Frost Damage Mitigation in Flowers and Fruitlets of Peach and Almond from the Application of a Multi-Attribute Approach Biostimulant
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Trial Location
2.2. Treatments and Experimental Design
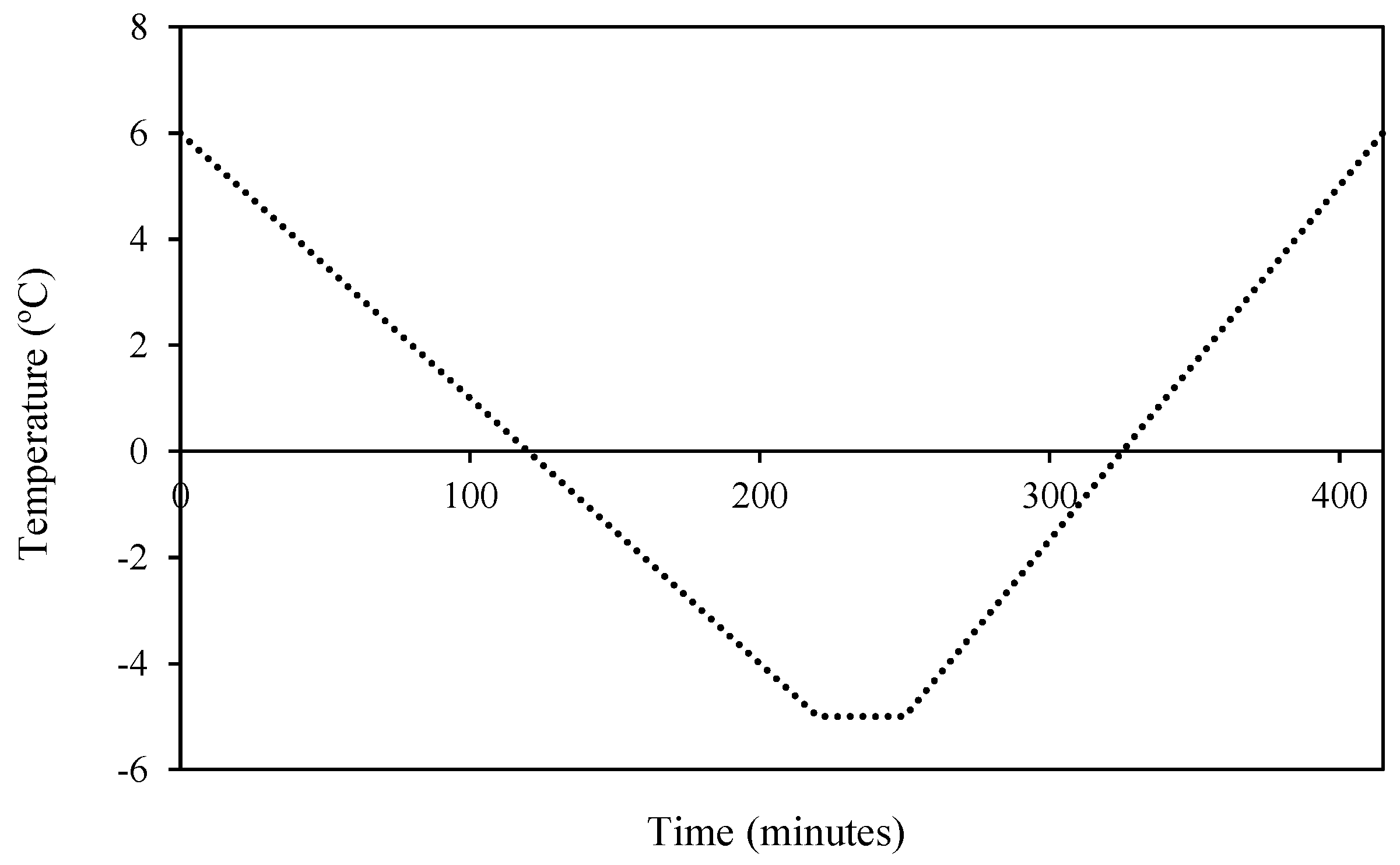
2.3. Frost Cycles Simulations
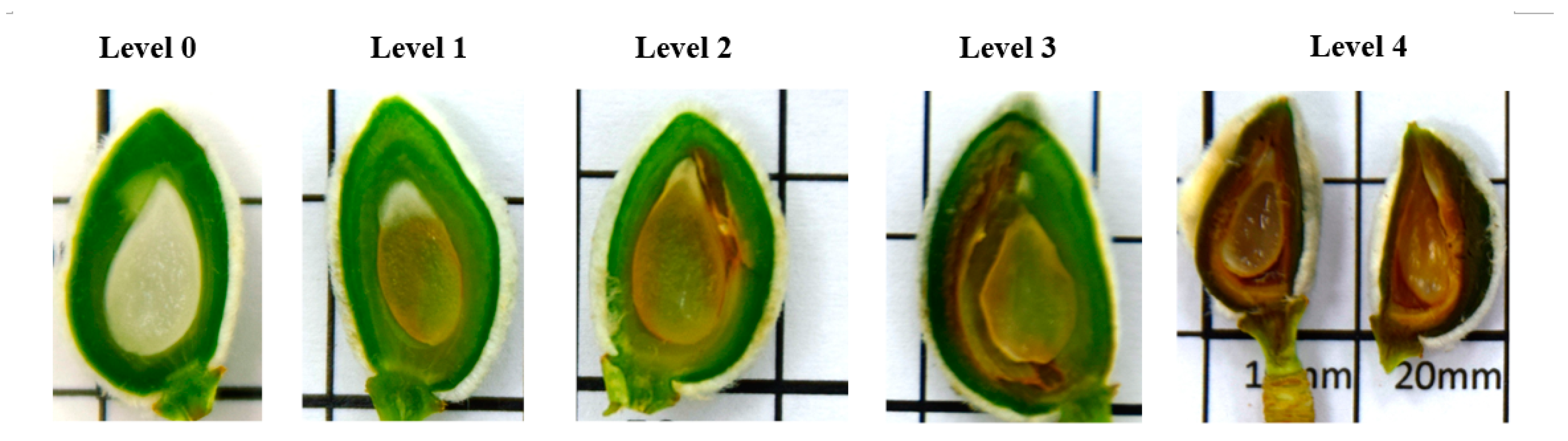
2.4. Frost Damage Evaluation
2.5. Statistical Analysis
3. Results
3.1. Experiment 1
3.1.1. Peach Blossom
3.1.2. Almond Blossom
3.2. Experiment 2
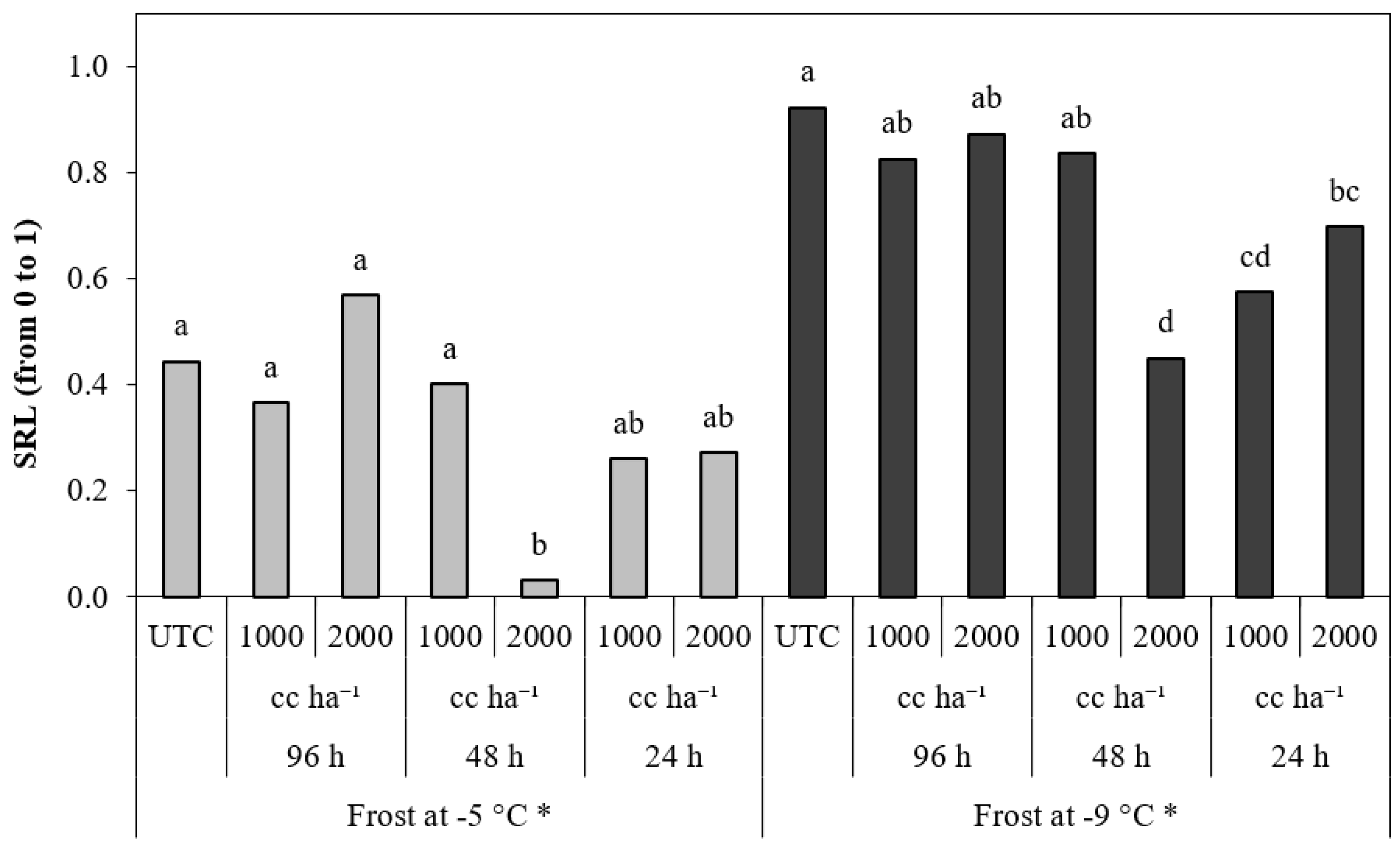
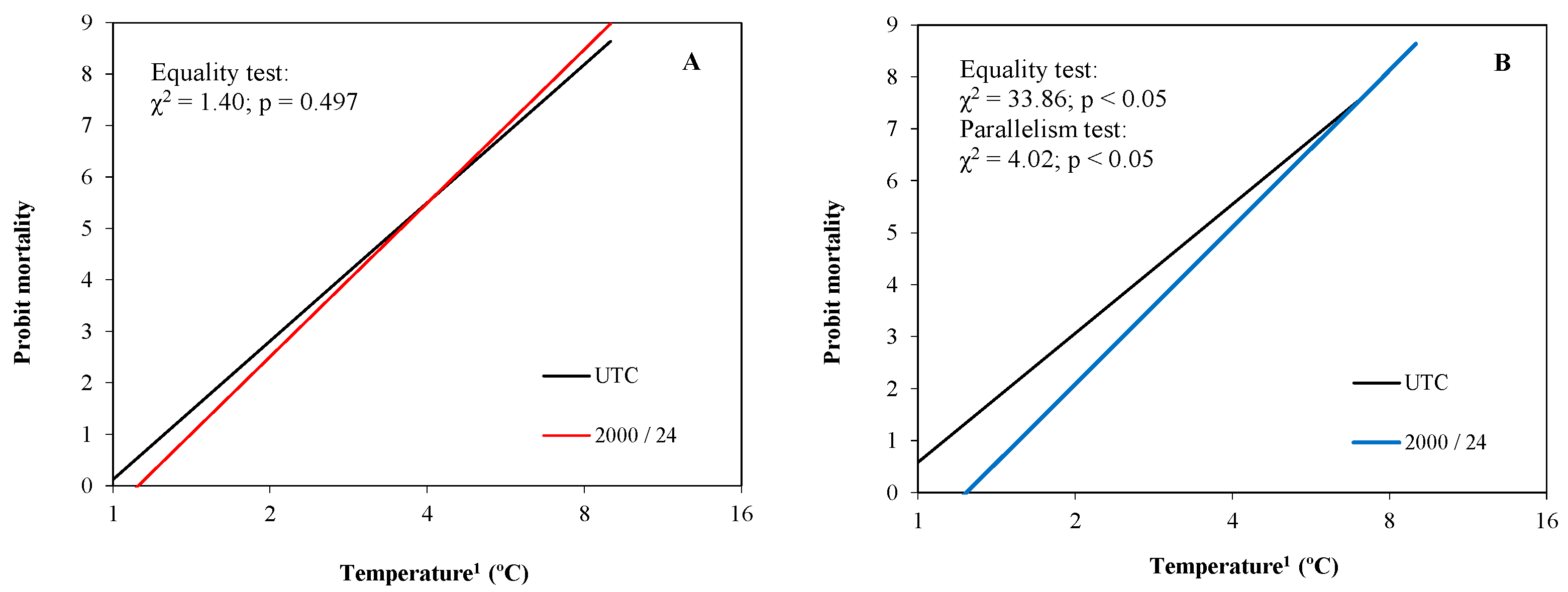
3.2.1. Peach Fruitlets
3.2.2. Almond Fruitlets
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drepper, B.; Bamps, B.; Gobin, A.; Van Orshoven, J. Strategies for managing spring frost risks in orchards: Effectiveness and conditionality—A systematic review protocol. Environ. Evid. 2021, 10, 32. [Google Scholar] [CrossRef]
- Plaza, F. Frost Damages the Production of up to 40 Catalan Fruit and Almond Cooperatives. Available online: https://www.freshplaza.com/europe/article/9418325/frost-damages-the-production-of-up-to-40-catalan-fruit-and-almond-cooperatives/ (accessed on 13 April 2022).
- Vautard, R.; van Oldenborgh, G.J.; Bonnet, R.; Li, S.; Robin, Y.; Kew, S.; Philip, S.; Soubeyroux, J.-M.; Dubuisson, B.; Viovy, N. Human influence on growing-period frosts like the early April 2021 in Central France. Nat. Hazards Earth Syst. Sci. Discuss. 2022, 2022, 1–25. [Google Scholar] [CrossRef]
- Pearce, R. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef] [PubMed]
- Prudencio, A.S.; Martínez-Gómez, P.; Dicenta, F. Evaluation of breaking dormancy, flowering and productivity of extra-late and ultra-late flowering almond cultivars during cold and warm seasons in South-East of Spain. Sci. Hortic. 2018, 235, 3946. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Bravo, L.; Paneque, M.; Navia, R.; Cea, M. Chemical products for crop protection against freezing stress: A review. J. Agron. Crop Sci. 2021, 207, 391–403. [Google Scholar] [CrossRef]
- Fryer, M. The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ. 1992, 15, 381–392. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Noga, G.; Schmitz, M. Uptake characteristics and biological activity of exogenously applied α-tocopherol. In Antioxidants in Higher Plants; Noga, G., Schmitz, M., Eds.; Shaker Verlag: Aachen, Germany, 1998; pp. 112–122. [Google Scholar]
- Vardi, A.; Berman-Frank, I.; Rozenberg, T.; Hadas, O.; Kaplan, A.; Levine, A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Curr. Biol. 1999, 9, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Oxidative stress in plants. Acta Physiol. Plant 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Elstner, E. Oxygen radicals—Biochemical basis for their efficacy. Klin. Wochenschr. 1991, 69, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Schmitz-Eiberger, M.; Brauckmann, M.; Rademacher, W.; Noga, G. Use of prohexadione-calcium, vitamin E, and glycerine for the reduction of frost injury in apple (Malus domestica) flowers and leaves. Eur. J. Hortic. Sci. 2004, 69, 59–65. [Google Scholar]
- Jahed, K.R.; Saini, A.K.; Sherif, S.M. Coping with the cold: Unveiling cryoprotectants, molecular signaling pathways, and strategies for cold stress resilience. Front. Plant Sci. 2023, 14, 1246093. [Google Scholar] [CrossRef] [PubMed]
- Ketchie, D.; Beeman, C. Cold acclimation in ‘Red Delicious’ apple trees under natural conditions during four winters. J. Am. Soc. Hortic. Sci. 1973, 98, 257–261. [Google Scholar] [CrossRef]
- Moratiel, R.; Durán, J.; Snyder, R. Freezing resistance in tomato (Lycopersicum esculentum Mill.) using potential cryoprotectors. Eur. J. Hortic. Sci. 2011, 76, 12–17. [Google Scholar]
- Chen, S.; Ren, J.; Chen, R. 5.11—Cryopreservation and Desiccation Preservation of Cells. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 157–166. [Google Scholar]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Räisänen, M.; Repo, T.; Lehto, T. Cold acclimation of Norway spruce roots and shoots after nitrogen and boron fertilization. Plant Soil. 2009, 292, 271–282. [Google Scholar] [CrossRef]
- Rufat, J.; Arbonés, A. Foliar applications of boron to almond trees in dryland areas. Acta Hortic. 2006, 845, 219–226. [Google Scholar] [CrossRef]
- Torres, E.; Recasens, I.; Àvila, G.; Lordan, J.; Alegre, S. Early stage fruit analysis to detect a high risk of bitter pit in ‘Golden Smoothee’. Sci. Hortic. 2017, 219, 98–106. [Google Scholar] [CrossRef]
- Huang, L.; Bell, R.W.; Dell, B. Physiology and metabalism of boron in plants. In Advances in Plant and Animal Boron Nutrition; Springer: Dordrecht, The Netherlands, 2007; pp. 31–46. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Malécange, M.; Pérez-Garcia, M.-D.; Citerne, S.; Sergheraert, R.; Lalande, J.; Teulat, B.; Mounier, E.; Sakr, S.; Lothier, J. Leafamine®, a free amino acid-rich biostimulant, promotes growth performance of deficit-irrigated lettuce. Int. J. Mol. Sci. 2022, 23, 7338. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An essential metabolite for plant growth and stress acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef] [PubMed]
- Starkutė, R.; Bundinienė, O.; Zalatorius, V.; Jankauskienė, J. The influence of biostimulant ‘Compo frost protect’ for plant resistance to a simulated frost. Sodininkystė Ir Daržininkystė 2017, 36, 73–88. [Google Scholar]
- Miranda, C.; Santesteban, L.G.; Royo, J.B. Variability in the relationship between frost temperature and injury level for some cultivated Prunus species. HortScience 2005, 40, 357–361. [Google Scholar] [CrossRef]
- Throne, J.E.; Weaver, D.K.; Baker, J.E. Probit analysis: Assessing goodness-of-fit based on backtransformation and residuals. J. Econ. Entomol. 1995, 88, 1513–1516. [Google Scholar] [CrossRef]
- Gang, G.-H.; Cho, H.J.; Kim, H.S.; Kwack, Y.-B.; Kwak, Y.-S. Analysis of fungicide sensitivity and genetic diversity among Colletotrichum species in sweet persimmon. Plant Pathol. J. 2015, 31, 115. [Google Scholar] [CrossRef] [PubMed]
- Vassilakos, T.N.; Riudavets, J.; Castañé, C.; Iturralde-Garcia, R.D.; Athanassiou, C.G. Efficacy of modified atmospheres on Trogoderma granarium (Coleoptera: Dermestidae) and Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2019, 112, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Bosch Serra, D.; Rodríguez García, M.; Depalo, L.; Avilla Hernández, J. Determination of the baseline susceptibility of European populations of Cydia pomonella (Lepidoptera: Tortricidae) to chlorantraniliprole and the role of cytochrome P450 monooxygenases. J. Econ. Entomol. 2018, 111, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Proebsting, E.; Mills, H. Low temperature resistance of developing flower buds of six deciduous fruit Species1. J. Am. Soc. Hortic. Sci. 1978, 103, 192–198. [Google Scholar] [CrossRef]
- Reig, G.; Iglesias, I.; Miranda, C.; Gatius, F.; Alegre, S. How does simulated frost treatment affect peach [Prunus persica (L.)] flowers of different cultivars from worldwide breeding programmes? Sci. Hortic. 2013, 160, 70–77. [Google Scholar] [CrossRef]
- Lindén, L.; Rita, H.; Suojala, T. Logit models for estimating lethal temperatures in apple. HortScience 1996, 31, 91–93. [Google Scholar] [CrossRef]
- Masip, D.; Torguet, L.; Batlle, I.; Alegre, S.; Miarnau, X. Almond fruit tolerance to frost temperatures in new Spanish cultivars. Acta Hortic. 2017, 1219, 67–72. [Google Scholar] [CrossRef]
- Moblidowska, I. Effects of some growth regulators on frost damage. Cryobiology 1968, 5, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Jones, K.M. Screening of surfactant polymers for cryoprotectant activity on flowering blackcurrants. Sci. Hortic. 1983, 19, 105–111. [Google Scholar] [CrossRef]
- Perry, K.B.; Bonanno, A.R.; Monks, D.W. Two Putative Cryoprotectants do not Provide Frost and Freeze Protection in Tomato and Pepper. HortScience 1992, 27, 26–27. [Google Scholar] [CrossRef]
- Aoun, M.F.; Perry, K.B.; Swallow, W.H.; Werner, D.J.; Parker, M.L. Antitranspirant and Cryoprotectant Do Not Prevent Peach Freezing Injury. HortScience 1993, 28, 343. [Google Scholar] [CrossRef]
- Wölfel, D.; Noga, G. The effect of alpha-tocopherol and glycerol on preventing blossom freezing injury. Acta Hortic. 1998, 95–102. [Google Scholar] [CrossRef]
- Hołubowicz, T.; Basak, A.; Pacholak, E. Effectiveness of HELP application on the protection of fruit plant flowers against frost. Folia Hort. 2004, 16, 65–69. [Google Scholar]
- Ma, Q.; Huang, J.-G.; Hänninen, H.; Berninger, F. Divergent trends in the risk of spring frost damage to trees in Europe with recent warming. Glob. Chang. Biol. 2019, 25, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Uemura, M.; Kawamura, Y. Freezing Tolerance of Plant Cells: From the Aspect of Plasma Membrane and Microdomain. Adv. Exp. Med. Biol. 2018, 1081, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Francko, D.A.; Wilson, K.G.; Li, Q.Q.; Equiza, M.A. A Topical Spray to Enhance Plant Resistance to Cold Injury and Mortality. HortTechnology 2011, 21, 109–118. [Google Scholar] [CrossRef]
- Tan, W.-J.; Yang, Y.-C.; Zhou, Y.; Huang, L.-P.; Xu, L.; Chen, Q.-F.; Yu, L.-J.; Xiao, S. Diacylglycerol acyltransferase and diacylglycerol kinase Modulate Triacylglycerol and Phosphatidic Acid Production in the Plant Response to Freezing Stress. Plant Physiol. 2018, 177, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A. Does FreezePruf Topical Spray Increase Plant Resistance to Freezing Stress? HortTechnology 2012, 22, 542–546. [Google Scholar] [CrossRef]
- Räisänen, M.; Repo, T.; Lehto, T. Effect of thawing time, cooling rate and boron nutrition on freezing point of the primordial shoot in norway spruce buds. Ann. Bot. 2006, 97, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G. Water content of plant tissues: So simple that almost forgotten? Plants 2023, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J. Spring frosts in deciduous fruit trees—Morphological damage and flower hardiness. Sci. Hortic. 2000, 85, 155–173. [Google Scholar] [CrossRef]
- Montañés Millán, L.; Val, J.; Betrán, J.; Monge, E.; Moreno, M.A.; Montañés, L. Floral analysis: Fresh and dry weight of flowers from different fruit tree species. Acta Hortic. 1997, 448, 233–240. [Google Scholar] [CrossRef]





| Experiment | Treatment | Dose (1) (cc hl−1) | Application Volume (L ha−1) | Dosage (cc ha−1) | Application Time (2) (HBF) | Phenological Stage (BBCH Scale) | Frost Objective Temperature (°C) |
|---|---|---|---|---|---|---|---|
| 1 | UTC (3) | - | 1000 | - | - | 50–75% full bloom (BBCH 65) | −1; −3; −5; −7; −9. |
| 1000/24 | 200 | 500 | 1000 | 24 | |||
| 2000/24 | 1000 | 2000 | |||||
| 1000/48 | 500 | 1000 | 48 | ||||
| 2000/48 | 1000 | 2000 | |||||
| 1000/96 | 500 | 1000 | 96 | ||||
| 2000/96 | 1000 | 2000 | |||||
| 2 | UTC (3) | - | 1000 | - | 24 | 100% jacket split (4) (BBCH 72) | −1; −3; −5; −7; −9; −11 |
| 2000/24 | 200 | 2000 |
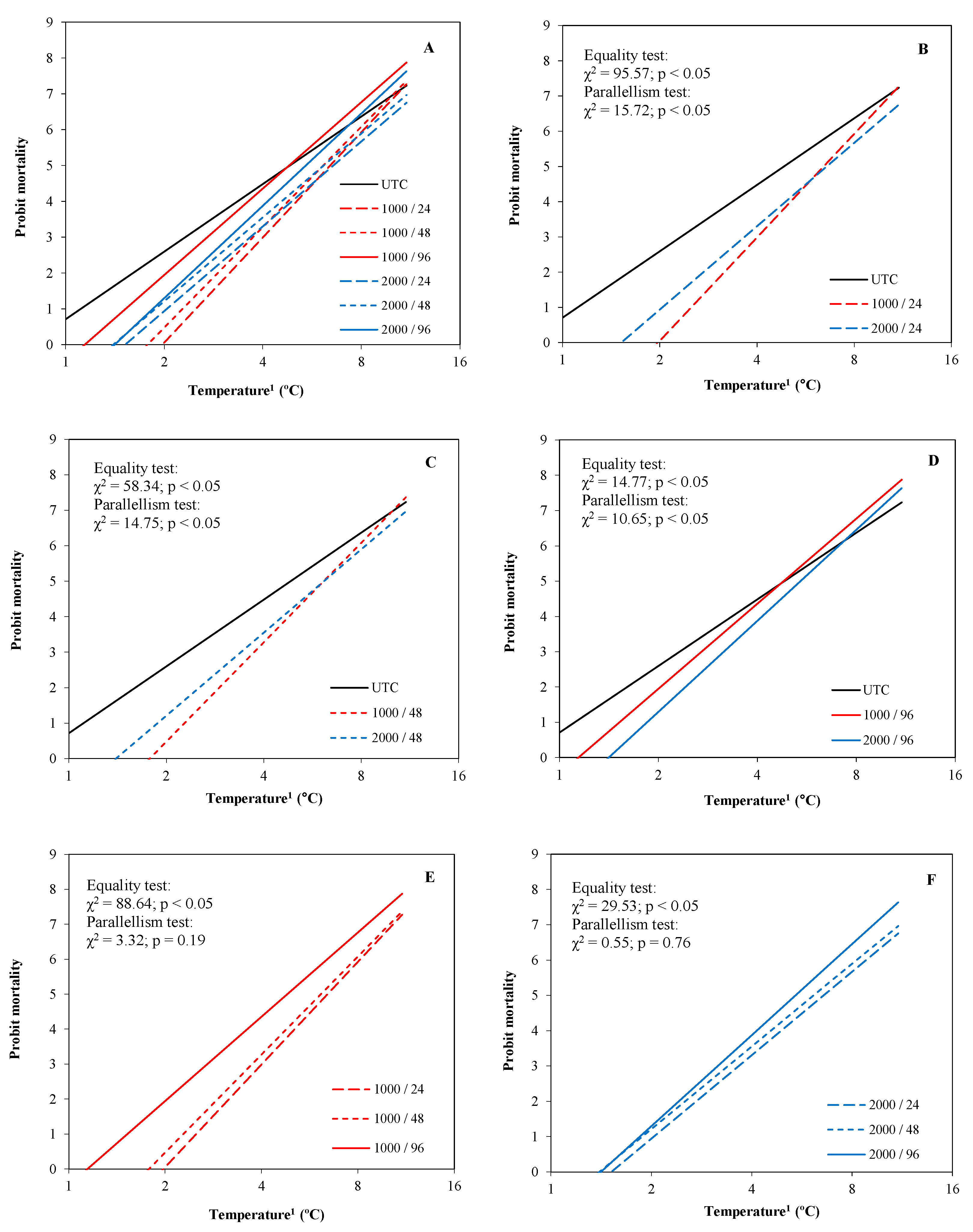
| Treatment | Num. Flowers | Slope (±SE) | LT10 (FL at 95%) * | LT50 (FL at 95%) * | LT90 (FL at 95%) * | Ratio LT50 |
|---|---|---|---|---|---|---|
| UTC | 761 | 6.26 ± 0.39 | −3.0 (−2.6/−3.3) a | −4.8 (−4.6/−5.1) a | −7.8 (−7.2/−8.3) ab | - |
| 1000/24 | 702 | 9.75 ± 0.91 | −4.7 (−4.3/−5.1) b | −6.4 (−6.1/−6.7) b | −8.7 (−8.3/−9.3) bc | 0.79 |
| 2000/24 | 735 | 7.86 ± 0.69 | −4.5 (−4.1/−4.8) b | −6.6 (−6.2/−6.9) b | −9.6 (−8.9/−10.6) c | 0.79 |
| 1000/48 | 823 | 9.31 ± 0.81 | −4.5 (−4.1/−4.8) b | −6.1 (−5.9/−6.4) b | −8.4 (−8.0/−9.0) bc | 0.79 |
| 2000/48 | 572 | 7.78 ± 0.77 | −4.2 (−3.7/−4.6) b | −6.1 (−5.7/−6.5) b | −9.0 (−8.3/−10.0) c | 0.79 |
| 1000/96 | 768 | 8.01 ± 0.58 | −3.3 (−3.1/−3.5) a | −4.8 (−4.6/−5.0) a | −7.0 (−6.5/−7.6) a | 1.01 |
| 2000/96 | 623 | 8.56 ± 0.80 | −3.8 (−3.4/−4.2) b | −5.4 (−5.0/−5.7) a | −7.6 (−7.2/−8.2) ab | 0.89 |
| Treatment | Num. Flowers | Slope (±SE) | LT10 * (FL at 95%) | LT50 * (FL at 95%) | LT90 ns (FL at 95%) | Ratio LT50 |
|---|---|---|---|---|---|---|
| UTC | 783 | 5.16 ± 0.38 | −3.4 (−3.0/−3.8) a | −6.1 (−5.7/−6.4) a | −10.8 (−10.0/−11.9) | - |
| 1000/24 | 830 | 8.72 ± 0.77 | −5.4 (−5.0/−5.7) b | −7.6 (−7.3/−7.8) b | −10.6 (−10.1/−11.4) | 0.80 |
| 2000/24 | 701 | 11.14 ± 0.98 | −6.1 (−5.7/−6.4) c | −8.0 (−7.7/−8.2) bc | −10.4 (−9.9/−11.1) | 0.76 |
| 1000/48 | 650 | 6.69 ± 0.73 | −4.9 (−4.3/−5.3) b | −7.6 (−7.2/−8.0) b | −11.8 (−10.7/−13.7) | 0.80 |
| 2000/48 | 627 | 7.23 ± 0.88 | −5.6 (−5.0/−6.0) bc | −8.4 (−8.0/−8.9) c | −12.6 (−11.3/−15.0) | 0.72 |
| 1000/96 | 791 | 5.32 ± 0.43 | −3.6 (−3.2/−4.0) a | −6.4 (−6.0/−6.7) a | −11.1 (−10.2/−12.2) | 0.96 |
| 2000/96 | 821 | 4.56 ± 0.37 | −3.5 (−3.0/−3.9) a | −6.6 (−6.2/−7.0) a | −12.6 (−11.0/−14.5) | 0.92 |
| Crop | Treatment | Num. Fruit | Slope (±SE) | LT10 * (FL at 95%) | LT50 * (FL at 95%) | LT90 ns (FL at 95%) | Ratio LT50 |
|---|---|---|---|---|---|---|---|
| Peach | UTC | 320 | 8.92 ± 0.34 | −2.5 (−2.4/−2.7) a | −3.4 (−3.4/−3.7) a | −4.9 (−4.7/−5.2) | - |
| 2000/24 | 319 | 9.92 ± 0.40 | −2.7 (−2.4/−2.9) a | −3.6 (−3.4/−3.8) a | −4.8 (−4.5/−5.3) | 1.1 | |
| Almond | UTC | 991 | 8.25 ± 0.39 | −2.4 (−2.1/−2.7) a | −3.4 (−3.2/−3.6) a | −4.9 (−4.7/−5.3) | - |
| 2000/24 | 1035 | 10.02 ± 0.38 | −2.9 (−2.7/−3.1) b | −3.9 (−3.8/−4.0) b | −5.2 (−5.0/−5.6) | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, E.; Miarnau, X. Frost Damage Mitigation in Flowers and Fruitlets of Peach and Almond from the Application of a Multi-Attribute Approach Biostimulant. Plants 2024, 13, 1603. https://doi.org/10.3390/plants13121603
Torres E, Miarnau X. Frost Damage Mitigation in Flowers and Fruitlets of Peach and Almond from the Application of a Multi-Attribute Approach Biostimulant. Plants. 2024; 13(12):1603. https://doi.org/10.3390/plants13121603
Chicago/Turabian StyleTorres, Estanis, and Xavier Miarnau. 2024. "Frost Damage Mitigation in Flowers and Fruitlets of Peach and Almond from the Application of a Multi-Attribute Approach Biostimulant" Plants 13, no. 12: 1603. https://doi.org/10.3390/plants13121603





