furthermore, TPK1b showed a pronounced induction of expression in both the W3-OE and W3-Ri lines at 12 h post inoculation with B. cinerea. Notably, the relative expression of TPK1b in the W3-Ri line was generally higher than that observed in the W3-OE line when inoculated with B. cinerea. TPK1b expression was induced at 48 h post inoculation with B. cinerea in the WT.
2.6. SlWRKY3 Can Regulate Downstream SA and ROS Signaling Pathways
To investigate which signal pathways are involved in TPK1b regulation by SlWRKY3 to change resistance to B. cinerea, we detected the expression levels of some signal pathway marker genes in W3-OE and W3-Ri plants at 0, 6, 12, 24, and 48 h after B. cinerea infection. These included hormone signaling pathways such as PI-I and PI-II of the JA signaling pathway, PR1 of the salicylic acid signaling pathway, ERF1b of the ethylene signaling pathway, and CAT1 and CAT2 of the reactive oxygen species (ROS) signaling pathway. PR1’s relative expression levels in the W3-OE line at 48 h after B. cinerea inoculation were significantly lower than in WT, while in the W3-Ri line they were significantly higher (Figure 6A). There was no obvious change in the expression levels of marker genes in the JA and ethylene signaling pathwayS (Figure 6B–D). In the ROS signaling pathway, CAT1’s relative expression levels in W3-OE lines were significantly higher than in W3-Ri lines and WT at 48 h after B. cinerea inoculation (Figure 6E). When the plants were not inoculated with B. cinerea, the relative expression level of the CAT2 gene in SlWRKY3 transgenic plants was significantly lower than that in WT. After inoculation with B. cinerea, there was no significant difference in CAT2’s relative expression level between SlWRKY3 transgenic plants and WT (Figure 6F). These results suggest that SlWRKY3 may regulate resistance to B. cinerea by regulating downstream SA and ROS signaling pathways, but whether SlWRKY3 regulates other pathways remains to be verified.
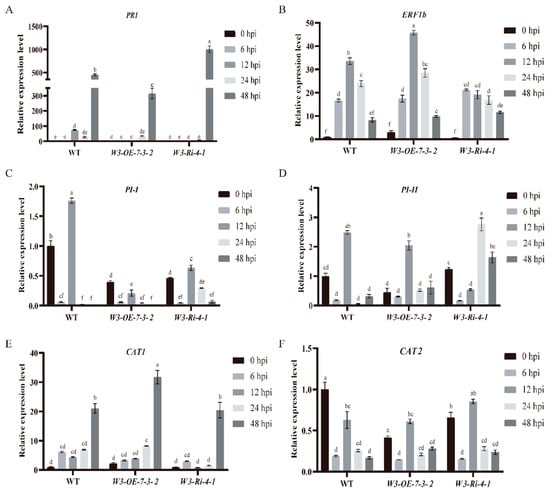
Figure 6.
Expression level detection of related genes in SlWRKY3 transgenic plants after B. cinerea inoculation. (A–F): PR1, ERF1b, PI-I, PI-II, CAT1, CAT2 expression levels. Different letters indicate significant differences in gene expression between different time points. The relative gene expression levels at 0, 6, 12, 24, and 48 h post inoculation (hpi) of B. cinerea were detected by real-time fluorescent quantitative PCR technology. Statistically significant differences were determined using a One-Way ANOVA. Different letters indicate statistically significant differences.
2.7. SlWRKY3 Regulates ROS Production
The immune response caused by pathogen infection can cause a ROS outbreak in plants, and then trigger a series of immune responses. However, ROS can promote infection by necrotic pathogens. To determine the effect of SlWRKY3 on ROS production, we used DAB staining to detect ROS production in WT, W3-OE and W3-Ri line leaves at 0, 12, 24, 48 and 72 h after B. cinerea infection. ROS produced in W3-OE plants was significantly higher than in WT at 48 and 72 h after being infected by B. cinerea, while in W3-Ri plants ROS was significantly lower (Figure 7). This indicates that SlWRKY3 could positively regulate ROS production after B. cinerea infection, and we speculate that this aggravates the hypersensitivity reaction caused by ROS, leading to more cell death, which is more conducive to further B. cinerea infection.
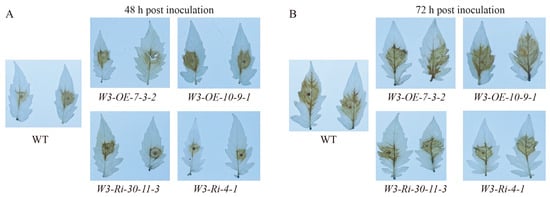
Figure 7.
Visualization of ROS production in SlWRKY3 transgenic plants after B. cinerea inoculation. The leaves of SlWRKY3 transgenic plants were inoculated with B. cinerea, and DAB staining was performed at 48 h (A) and 72 h (B) post inoculation.
3. Discussion
B. cinerea seriously threatens tomato growth, resulting in reduced or even no tomato harvest, and presently, there is a lack of B. cinerea-resistant tomato varieties. Although many studies have investigated tomato resistance to B. cinerea, little is known about its molecular mechanism. Therefore, it is crucial to further analyze the tomato resistance mechanism against B. cinerea in breeding and production. In 2008, the tomato TPK1b protein, a cytoplasmic receptor-like protein kinase localized in the plasma membrane, was induced by B. cinerea or other bacteria and stresses and shown to positively regulate tomato resistance to B. cinerea and insect infestation. Upstream of TPK1b, a tomato receptor kinase PORK1 can directly phosphorylate TPK1b and regulate its role in immune response and signal transduction [18,37]. To further explore the molecular mechanism, we cloned the TPK1b promoter and conducted a yeast one hybridization screening library experiment. We found that many transcription factors can bind to the TPK1b promoter, but not all of them can participate in the immune signal transduction pathway mediated by TPK1b. SlWRKY3 plays a regulatory role in TPK1b-mediated immune response, and it solely regulates tomato resistance to B. cinerea. According to the WRKY protein classification rules, the SlWRKY3 protein is an evolutionarily conserved type I WRKY transcription factor. Further studies have found that SlWRKY3 can bind to the TPK1b promoter through two W-boxes and have a regulatory effect on TPK1b transcription.
Certain plant WRKY proteins are known to be integral to the immune response in plants. The WRKY transcription factor RhWRKY30 can promote lignin biosynthesis, improve the resistance of rose petals to B. cinerea, and regulate the expression of RhCAD1 [38]. Similarly, RNA interference (RNAi) targeting FaWRKY29 and FaWRKY64 has been demonstrated to enhance the resistance of strawberries to B. cinerea. This is achieved by modulating ABA and JA signals, adjusting the composition of the plant cell wall, and regulating the expression of genes associated with ROS [39]. Furthermore, the overexpression of SlWRKY46 has been observed to suppress the expression of SA and JA marker genes, including PR1 and protease inhibitors (PI-I and PI-II), thereby negatively impacting tomato resistance to B. cinerea [40]. Additionally, SlWRKY3 has been identified as a positive regulator of tomato defense responses to nematode invasion and salt stress [35,41]. Herein, we discovered that the WRKY transcription factor SlWRKY3 is capable of binding to the TPK1b promoter, thereby regulating the immune response mediated by TPK1b and ultimately exerting a negative regulatory effect on tomato plant resistance to B. cinerea.
Salicylic acid (SA) is a plant hormone that plays an important role in plant immune response, and, more specifically, can mediate the innate antiviral immune response in plants [42]. SA has a close relationship with systemic acquired resistance (SAR) and pathogenesis-related proteins (PRs). SAR reactions are generally thought to be caused by increased PR protein expression [43]. Exogenous SA can stimulate the transfer of PR proteins to generate an SAR immune response. High SA accumulation in plants can enhance pathogen resistance and increase PR protein expression [44]. Conversely, when SA accumulation is blocked, the plant cannot normally produce the ETI or SAR immune response [45]. In plants, multiple WRKY transcription factor family members can participate in SA biosynthesis and SA-mediated immune responses [46]. PR1 is accumulated downstream of SA and is a marker gene in SA-mediated SAR response. Moreover, the WRKY transcription factor can regulate PR1 gene expression [47]. We found a significant difference in PR1 gene expression between SlWRKY3 overexpression and interference lines after B. cinerea inoculation. PR1 expression level in W3-OE lines decreased, while in W3-Ri lines it significantly increased. Therefore, we speculate that SlWRKY3 could participate in the SA hormone signaling pathway through PR1 expression regulation, and participate in the SAR immune response mediated by SA, thus achieving a negative regulatory effect on tomato’s defense against B. cinerea infection. However, since binding of SlWRKY3 and PR1 promoters has not been verified, it is still unclear whether SlWRKY3’s regulation of PR1 expression is a direct relationship.
Oxidation, as the second messenger, transmits immune signals and causes hypersensitivity reactions leading to cell death, thus resisting the invasion of some pathogens. However, ROS will weaken plant immunity to necrotic pathogens because cell death will accelerate their invasion. As a typical necrotic pathogen, B. cinerea has certain resistance to ROS outbreak, can promote its outbreak in host plants, and produce ROS by itself [48]. ROS in plant disease interactions mainly include superoxide anion O2-, hydroxyl free group ·OH, and hydrogen peroxide H2O2. CAT, as a catalase in plants, can catalyze the H2O2 decomposition generated by ROS outbreak. Elimination of excess H2O2 can reduce the plant cell damage and HR response caused by ROS [49]. We detected expression levels of the CAT marker genes CAT1 and CAT2 in the SlWRKY3 transgenic line after inoculation with B. cinerea, and found that the CAT1 expression level in SlWRKY3-OE was significantly higher than SlWRKY3-Ri. However, there was no significant difference in the relative expression level of CAT2 between SlWRKY3 transgenic plants and WT after B.cinerea inoculation, so it was impossible to determine how SlWRKY3 regulates ROS production after B. cinerea inoculation. DAB staining was performed on the ROS produced in SlWRKY3 transgenic plants after B. cinerea inoculation, and the ROS produced in SlWRKY3-OE after B. cinerea infection was significantly higher than in SlWRKY3-Ri. These results indicate that SlWRKY3 can positively regulate ROS production, thereby promoting an ROS-mediated hypersensitivity response in plant cells, which may also explain why SlWRKY3 negatively regulates tomato resistance to B. cinerea. The CAT1 expression level in W3-OE lines was higher than W3-Ri lines after B. cinerea inoculation. However, it is possible that there are other factors contributing to the increased expression of CAT1 in W3-OE lines, which may not necessarily lead to a reduction in ROS production.
Since the yeast interaction verified the binding of SlWRKY3 and TPK1b promoter, their regulatory relationship is a more concerning issue in this study. Based on the mode of tomato resistance to B. cinerea, we speculate that SlWRKY3 could negatively regulate TPK1b expression and transcription. However, there are other molecular substances upstream of TPK1b such as PORK1 that can regulate TPK1b expression [37]. SlWRKY3 may not only regulate TPK1b transcription through direct binding with the TPK1b promoter but also achieve indirect regulatory effects on TPK1b through interacting with other factors upstream of TPK1b or interacting with TPK1b itself. Overall, TPK1b expression was negatively correlated with SlWRKY3 in SlWRKY3 transgenic plants, but the regulatory mechanism of SlWRKY3 on TPK1b needs further study.
TPK1b has been reported to mediate the immune response of tomatoes to necrotic fungi through the ET pathway as a signaling component. We showed that the transcription factor SlWRKY3 not only acts upstream of TPK1b but also negatively regulates the immune response mediated by TPK1b. It can also regulate immune pathways such as SA and ROS in plants, thereby constructing an immune signal transduction network mediated by SlWRKY3 that negatively regulates tomato resistance to B. cinerea.
4. Materials and methods
4.1. Plant Materials
Tomato (Solanum lycopersicum L.) plants used in this experiment were planted in a greenhouse at 25 °C, 16 h/8 h day and night cycle, and 55% relative humidity. Using the tomato cultivar ‘Alisa Craig’ as the background material, we successfully achieved genetic transformation to obtain the SlWRKY3 transgenic material. SlWRKY3 transgenic materials were SlWRKY3 overexpression transgenic lines W3-OE-7-3-2 and W3-OE-10-9-1 and SlWRKY3 RNA interference transgenic lines W3-Ri-19-1-2 and W3-Ri-4-1.
4.2. Genetic Transformation
We successfully integrated the complete coding sequence of SlWRKY3 into the overexpression vector pHellsgate8, resulting in the construction of the SlWRKY3 overexpression vector (designated as W3-OE). Additionally, a 243-base-pair-specific fragment of SlWRKY3 was precisely inserted into the plasmid pHellsgate2, yielding the SlWRKY3 RNA interference vector (designated as W3-Ri). The primer sequences for constructing overexpression and RNA interference vectors are listed in Supplementary Table S1. Agrobacterium tumefaciens-mediated transformation was performed to generate SlWRKY3 transgenic plants [50]. Tomato cultivar ‘Alisa Craig’ (LA2838A) was used as the wild-type background. Tomato seeds were soaked in distilled water for 30 min and then decanted. Then, 75% alcohol was added, shaken for 30~60 s, and then decanted, and 2% sodium hypochlorite solution was added and shaken for 15 min, and then the seeds washed with sterile water 3 times. Cleaned seeds were inoculated on 1/2 MS medium and cultured for 6~8 days until two cotyledons had fully unfolded. Explants with 2–3 segments of cotyledons were cultured in the dark on KCMS medium for 1 day. The activated Agrobacterium line was cultured overnight, centrifuged at 3500 r·min−1 for 5 min, and then the supernatant was removed and suspended in 0.2 MS liquid medium. Pre-cultured cotyledons were transferred to 0.2 MS liquid medium for re-suspension. An appropriate amount of Agrobacterium tumefaciens was added for re-suspension, shaken, and infected for 4 min. It was then decanted and the bacteria liquid was thoroughly absorbed with sterile filter paper, and then the cotyledons were transferred to pre-culture medium for dark culture for 2 days. Cotyledons were then cultured on 2 Z medium for c.15 days, and after callus formation, they were cultured on 0.2 Z medium for redifferentiation and screening, and subcultured once for c.15 days. When adventitious buds had grown to 2~3 cm, they were transferred to rooting medium to induce rooting. Transplant transgenic seedlings were planted in nutrient soil, and cultured in the greenhouse.
4.3. RT-qPCR Detection and Analysis
SlWRKY3 transgenic line and control line (WT) seeds were evenly placed in a culture medium, covered with filter paper, and then transferred to a 28 °C incubator for germination. After germination, they were planted in plug trays, and transferred to the laboratory greening room. When the seedlings were c. 20 cm, B. cinerea was inoculated in vivo (i.e., sprayed with B. cinerea spore suspension), and the young leaves were taken and stored in liquid nitrogen at 0, 6, 12, 24, and 48 h after inoculation. RNA was extracted by Trizol reagent (Invitrogen, Waltham, MA, USA); cDNA was obtained by reverse transcription. Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to detect gene transcription levels, and Roche LightCycler 480 system was used to detect the relative gene expression at different time points after inoculation with B. cinerea [51]. The primer sequence design and experimental procedure for RT-qPCR are based on previous studies [52]. When designing RT-qPCR primers, we take into account that the amplification fragment is a specific segment of the gene, with the length of the amplified product ranging from 80 to 150 bp. The primers for RT-qPCR are listed in Supplementary Table S2. Actin gene (SGN-U580609) expression was used as an internal control. The reaction system, a total volume of 10 µL, was carefully assembled, consisting of a 5 µL SYBR Green master mix, 0.5 µL each of 10 µM of forward and reverse primers, and 4 µL of cDNA. The amplification protocol commenced with an initial denaturation at 95 °C for 60 s, succeeded by 45 cycles comprising denaturation at 95 °C for 10 s, annealing at 58 °C for 15 s, and extension at 72 °C for 20 s. The relative expression levels were quantified using the 2−△△Ct method [51].
4.4. Yeast One-Hybrid Assays (Y1H)
The full-length ERF118-like, SlWRKY3, JERF, B-box, and C3H4 were amplified with tomato cDNA as the template and inserted into pGADT7 to obtain the prey vector (ERF118-like, JERF, BPE-like, bHLH130-like, SlWRKY3-AD). Fragments of the TPK1b promoters were amplified with tomato genomic DNA as the template and cloned into pAbAi to obtain a bait vector (pAbAi-TPK1b). The primer list of pAbAi-TPK1b and different truncated pAbAi-TPK1b and SlWRKY3-AD is provided in Supplementary Table S1. The yeast one-hybrid assays were performed using the Matchmaker Gold Yeast One-Hybrid System as previously described [53]. The yeast strain Y1H Gold was cultured in 2 × YPDA liquid medium at 28 °C, and shaken at 200 r·min−1 on a shaking table for 14 h. Bacterial fluid was collected twice by centrifuging at 12,000 r·min−1 for 30 s. An amount of 2 g·L−1 carrier DNA was prepared at 100 °C for 5 min, then denatured and placed on ice for later use. In the centrifuge tube of step 1, 50% PEG3350 240 μL, 1 mol·L−1 LiAc 34 μL, 2 g·L−1 carrier DNA 50 μL, and plasmid 10 μL were added and then the tube was soaked in water at 42 °C for 90 min. The sample was centrifuged at 12000 r·min−1 for 1 min, and 500 μL ddH2O was added into the centrifuge tube. A 100 μL bacterial solution was absorbed and evenly coated on SD/-Leu-Ura medium, and cultured at 30 °C for 3 d. Plates were examined after 3 d.
4.5. Identification of B. cinerea Resistance in Transgenic Plants
Transgenic tomato plants and WT were inoculated with B. cinerea as previously described [6], and then the third and fourth compound leaves from the top were inoculated with 105 mL−1 suspension of B. cinerea spores. After 2~3 days, disease status was observed and photographed. Disease lesion size (mm2) was calculated using LA-S software (Hangzhou Wanshen Detection Technology Co., Ltd., China) and compared with WT. Then, plant resistance was analyzed.
4.6. Detection of Hydrogen Peroxide Content
Detached leaves of transgenic SlWRKY3 and WT were inoculated with B. cinerea. Leaf samples were collected at 48 h and 72 h after inoculation, and stored in DAB (3,3’-diaminobenzidine) solution for staining [54]. The accumulation of reactive oxygen species was observed. Bottles were sealed and kept at 25 °C for 12 to 24 h under dark conditions. During this time, the dye solution was shaken several times to ensure full contact with the leaves. After incubation, the leaves were removed and bathed in 95% ethanol and heated in boiling water until the leaf chlorophyll had completely faded. The grayish brown spots formed by the reaction with DAB on leaves were observed and compared.
4.7. Statistical Analysis
GraphPad prism 8.0, SPSS 26.0 and Microsoft Excel were used for statistical analysis. All experiments were repeated at least three times. Statistically significant differences were determined by one-way ANOVA. Data are reported as mean ± SE. * indicates p < 0.05, and ** indicates p < 0.01. Different lowercase letters indicate significant differences.
5. Conclusions
Herein, our research showed that SlWRKY3 plays a pivotal role in the regulation of tomato resistance to B. cinerea by repressing TPK1b transcription, which in turn affects the SA and ROS pathways. This regulatory mechanism provides valuable insights into the complex interactions between plants and pathogens and can inform strategies for developing tomatoes with improved resistance to B. cinerea. Understanding these molecular mechanisms can guide future breeding efforts and genetic engineering approaches to enhance crop resistance to diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13121597/s1, Table S1. List of primers used in this study; Table S2. qRT-PCR primer sequences used to quantify the expression genes.
Author Contributions
D.L. and T.W. conceived and designed the research. J.C., W.S. and G.H. performed experiments and fieldwork. J.C. and D.L. analyzed the data and wrote the manuscript. D.L., W.S., Q.Y. and T.W. advised the research and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32302585), the Science and Technology Planning Project of Guangxi (GuikeAA22068088-1), the Basic Research Program of Shanxi Province (202203021212423), the Shanxi Agricultural University Doctoral Research Startup Project (2021BQ116), and the Shanxi Province Excellent Doctoral Work Award-Scientific Research Project (SXBYKY2022063).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
All authors declare no competing interests.
References
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, L.; Liu, T.; Ma, J.; Huang, K.; Guo, H.; Huang, Y.; Zhang, L.; Zhao, J.; Tsuda, K.; et al. Suppression of ETI by PTI priming to balance plant growth and defense through an MPK3/MPK6-WRKYs-PP2Cs module. Mol. Plant 2023, 16, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 4463. [Google Scholar] [CrossRef] [PubMed]
- Tzipilevich, E.; Russ, D.; Dangl, J.L.; Benfey, P.N. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 2021, 29, 1507–1520.e4. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Sun, W.; Cai, J.; Hu, G.; Zhang, D.; Zhang, X.; Larkin, R.M.; Zhang, J.; Yang, C.; Ye, Z.; et al. SlBBX20 attenuates JA signalling and regulates resistance to Botrytis cinerea by inhibiting SlMED25 in tomato. Plant Biotechnol. J. 2023, 21, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.K.; Liang, G. Fe deficiency-induced ethylene synthesis confers resistance to Botrytis cinerea. New Phytol. 2023, 237, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Vyhnánek, T.; Zargar, S.M.; Kahraman, A.; Topal, A.; Gezgin, S. Exploring strigolactones for inducing abiotic stress tolerance in plants. Czech J. Genet. Plant Breed. 2024, 60, 55–69. [Google Scholar] [CrossRef]
- Daymi, C.; Ángel, G.-C.; Alexander, M. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar]
- Adam, Z.; Francisco, J.C.; Matteo, C.; Michael, W. CYSTEINE-RICH RECEPTOR-LIKE PROTEIN KINASES: Their evolution, structure, and roles in stress response and development. J. Exp. Bot. 2023, 74, 4910–4927. [Google Scholar]
- Qingfeng, Z.; Yanzhao, F.; Jiao, X.; Pei, C.; Aixia, Z.; Yang, Y. Advances in Receptor-like Protein Kinases in Balancing Plant Growth and Stress Responses. Plants 2023, 12, 427. [Google Scholar]
- Xiyu, M.; Lucas, A.N.C.; Michelle, E.L.; Kai, T.; Zhiping, W.; Jun, L.; Xiao, Y.; Bo, L.; Jinggeng, Z.; Daniel, V.S.; et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature 2020, 581, 199–203. [Google Scholar]
- Veronese, P.; Nakagami, H.; Bluhm, B.; Abuqamar, S.; Chen, X.; Salmeron, J.; Dietrich, R.A.; Hirt, H.; Mengiste, T. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 2006, 18, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Yu, L.; Zhou, Z.; Liang, X.; Liu, Z.; Cai, G.; Gao, L.; Zhang, X.; Wang, Y.; et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 2014, 15, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Laluk, K.; Luo, H.; Chai, M.; Dhawan, R.; Lai, Z.; Mengiste, T. Biochemical and genetic requirements for function of the immune response regulator BOTRYTIS-INDUCED KINASE1 in plant growth, ethylene signaling, and PAMP-triggered immunity in Arabidopsis. Plant Cell 2011, 23, 2831–2849. [Google Scholar] [CrossRef] [PubMed]
- Lal, N.K.; Nagalakshmi, U.; Hurlburt, N.K.; Flores, R.; Bak, A.; Sone, P.; Ma, X.; Song, G.; Walley, J.; Shan, L.; et al. The Receptor-like Cytoplasmic Kinase BIK1 Localizes to the Nucleus and Regulates Defense Hormone Expression during Plant Innate Immunity. Cell Host Microbe 2018, 23, 485–497.e5. [Google Scholar] [CrossRef] [PubMed]
- Abuqamar, S.; Chai, M.F.; Luo, H.; Song, F.; Mengiste, T. Tomato protein kinase 1b mediates signaling of plant responses to necrotrophic fungi and insect herbivory. Plant Cell 2008, 20, 1964–1983. [Google Scholar] [CrossRef] [PubMed]
- Podell, S.; Gribskov, M. Predicting N-terminal myristoylation sites in plant proteins. BMC Genom. 2004, 5, 37. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Qiu, D.; Yuan, J.; Yang, X. Comparison of cerato-platanin family protein BcSpl1 produced in Pichia pastoris and Escherichia coli. Protein Expr. Purif. 2017, 136, 20–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qiu, D.; Zeng, H.; Guo, L.; Yang, X. BcGs1, a glycoprotein from Botrytis cinerea, elicits defence response and improves disease resistance in host plants. Biochem. Biophys. Res. Commun. 2015, 457, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Guo, Z.J.; Wang, H.H.; Li, J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005, 12, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012, 287, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Talha, J.; San-Ji, G. WRKY transcription factors in plant defense. Trends Genet. 2023, 39, 787–801. [Google Scholar]
- Tianjiao, C.; Dan, Z.; Jie, Y.; Yunyan, H.; Hongcheng, W.; Lei, G.; Bin, Z.; Huinan, W.; Xuye, D. Full-Length Transcriptome Sequencing Analysis and Characterization of WRKY Transcription Factors Responsive to Cadmium Stress in Arabis paniculata. Plants 2023, 12, 3779. [Google Scholar]
- Oh, S.K.; Baek, K.H.; Park, J.M.; Yi, S.Y.; Yu, S.H.; Kamoun, S.; Choi, D. Capsicum annuum WRKY protein CaWRKY1 is a negative regulator of pathogen defense. New Phytol. 2008, 177, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.S.; Deslandes, L.; Auriac, M.C.; Marco, Y.; Somssich, I.E. The Arabidopsis transcription factor WRKY27 influences wilt disease symptom development caused by Ralstonia solanacearum. Plant J. 2008, 56, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Journot-Catalino, N.; Somssich, I.E.; Roby, D.; Kroj, T. The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 2006, 18, 3289–3302. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Vinod, K.; Zheng, Z.; Fan, B.; Chen, Z. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol. 2008, 8, 68. [Google Scholar] [CrossRef]
- Hu, L.; Ye, M.; Li, R.; Zhang, T.; Zhou, G.; Wang, Q.; Lu, J.; Lou, Y. The Rice Transcription Factor WRKY53 Suppresses Herbivore-Induced Defenses by Acting as a Negative Feedback Modulator of Mitogen-Activated Protein Kinase Activity. Plant Physiol. 2015, 169, 2907–2921. [Google Scholar]
- Jing, G.; Yao, Y.; An, L.; Cui, Y.; Bai, Y.; Li, X.; Yao, X.; Wu, K. Identification and functional analysis of the HvWRKY1 gene associated with Qingke (Hordeum vulgare L. var. nudum Hook. f.) leaf stripe disease. Czech J. Genet. Plant Breed. 2023, 59, 263–277. [Google Scholar] [CrossRef]
- Ishihama, N.; Yamada, R.; Yoshioka, M.; Katou, S.; Yoshioka, H. Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. Plant Cell 2011, 23, 1153–1170. [Google Scholar] [CrossRef] [PubMed]
- Birkenbihl, R.P.; Diezel, C.; Somssich, I.E. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012, 159, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hong, Y.B.; Zhang, Y.F.; Li, X.H.; Huang, L.; Zhang, H.J.; Li, D.Y.; Song, F.M. Tomato WRKY transcriptional factor SlDRW1 is required for disease resistance against Botrytis cinerea and tolerance to oxidative stress. Plant Sci. 2014, 227, 145–156. [Google Scholar] [CrossRef]
- Hichri, I.; Muhovski, Y.; Zizkova, E.; Dobrev, P.I.; Gharbi, E.; Franco-Zorrilla, J.M.; Lopez-Vidriero, I.; Solano, R.; Clippe, A.; Errachid, A.; et al. The Solanum lycopersicum WRKY3 Transcription Factor SlWRKY3 Is Involved in Salt Stress Tolerance in Tomato. Front. Plant Sci. 2017, 8, 1343. [Google Scholar] [CrossRef]
- Cui, J.; Xu, P.; Meng, J.; Li, J.; Jiang, N.; Luan, Y. Transcriptome signatures of tomato leaf induced by Phytophthora infestans and functional identification of transcription factor SpWRKY3. Theor. Appl. Genet. 2018, 131, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liao, C.J.; Jaiswal, N.; Lee, S.; Yun, D.J.; Lee, S.Y.; Garvey, M.; Kaplan, I.; Mengiste, T. Tomato PEPR1 ORTHOLOG RECEPTOR-LIKE KINASE1 Regulates Responses to Systemin, Necrotrophic Fungi, and Insect Herbivory. Plant Cell 2018, 30, 2214–2229. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Wang, Z.; Wang, H.; Gao, J.; Liu, X.; Zhang, Z. Transcription factors RhbZIP17 and RhWRKY30 enhance resistance to Botrytis cinerea by increasing lignin content in rose petals. J. Exp. Bot. 2024, 75, 1633–1646. [Google Scholar] [CrossRef]
- Lee, M.; Han, H.; Lee, S. The role of WRKY transcription factors, FaWRKY29 and FaWRKY64, for regulating Botrytis fruit rot resistance in strawberry (Fragaria × ananassa Duch.). BMC Plant Biol. 2023, 23, 420. [Google Scholar] [CrossRef]
- Shu, P.; Zhang, S.; Li, Y.; Wang, X.; Yao, L.; Sheng, J.; Shen, L. Over-expression of SlWRKY46 in tomato plants increases susceptibility to Botrytis cinerea by modulating ROS homeostasis and SA and JA signaling pathways. Plant Physiol. Biochem. 2021, 166, 1–9. [Google Scholar] [CrossRef]
- Chinnapandi, B.; Bucki, P.; Fitoussi, N.; Kolomiets, M.; Borrego, E.; Braun Miyara, S. Tomato SlWRKY3 acts as a positive regulator for resistance against the root-knot nematode Meloidogyne javanica by activating lipids and hormone-mediated defense-signaling pathways. Plant Signal. Behav. 2019, 14, 1601951. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.A.; Naqvi, R.Z.; Amin, I.; Mansoor, S. Salicylic acid-driven innate antiviral immunity in plants. Trends Plant Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Tang, Y.; Ding, W. NtPR1a regulates resistance to Ralstonia solanacearum in Nicotiana tabacum via activating the defense-related genes. Biochem. Biophys. Res. Commun. 2019, 508, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Choi, S.M.; Kang, M.J.; Yun, S.H.; Kwon, D.J.; Noh, Y.S.; Noh, B. Salicylic acid-induced transcriptional reprogramming by the HAC-NPR1-TGA histone acetyltransferase complex in Arabidopsis. Nucleic Acids Res. 2018, 46, 11712–11725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, T.; Jia, Z.; Jia, X.; Liu, Y.; Xuan, J.; Wang, G.; Zhang, F. Transcriptome Analysis Reveals a Comprehensive Virus Resistance Response Mechanism in Pecan Infected by a Novel Badnavirus Pecan Virus. Int. J. Mol. Sci. 2022, 23, 13576. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.S.; Peng, T.; Wang, M.; Liu, J.H. Genome-wide identification and comparative expression profiling of the WRKY transcription factor family in two Citrus species with different Candidatus Liberibacter asiaticus susceptibility. BMC Plant Biol. 2023, 23, 159. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.F.; Sheikh, A.H.; Haider, I.; Quareshy, M.; Linthorst, H.J.M. Arabidopsis WRKY50 and TGA Transcription Factors Synergistically Activate Expression of PR1. Front. Plant Sci. 2018, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- Schouten, A.; Tenberge, K.B.; Vermeer, J.; Stewart, J.; Wagemakers, L.; Williamson, B.; van Kan, J.A. Functional analysis of an extracellular catalase of Botrytis cinerea. Mol. Plant Pathol. 2002, 3, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29, 345–350. [Google Scholar] [CrossRef]
- Xiong, C.; Luo, D.; Lin, A.; Zhang, C.; Shan, L.; He, P.; Li, B.; Zhang, Q.; Hua, B.; Yuan, Z.; et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2019, 221, 279–294. [Google Scholar] [CrossRef]
- Wenbin, L.; Diva, C.T.; John, S.H.; Qi, H.; Yongping, D.; Lijuan, Z.; Jianchi, C.; Hong, L.; Silvio, L.; A Juliano, A.; et al. Development and systematic validation of qPCR assays for rapid and reliable differentiation of Xylella fastidiosa strains causing citrus variegated chlorosis. J. Microbiol. Methods 2012, 92, 79–89. [Google Scholar]
- Lizandra, P.-B.; Alejandro, G.-M.; Juan, J.C.; Belen, J.-J.; Belen, R.; Jesus, G.-L.; David, C.-G. Design and Validation of Primer Sets for the Detection and Quantification of Antibiotic Resistance Genes in Environmental Samples by Quantitative PCR. Microb. Ecol. 2024, 87, 71. [Google Scholar]
- Luo, D.; Xiong, C.; Lin, A.; Zhang, C.; Sun, W.; Zhang, J.; Yang, C.; Lu, Y.; Li, H.; Ye, Z.; et al. SlBBX20 interacts with the COP9 signalosome subunit SlCSN5-2 to regulate anthocyanin biosynthesis by activating SlDFR expression in tomato. Hortic. Res. 2021, 8, 163. [Google Scholar] [CrossRef]
- Md Ziaur, R.B.; Shyam, S.; Luis E, D.R.M.; Pawel, B.; Dilip K, L.; Aiming, Q.; Gazala, A.; Mohamed F R, K. Histopathological Investigation of Varietal Responses to Cercospora beticola Infection Process on Sugar Beet Leaves. Plant Dis. 2023, 107, 3906–3912. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).