Phytochemical Profiling and Bioactive Potential of Grape Seed Extract in Enhancing Salinity Tolerance of Vicia faba
Abstract
1. Introduction
2. Results
2.1. Identification of Phytochemical Components in Vitis vinifera Ethanolic Seed Extract
2.2. Nutrient Content and Antioxidant Capacity of Grape Seed Extract
2.3. Effects of Grape Seed Extract on Growth Parameters
2.4. Effects of Grape Seed Extract on Photosynthetic Activity
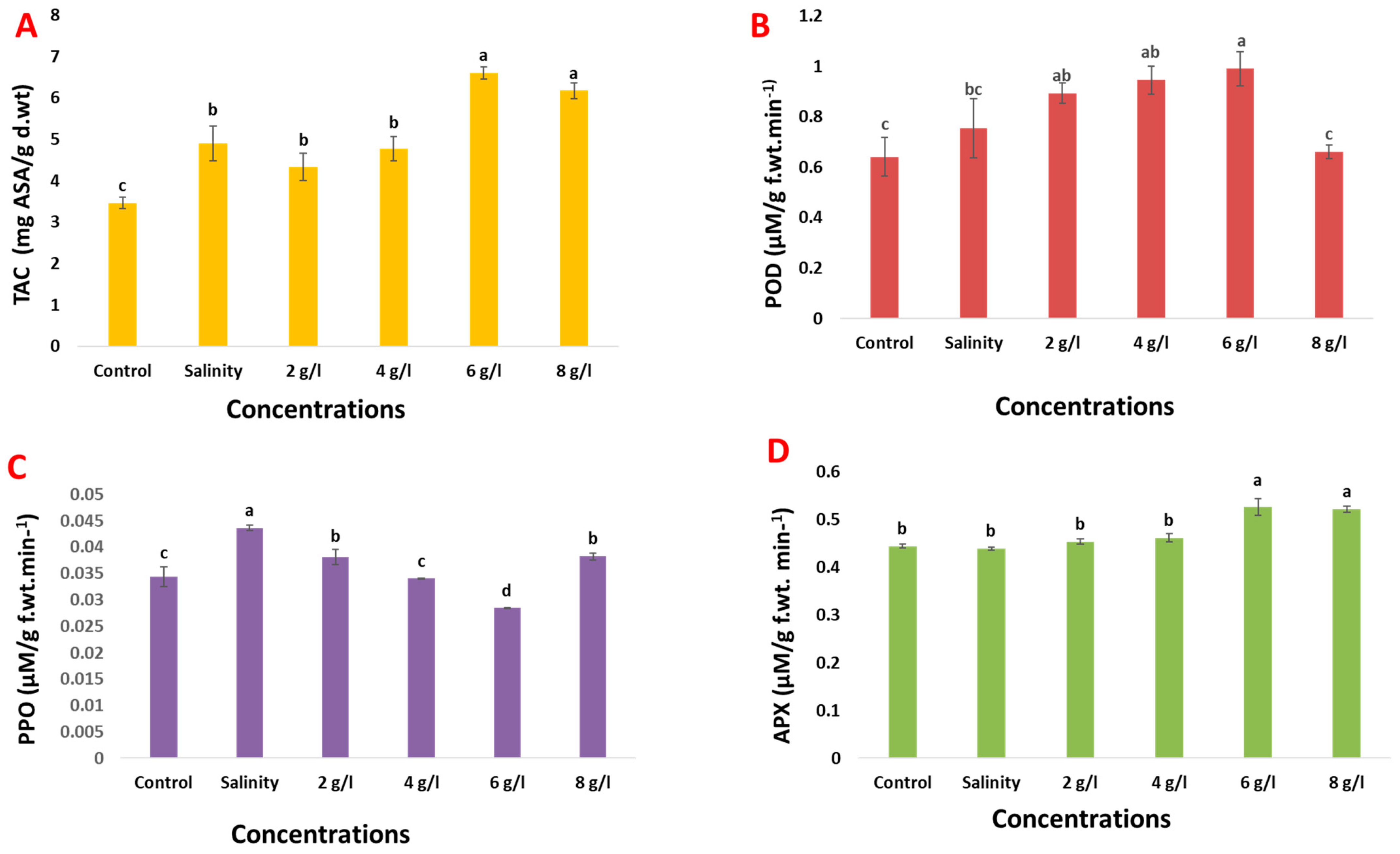
2.5. Effects of Grape Seed Extract on Oxidative Stress
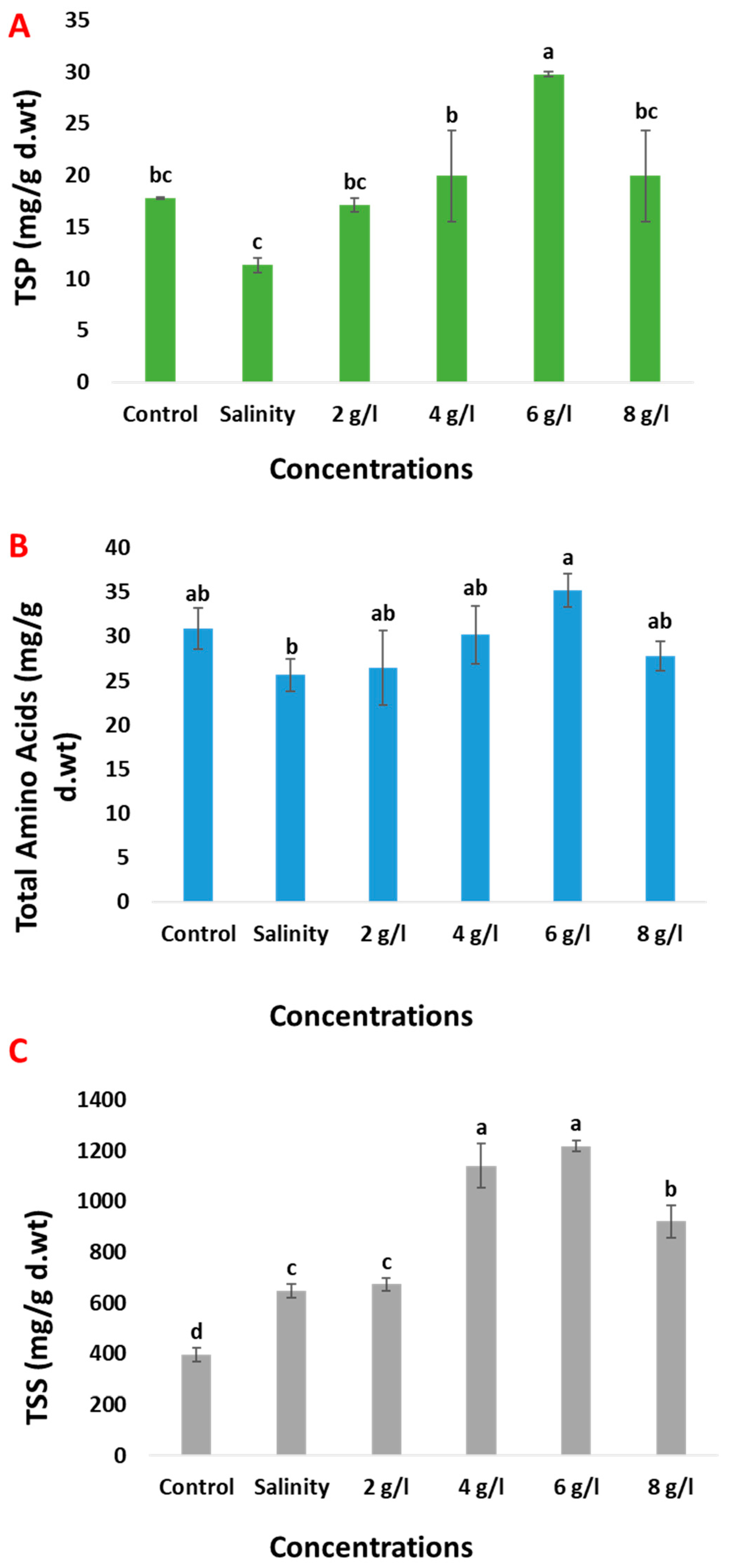
2.6. Effects of Grape Seed Extract on Osmolytes
2.7. Effects of Grape Seed Extract on Antioxidants
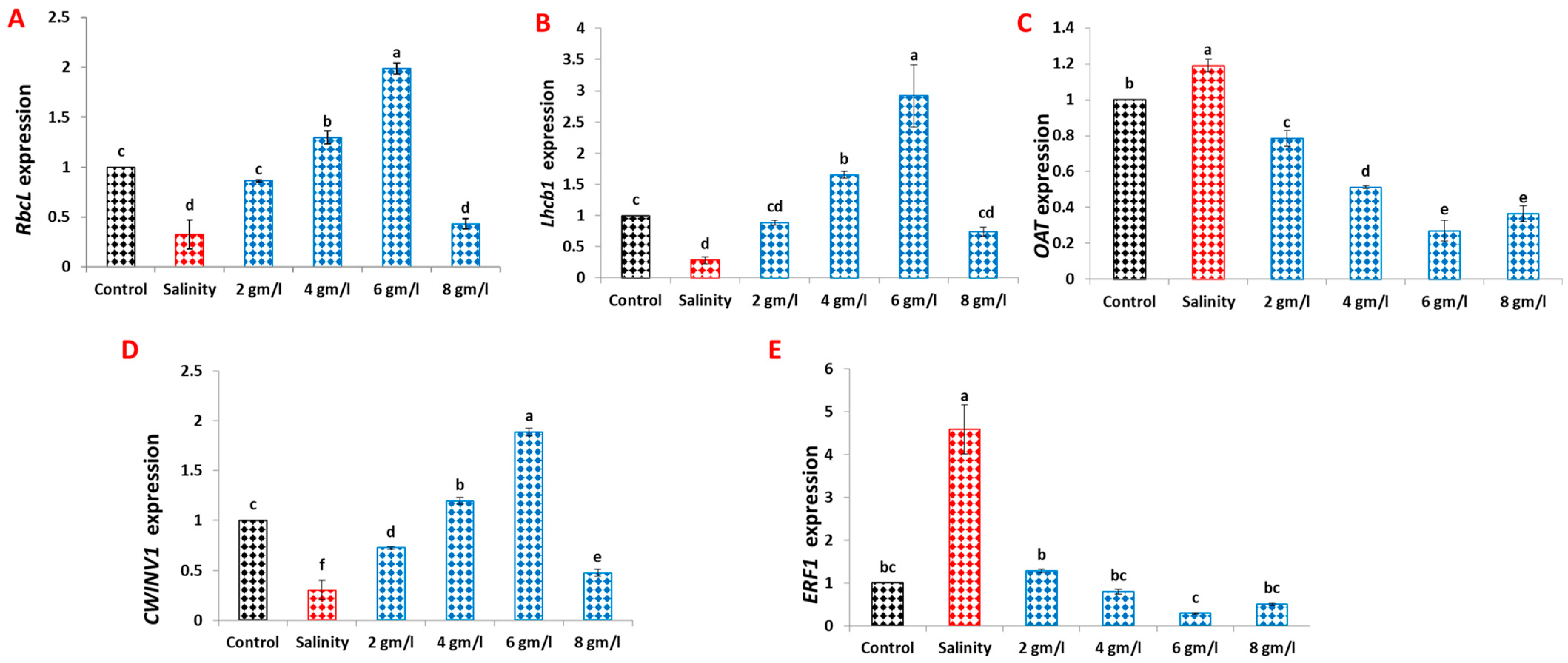
2.8. Effects of Grape Seed Extract on Relative Gene Expression
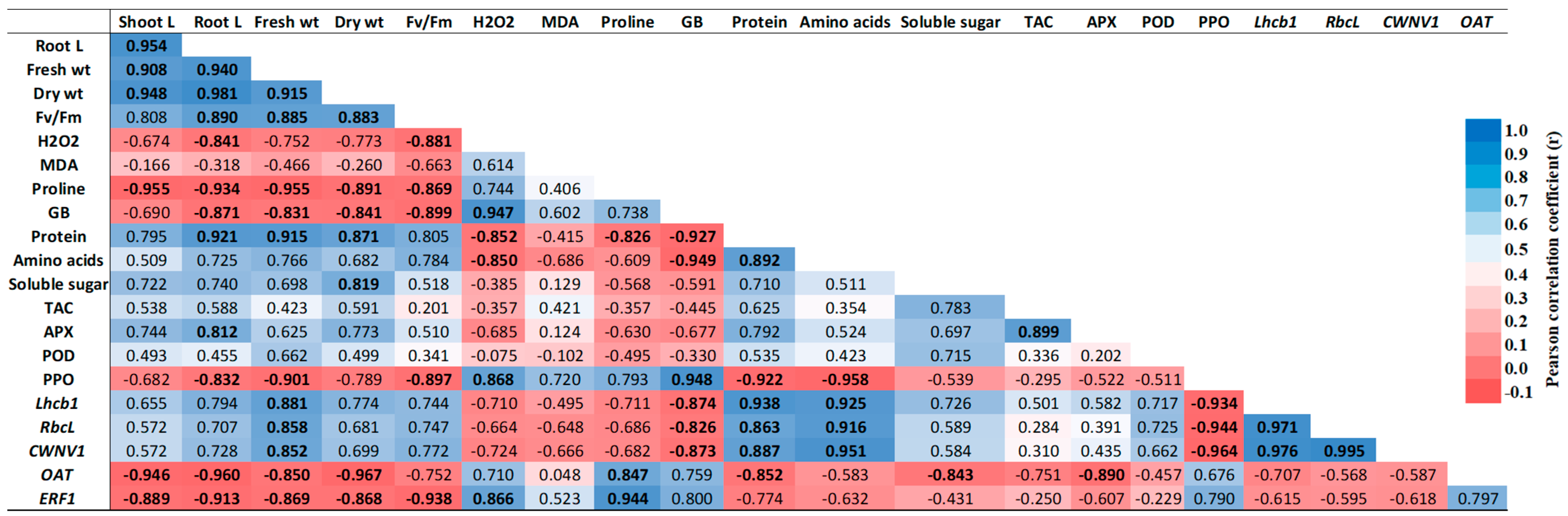
2.9. Correlation Analysis of Physiological and Biochemical Traits
3. Discussion
4. Materials and Methods
4.1. Preparation of Grape Seed Extract
4.2. Characterizations of Grape Seed Extract Using GC–MS Spectrometry
4.3. Experimental Design
4.4. Measurement of Photosynthetic Activity (Fv/Fm)
4.5. Measurement of Oxidative Stress Markers
4.6. Measurement of Osmo-Protectants
4.7. Measurement of Antioxidants
4.8. Determination of Peroxidase, Ascorbate Peroxidase and Polyphenol Oxidase
4.9. Expression Profiling of Selected Genes
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Choudhary, M.; Halder, T.; Prakash, N.R.; Singh, V.; Vineeth, T.V.; Sheoran, S.; Ravikiran, K.T.; Longmei, N.; Rakshit, S.; et al. Salinity Stress Tolerance and Omics Approaches: Revisiting the Progress and Achievements in Major Cereal Crops. Heredity 2022, 128, 497–518. [Google Scholar] [CrossRef]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura. Global Map of Salt Affected Soils; Organización de Las Naciones Unidas Para La Alimentación y La Agricultura: Rome, Italy, 1990. [Google Scholar]
- Zhang, X.; Liu, P.; Qing, C.; Yang, C.; Shen, Y.; Ma, L. Comparative Transcriptome Analyses of Maize Seedling Root Responses to Salt Stress. PeerJ 2021, 9, e10765. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Tamanadar, E.; Moradi Pour, M.; Thakur, V.K. Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It. Sustainability 2021, 13, 12758. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication between Plants and Plant Growth-Promoting Microorganisms under Stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Ullah, F.; Saqib, S.; Khan, W.; Ayaz, A.; Batool, A.; Wang, W.-Y.; Xiong, Y.-C. The Multifaceted Role of Sodium Nitroprusside in Plants: Crosstalk with Phytohormones under Normal and Stressful Conditions. Plant Growth Regul. 2024, 6, 1–8. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin Function and Crosstalk with Other Phytohormones under Normal and Stressful Conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef]
- Mahdi, A.H.A. Improvement of Salt Tolerance in Vicia faba (L.) Plants by Exogenous Application of Polyamines. Egypt. J. Agron. 2016, 38, 1–21. [Google Scholar] [CrossRef]
- Desouky, A.F.; Ahmed, A.H.H.; Reda, A.S.A.; Stȕtzel, H.; Hanafy, M.S. Physiological and Biochemical Responses of Two Faba Bean (Vicia faba L.) Varieties Grown in Vitro to Salt Stress. J. Crop Sci. Biotechnol. 2023, 26, 151–160. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Taha, S.S. Main Sulphur Content in Essential Oil of Eruca Sativa as Affected by Nano Iron and Nano Zinc Mixed with Organic Manure. Agriculture 2018, 64, 65–79. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; EL-Maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and Ammi Seed Extracts Modulate Antioxidant Defence System and Alleviate Salinity Stress in Cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of Yield, Nutritional and Nutraceutical Properties of Two Common Bean Cultivars Following the Application of Seaweed Extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef]
- Suryaman, M.; Sunarya, Y.; Istarimila, I.; Fudholi, A. Effect of Salinity Stress on the Growth and Yield of Mungbean (Vigna radiata (L.) R. Wilczek) Treated with Mangosteen Pericarp Extract. Biocatal. Agric. Biotechnol. 2021, 36, 102132. [Google Scholar] [CrossRef]
- Latif, H.H.; Mohamed, H.I. Exogenous Applications of Moringa Leaf Extract Effect on Retrotransposon, Ultrastructural and Biochemical Contents of Common Bean Plants under Environmental Stresses. S. Afr. J. Bot. 2016, 106, 221–231. [Google Scholar] [CrossRef]
- Latef, A.; Abu Alhmad, A.A.H.; Ahmad, M. Foliar Application of Fresh Moringa Leaf Extract Overcomes Salt Stress in Fenugreek (Trigonella foenum-graecum) Plants. Egypt. J. Bot. 2017, 57, 157–179. [Google Scholar]
- Merwad, A.-R.M. Effect of Humic and Fulvic Substances and Moringa Leaf Extract on Sudan Grass Plants Grown under Saline Conditions. Can. J. Soil Sci. 2017, 97, 703–716. [Google Scholar] [CrossRef]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grape Seed Oil: A Potential Functional Food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Haroutounian, S.A. Antilisterial Activities of Polyphenol-Rich Extracts of Grapes and Vinification Byproducts. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Ignelzi, S.; Shirley, J.R. A Comparison of Methods for Quantifying Oligomeric Proanthocyanidins from Grape Seed Extracts. Am. J. Enol. Vitic. 2000, 51, 383–389. [Google Scholar] [CrossRef]
- Hernandez-Jimenez, A.; Gomez-Plaza, E.; Martinez-Cutillas, A.; Kennedy, J.A. Grape Skin and Seed Proanthocyanidins from Monastrell × Syrah Grapes. J. Agric. Food Chem. 2009, 57, 10798–10803. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis vinifera Varieties Grown in a Warm Climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Matthäus, B. Virgin Grape Seed Oil: Is It Really a Nutritional Highlight? Eur. J. Lipid Sci. Technol. 2008, 110, 645–650. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia. Europe and the Nile Valley; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Ahmad, P.; Alyemeni, M.N.; Ahanger, M.A.; Egamberdieva, D.; Wijaya, L.; Alam, P. Salicylic Acid (SA) Induced Alterations in Growth, Biochemical Attributes and Antioxidant Enzyme Activity in Faba Bean (Vicia faba L.) Seedlings under NaCl Toxicity. Russ. J. Plant Physiol. 2018, 65, 104–114. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Migdadi, H.M.; Ammar, M.H.; Paull, J.G.; Siddique, K.H.M. Faba Bean Genomics: Current Status and Future Prospects. Euphytica 2012, 186, 609–624. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, A.M. Quercetin Mediated Salt Tolerance in Tomato through the Enhancement of Plant Antioxidant Defense and Glyoxalase Systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef]
- Abdel-Naime, W.A.; Fahim, J.R.; Fouad, M.A.; Kamel, M.S. Antibacterial, Antifungal, and GC–MS Studies of Melissa Officinalis. S. Afr. J. Bot. 2019, 124, 228–234. [Google Scholar] [CrossRef]
- Ali, Q.; Anwar, F.; Ashraf, M.; Saari, N.; Perveen, R. Ameliorating Effects of Exogenously Applied Proline on Seed Composition, Seed Oil Quality and Oil Antioxidant Activity of Maize (Zea mays L.) under Drought Stress. Int. J. Mol. Sci. 2013, 14, 818–835. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The Oxylipin Pathways: Biochemistry and Function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, H.; Takagi, K.; Hirashima, T.; Nishiyama, Y.; Wada, H. Long-Chain Saturated Fatty Acids, Palmitic and Stearic Acids, Enhance the Repair of Photosystem II. Int. J. Mol. Sci. 2020, 21, 7509. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Zhang, Y.; Bu, N.; Wang, S.H. Alleviation Effect of Alginate-Derived Oligosaccharides on Vicia Faba Root Tip Cells Damaged by Cadmium. Bull. Environ. Contam. Toxicol. 2010, 84, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sumayo, M.S.; Kwon, D.-K.; Ghim, S.-Y. Linoleic Acid-Induced Expression of Defense Genes and Enzymes in Tobacco. J. Plant Physiol. 2014, 171, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Wang, J.; Pan, J.; Li, H.; Wang, J.; Zhang, F.; Gao, Y. Effects of Sesame Seed Cake Allelochemicals on the Growth Cucumber (Cucumis sativus L. Cv. Jinchun 4). Allelopath. J. 2005, 16, 217–225. [Google Scholar]
- Scalschi, L.; Vicedo, B.; Camañes, G.; Fernandez-Crespo, E.; Lapeña, L.; González-Bosch, C.; García-Agustín, P. Hexanoic Acid Is a Resistance Inducer That Protects Tomato Plants against P Seudomonas syringae by Priming the Jasmonic Acid and Salicylic Acid Pathways. Mol. Plant Pathol. 2013, 14, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Janeeshma, E.; Arif, N.; Qudrat Ullah Farooqi, M.; Kumar, V.; Ansari, N.A.; Ghani, M.I.; Ahanger, M.A.; Hasanuzzaman, M. Understanding the Role of Beneficial Elements in Developing Plant Stress Resilience: Signalling and Crosstalk with Phytohormones and Microbes. Plant Stress 2023, 10, 100224. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of Salt Stress Effects on the Growth, Physio-Chemical Attributes and Yields of Phaseolus vulgaris L. Plants by the Combined Application of Salicylic Acid and Moringa Oleifera Leaf Extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Tomar, N.S.; Sharma, M.; Agarwal, R.M. Phytochemical Analysis of Jatropha Curcas L. during Different Seasons and Developmental Stages and Seedling Growth of Wheat (Triticum aestivum L.) as Affected by Extracts/Leachates of Jatropha curcas L. Physiol. Mol. Biol. Plants 2015, 21, 83–92. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Davy, A.J.; Fernández-Muñoz, F.; Castellanos, E.M.; Luque, T.; Figueroa, M.E. Growth and Photosynthetic Responses to Salinity of the Salt-Marsh Shrub Atriplex portulacoides. Ann. Bot. 2007, 100, 555–563. [Google Scholar] [CrossRef]
- Rady, M.O.A.; Semida, W.M.; Abd El-Mageed, T.A.; Hemida, K.A.; Rady, M.M. Up-Regulation of Antioxidative Defense Systems by Glycine Betaine Foliar Application in Onion Plants Confer Tolerance to Salinity Stress. Sci. Hortic. 2018, 240, 614–622. [Google Scholar] [CrossRef]
- Yaseen, R.; Aziz, O.; Saleem, M.H.; Riaz, M.; Zafar-ul-Hye, M.; Rehman, M.; Ali, S.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A.; et al. Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry. Sustainability 2020, 12, 6022. [Google Scholar] [CrossRef]
- Aazami, M.A.; Rasouli, F.; Ebrahimzadeh, A. Oxidative Damage, Antioxidant Mechanism and Gene Expression in Tomato Responding to Salinity Stress under in Vitro Conditions and Application of Iron and Zinc Oxide Nanoparticles on Callus Induction and Plant Regeneration. BMC Plant Biol. 2021, 21, 597. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Abolhassani, M.; Hadian-Deljou, M.; Feyzi, H.; Akbari, A.; Rasouli, F.; Koçak, M.Z.; Kulak, M.; Gohari, G. Proline-Functionalized Graphene Oxide Nanoparticles (GO-Pro NPs): A New Engineered Nanoparticle to Ameliorate Salinity Stress on Grape (Vitis vinifera L. Cv Sultana). Plant Stress 2023, 7, 100128. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Bulgaru, V.C.; Anghel, A.C.; Sărăcilă, M.; Vlassa, M.; Filip, M.; Taranu, I. Grape Seed Meal By-Product Is Able to Counteract Oxidative Stress Induced by Lipopolysaccharide and Dextran Sulphate in IPEC Cells and Piglets after Weaning. PLoS ONE 2023, 18, e0283607. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Blasco, B.; Martos, V. Combating Salinity through Natural Plant Extracts Based Biostimulants: A Review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Magaña, C.; Aguilar-Caamal, L.F.; Echevarría-Machado, I.; Medina-Lara, F.; Cach, L.S.; Martínez-Estévez, M. Contribution of Glycine Betaine and Proline to Water Deficit Tolerance in Pepper Plants. HortScience 2019, 54, 1044–1054. [Google Scholar] [CrossRef]
- Malekzadeh, P. Influence of Exogenous Application of Glycinebetaine on Antioxidative System and Growth of Salt-Stressed Soybean Seedlings (Glycine max L.). Physiol. Mol. Biol. Plants 2015, 21, 225–232. [Google Scholar] [CrossRef]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of Proline in Plants under Contaminated Soils-Are We on the Same Page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B. Effects of Salt on Growth, Ion Accumulation, Photosynthesis and Leaf Anatomy of the Mangrove, Bruguiera Parviflora. Trees 2004, 18, 167–174. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Effects of NaCl Stress on Nitrogen and Phosphorous Metabolism in a True Mangrove Bruguiera parviflora Grown under Hydroponic Culture. J. Plant Physiol. 2004, 161, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and Crop Responses: A Review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.A. Mitigation of Salinity Stress Effects on Growth, Yield and Nutrient Uptake of Wheat by Application of Organic Extracts. Commun. Soil Sci. Plant Anal. 2020, 51, 1150–1160. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global Plant-Responding Mechanisms to Salt Stress: Physiological and Molecular Levels and Implications in Biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Alharby, H.F.; Bamagoos, A.A.; Abdelhamid, M.T.; Rady, M.M. Sequenced Application of Glutathione as an Antioxidant with an Organic Biostimulant Improves Physiological and Metabolic Adaptation to Salinity in Wheat. Plant Physiol. Biochem. 2021, 158, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Bishi, S.K.; Goswami, N.; Singh, A.L.; Zala, P.V. Differential Fine-Regulation of Enzyme Driven ROS Detoxification Network Imparts Salt Tolerance in Contrasting Peanut Genotypes. Environ. Exp. Bot. 2016, 128, 79–90. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Oyeniyi, T.A.; Odekanyin, O.O.; Kuku, A.; Otusanya, O.O. Allelopathic Effects of Tithonia diversifolia Extracts on Biochemical Parameters and Growth of Vigna unguiculata. Int. J. Biol. 2016, 8, 45. [Google Scholar] [CrossRef]
- Asha Devi, S.; Sagar Chandrasekar, B.K.; Manjula, K.R.; Ishii, N. Grape Seed Proanthocyanidin Lowers Brain Oxidative Stress in Adult and Middle-Aged Rats. Exp. Gerontol. 2011, 46, 958–964. [Google Scholar] [CrossRef]
- Demir, Y.; Kocaçalişkan, I. Effects of NaCl and Proline on Polyphenol Oxidase Activity in Bean Seedlings. Biol. Plant. 2001, 44, 607–609. [Google Scholar] [CrossRef]
- Thipyapong, P.; Hunt, M.D.; Steffens, J.C. Antisense Downregulation of Polyphenol Oxidase Results in Enhanced Disease Susceptibility. Planta 2004, 220, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant Responses to Stresses: Role of Ascorbate Peroxidase in the Antioxidant Protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. Tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Howladar, S.M. A Novel Moringa Oleifera Leaf Extract Can Mitigate the Stress Effects of Salinity and Cadmium in Bean (Phaseolus vulgaris L.) Plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef]
- Jansson, S. A Guide to the Lhc Genes and Their Relatives in Arabidopsis. Trends Plant Sci. 1999, 4, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Sun, J.; Liu, L.; He, W.; Zhao, B. Transcriptome Analysis of Differentially Expressed Genes in Wild Jujube Seedlings under Salt Stress. J. Am. Soc. Hortic. Sci. 2020, 145, 174–185. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Shi, X.; Qian, W.; Mehmood, J.; Yin, Y.; Jia, H. Identification of the Light-Harvesting Chlorophyll a/b Binding Protein Gene Family in Peach (Prunus persica L.) and Their Expression under Drought Stress. Genes 2023, 14, 1475. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, C. Effects of Salt Stress on Seed Germination, Photosynthetic Characteristics, Chlorophyll Fluorescence and Metabolism of Inorganic Ions of Coleus scutellarioides. J. West China For. Sci. 2018, 47, 78–84. [Google Scholar]
- Xu, Y.-H.; Liu, R.; Yan, L.; Liu, Z.-Q.; Jiang, S.-C.; Shen, Y.-Y.; Wang, X.-F.; Zhang, D.-P. Light-Harvesting Chlorophyll a/b-Binding Proteins Are Required for Stomatal Response to Abscisic Acid in Arabidopsis. J. Exp. Bot. 2012, 63, 1095–1106. [Google Scholar] [CrossRef]
- Vitlin Gruber, A.; Feiz, L. Rubisco Assembly in the Chloroplast. Front. Mol. Biosci. 2018, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xia, W.; Wei, S.; Zhang, M.; Wang, W.; Zeng, D.; Liu, M.; Sun, Y.; Lu, W. Photosynthetic Performance of Rice Seedlings Originated from Seeds Exposed to Spaceflight Conditions. Photochem. Photobiol. 2019, 95, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Makino, A. Translational Downregulation of RBCL Is Operative in the Coordinated Expression of Rubisco Genes in Senescent Leaves in Rice. J. Exp. Bot. 2013, 64, 1145–1152, Erratum in J. Exp. Bot. 2018, 69, 3171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Shibata, S.; Goto, E. Time-Course of Changes in Photosynthesis and Secondary Metabolites in Canola (Brassica napus) under Different UV-B Irradiation Levels in a Plant Factory with Artificial Light. Front. Plant Sci. 2021, 12, 786555. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.-A.; Ruan, Y.-L. Posttranslational Elevation of Cell Wall Invertase Activity by Silencing Its Inhibitor in Tomato Delays Leaf Senescence and Increases Seed Weight and Fruit Hexose Level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Sellami, S.; Le Hir, R.; Thorpe, M.R.; Vilaine, F.; Wolff, N.; Brini, F.; Dinant, S. Salinity Effects on Sugar Homeostasis and Vascular Anatomy in the Stem of the Arabidopsis thaliana Inflorescence. Int. J. Mol. Sci. 2019, 20, 3167. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Albacete, A.; Martínez-Andújar, C.; Acosta, M.; Romero-Aranda, R.; Dodd, I.C.; Lutts, S.; Pérez-Alfocea, F. Hormonal Changes during Salinity-Induced Leaf Senescence in Tomato (Solanum lycopersicum L.). J. Exp. Bot. 2008, 59, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Balibrea Lara, M.E.; Gonzalez Garcia, M.-C.; Fatima, T.; Ehness, R.; Lee, T.K.; Proels, R.; Tanner, W.; Roitsch, T. Extracellular Invertase Is an Essential Component of Cytokinin-Mediated Delay of Senescence. Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; She, M.; Wang, K.; Riaz, B.; Ye, X. Biological Roles of Ornithine Aminotransferase (OAT) in Plant Stress Tolerance: Present Progress and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 3681. [Google Scholar] [CrossRef]
- Stránská, J.; Kopečný, D.; Tylichová, M.; Snégaroff, J.; Šebela, M. Ornithine δ-Aminotransferase: An Enzyme Implicated in Salt Tolerance in Higher Plants. Plant Signal. Behav. 2008, 3, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Roosens, N.H.C.J.; Thu, T.T.; Iskandar, H.M.; Jacobs, M. Isolation of the Ornithine-δ-Aminotransferase cDNA and Effect of Salt Stress on Its Expression in Arabidopsis thaliana. Plant Physiol. 1998, 117, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fan, Z.; Guo, L.; Li, Y.; Zhang, W.; Qu, L.J.; Chen, Z. Over-Expression of an Arabidopsis δ-OAT Gene Enhances Salt and Drought Tolerance in Transgenic Rice. Chin. Sci. Bull. 2003, 48, 2594–2600. [Google Scholar] [CrossRef]
- Wu, D.; Ji, J.; Wang, G.; Guan, C.; Jin, C. LchERF, a Novel Ethylene-Responsive Transcription Factor from Lycium chinense, Confers Salt Tolerance in Transgenic Tobacco. Plant Cell Rep. 2014, 33, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Fujisawa, M.; Shima, Y.; Ito, Y. The AP2/ERF Transcription Factor SlERF52 Functions in Flower Pedicel Abscission in Tomato. J. Exp. Bot. 2014, 65, 3111–3119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different Cis-Acting Elements in Response to Different Stress Signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Sommers, L.E. Chemical Analysis of Ecological Materials: By S. e. Allen, H. m. Grimshaw, J. a. Parkinson, and C. Quarmby. 1974. John Wiley & Sons, Inc., New York, NY 10016. 565 p. $39.95. J. Environ. Qual. 1976, 5, 494. [Google Scholar] [CrossRef]
- Gonçalves, J.F.d.C.; dos Santos Júnior, U.M. Utilization of the Chlorophyll a Fluorescence Technique as a Tool for Selecting Tolerant Species to Environments of High Irradiance. Braz. J. Plant Physiol. 2005, 17, 307–313. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198, reprinted in Arch. Biochem. Biophys. 2022, 726, 109248. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A.J.P.S. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid Assay for Determination of Water Soluble Quaternary Ammonium Compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Lee, Y.P.; Takahashi, T. An Improved Colorimetric Determination of Amino Acids with the Use of Ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Naguib, S.M.; Geiser, P.B.; Comstock, G.W. Response to a Program of Screening for Cervical Cancer. Public Health Rep. 1968, 83, 990–998. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C.; Logan, B.A. Acclimation of Foliar Antioxidant Systems to Growth Irradiance in Three Broad-Leaved Evergreen Species. Plant Physiol. 1996, 112, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Zhou, X.; Xiao, Y.; Meng, X.; Liu, B. Full Inhibition of Whangkeumbae Pear Polyphenol Oxidase Enzymatic Browning Reaction by L-Cysteine. Food Chem. 2018, 266, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Liu, C.L.; Yu, X.J.; Yang, J.D.; Feng, C.N. Screening of Reference Genes for Differentially Expressed Genes in Pyrus betulaefolia Plant under Salt Stress by qRT-PCR. Acta Hortic. Sin. 2022, 49, 1557–1570. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
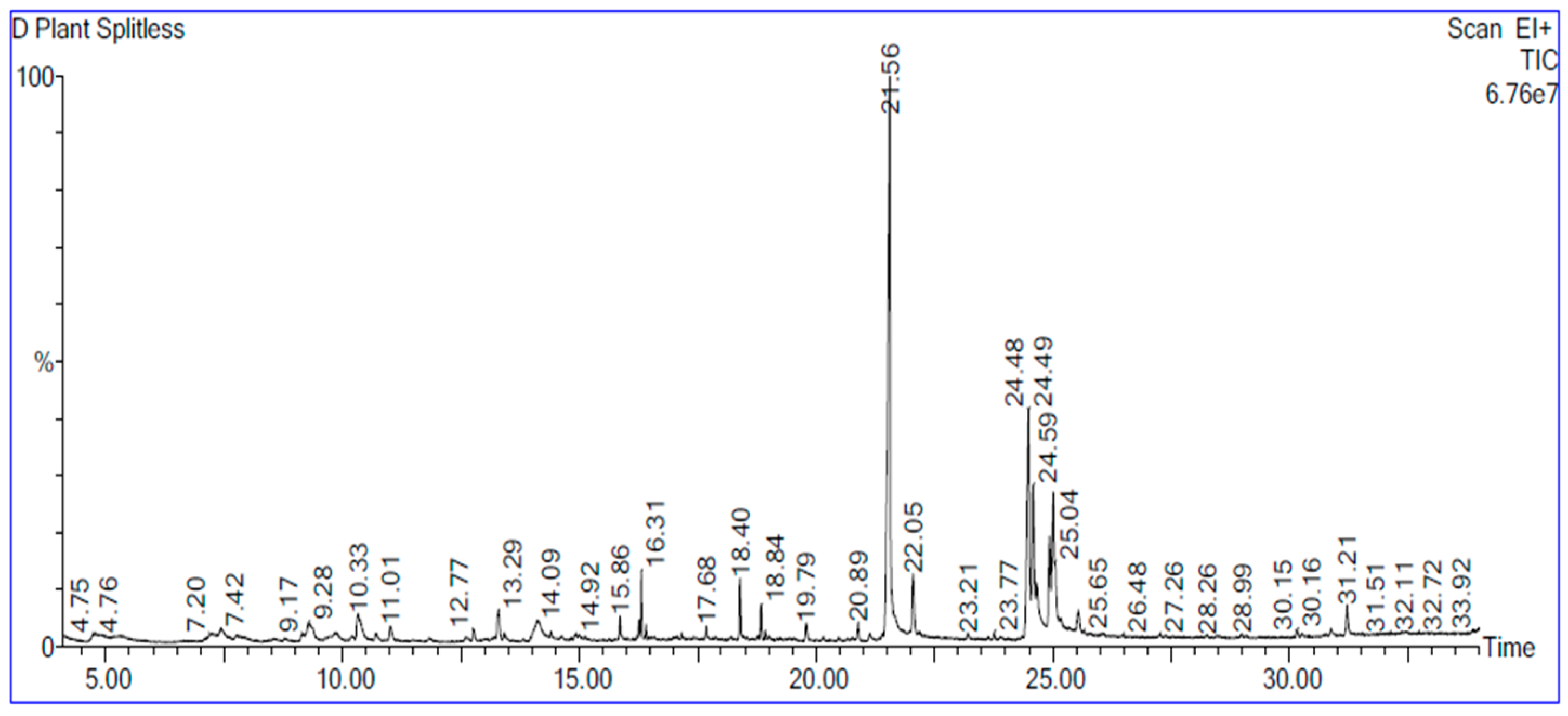




| NO | Compound Name | Molecular Formula | RT | Area % |
|---|---|---|---|---|
| 1. | Hexanoic acid | C6H12O2 | 4.799 | 1.690 |
| 2. | Octanoic Acid | C8H16O2 | 9.280 | 2.065 |
| 3. | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | C₆H₆O₃ | 10.331 | 2.203 |
| 4. | à-D-Glucopyranoside, O-à-D-glucopyranosyl-(1.fwdarw.3)-á-D-fructofuranosyl | C18H32O16 | 14.122 | 3.056 |
| 5. | n-Hexadecanoic acid | C₁₆H₃₂O₂ | 21.560 | 26.350 |
| 6. | Hexadecanoic acid, ethyl ester | C18H36O2 | 22.051 | 2.263 |
| 7. | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 24.492 | 7.442 |
| 8. | Hexadecenoic acid, Z-11- | C16H30O2 | 24.587 | 3.754 |
| 9. | Linoleic acid ethyl ester | C20H36O2 | 24.932 | 2.586 |
| 10. | Octadecanoic acid | C18H36O2 | 25.007 | 6.560 |
| Parameters | Results | Unit |
|---|---|---|
| N ions | 15.13 ± 0.4 | mg/g DM |
| P ions | 1.4 ± 0.4 | mg/g DM |
| Ca ions | 18.29 ± 0.1 | mg/g DM |
| Mg ions | 5.34 ± 0.1 | mg/g DM |
| Total antioxidant capacity | 9.85 ± 0.6 | mg ASA/g DM |
| Gene Name | Abbreviation | Forward (F) and Reverse (R) Primer 5′–3′ |
|---|---|---|
| Reference gene | GAPDH | F: 5′-AAGGTTATCAACGACAGGTTTG-3′ R: 5′-ATACCCTTAAGCTTGCCTTCTG-3′ |
| Chlorophyll a/b-binding protein of LHCII type 1-like (LOC114182484) | Lhcb1 | F: 5′-GGCTTTTGCTGAGTTGAAGG-3′ R: 5′-GTAAGCCCAGGCATTGTTGT-3′ |
| Ribulose bisphosphate carboxylase large chain-like. Number in gene bank (X01167) | RbcL | F: 5′-CTTGGTACCATCCAACCAATTCA-3′ R: 5′-GCTTGGAACCCAACCTTTGC-3′ |
| Cell wall Invertase I Number in gene bank (Z35162) | CWINV1 | F: 5′-GGGTTGGACCGTTTGGACTT-3′ R: 5′-CACGCCCGATTAAAACCATACT-3′ |
| Ornithine aminotransferase (loc107482012) | OAT | F: 5′-GAATACTGGCGCTGAAGGTGTG-3′ R: 5′-AGATGGCCAGGCAATAAAGGAC-3′ |
| Ethylene-responsive transcription Factor 1 Number in gene bank (EU543659) | ERF1 | F: 5′-TGCTGCTTTTCATTTTCGTG-3′ R: 5′-AGGCGCTGTAAGAGGCATAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsherif, D.E.; Safhi, F.A.; Subudhi, P.K.; Shaban, A.S.; El-Esawy, M.A.; Khalifa, A.M. Phytochemical Profiling and Bioactive Potential of Grape Seed Extract in Enhancing Salinity Tolerance of Vicia faba. Plants 2024, 13, 1596. https://doi.org/10.3390/plants13121596
Elsherif DE, Safhi FA, Subudhi PK, Shaban AS, El-Esawy MA, Khalifa AM. Phytochemical Profiling and Bioactive Potential of Grape Seed Extract in Enhancing Salinity Tolerance of Vicia faba. Plants. 2024; 13(12):1596. https://doi.org/10.3390/plants13121596
Chicago/Turabian StyleElsherif, Doaa E., Fatmah A. Safhi, Prasanta K. Subudhi, Abdelghany S. Shaban, Mai A. El-Esawy, and Asmaa M. Khalifa. 2024. "Phytochemical Profiling and Bioactive Potential of Grape Seed Extract in Enhancing Salinity Tolerance of Vicia faba" Plants 13, no. 12: 1596. https://doi.org/10.3390/plants13121596
APA StyleElsherif, D. E., Safhi, F. A., Subudhi, P. K., Shaban, A. S., El-Esawy, M. A., & Khalifa, A. M. (2024). Phytochemical Profiling and Bioactive Potential of Grape Seed Extract in Enhancing Salinity Tolerance of Vicia faba. Plants, 13(12), 1596. https://doi.org/10.3390/plants13121596







