Pollinator Diversity and Phenological Interplay: Exploring Mineral, Hormonal, Sugar, and Vitamin Contents in Vitis vinifera L. cv Bozcaada Çavuşu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cluster Properties and Must Composition
2.3. Mineral Analysis in Grape Varieties
2.4. HPLC Analysis for Hormone Identification in Grape Varieties
2.5. HPLC Analysis for Sugar Identification in Grape Varieties
2.6. HPLC Analysis for Vitamin Identification in Grape Varieties
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Effect of Sampling Time and Variety on Berry Traits and Maturity Parameters
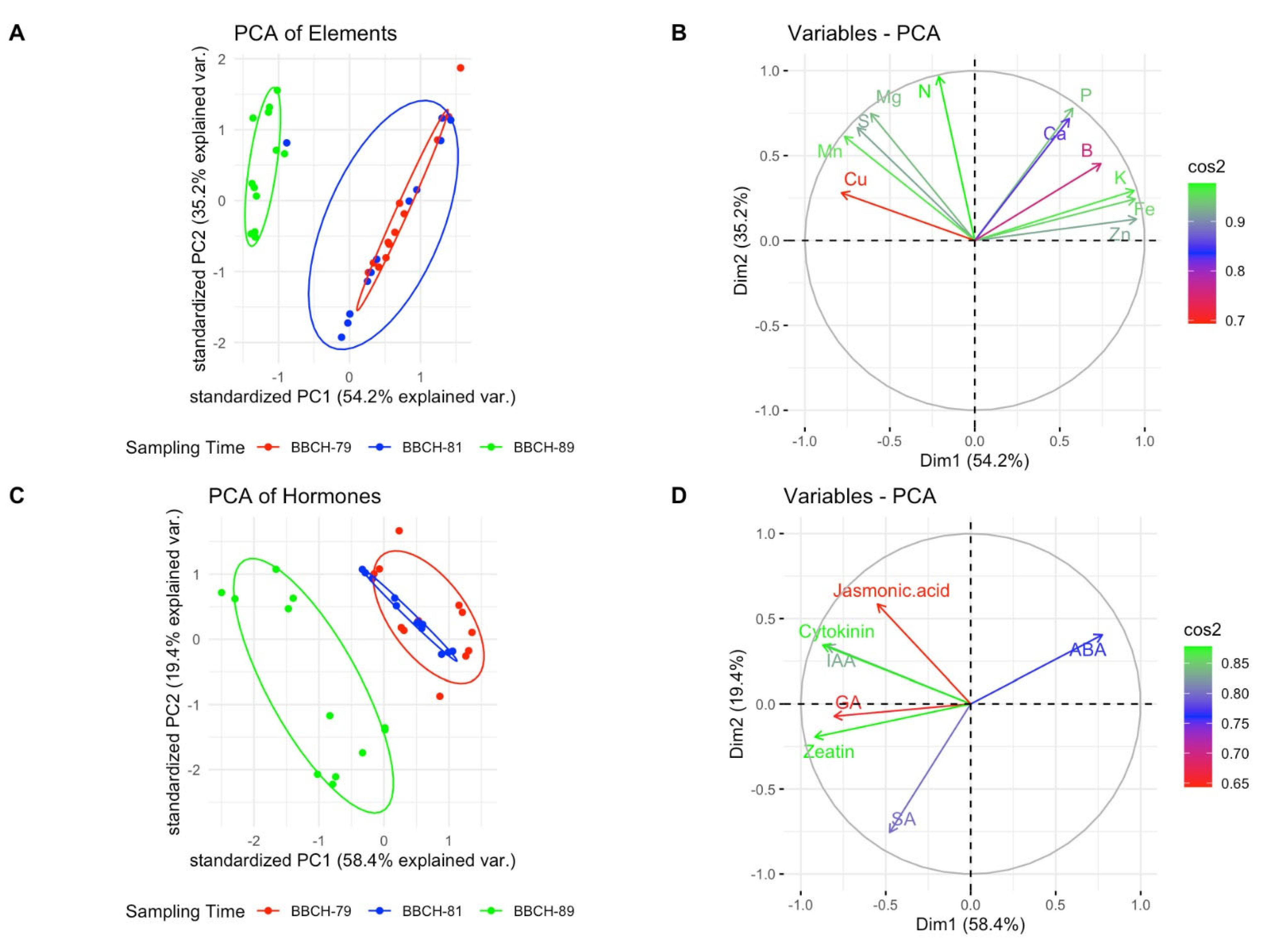
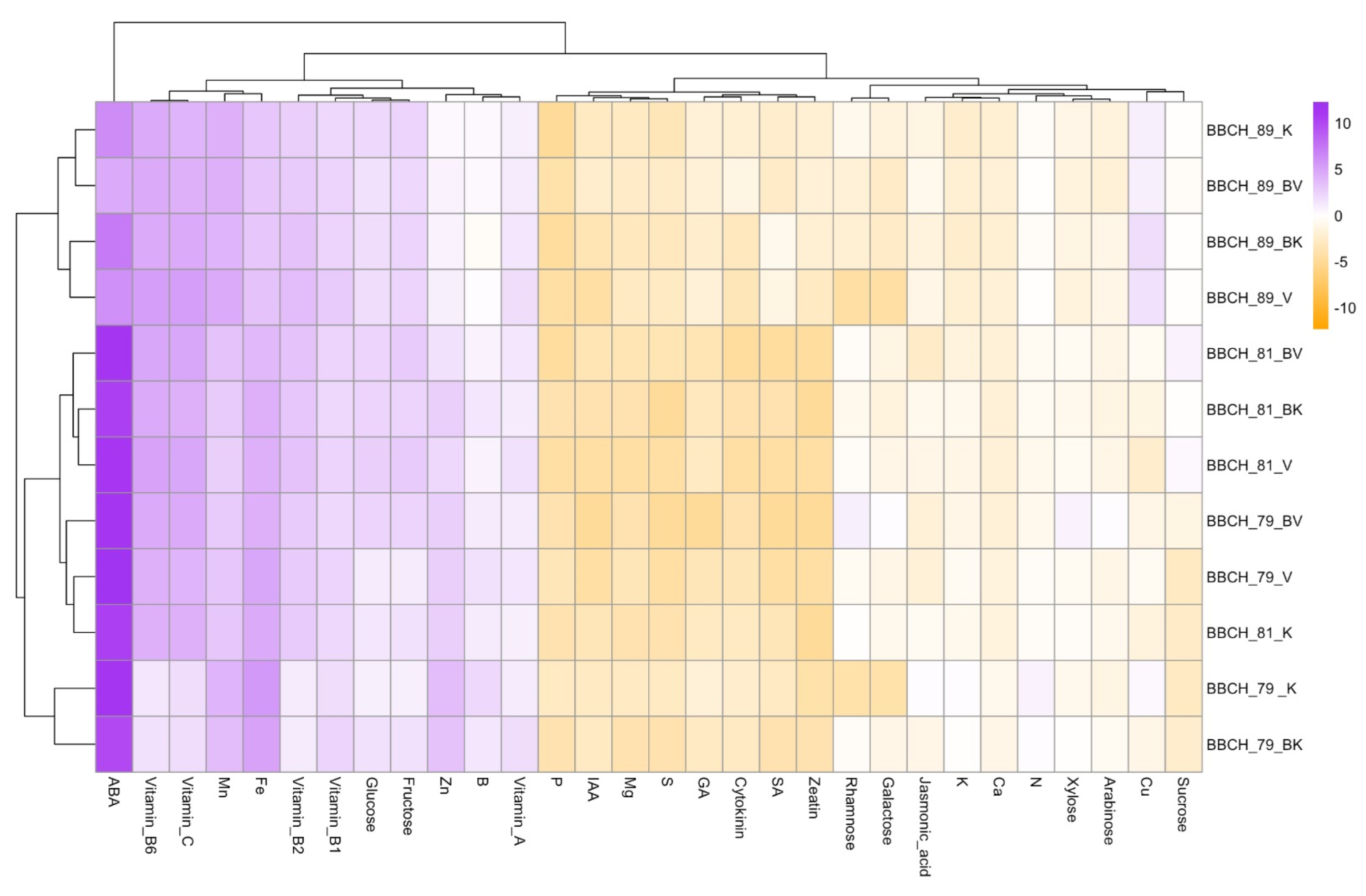
4.2. Effect of Sampling Time and Variety on Berry Mineral Content
4.3. Effect of Sampling Time and Variety on Berry Hormonal Content
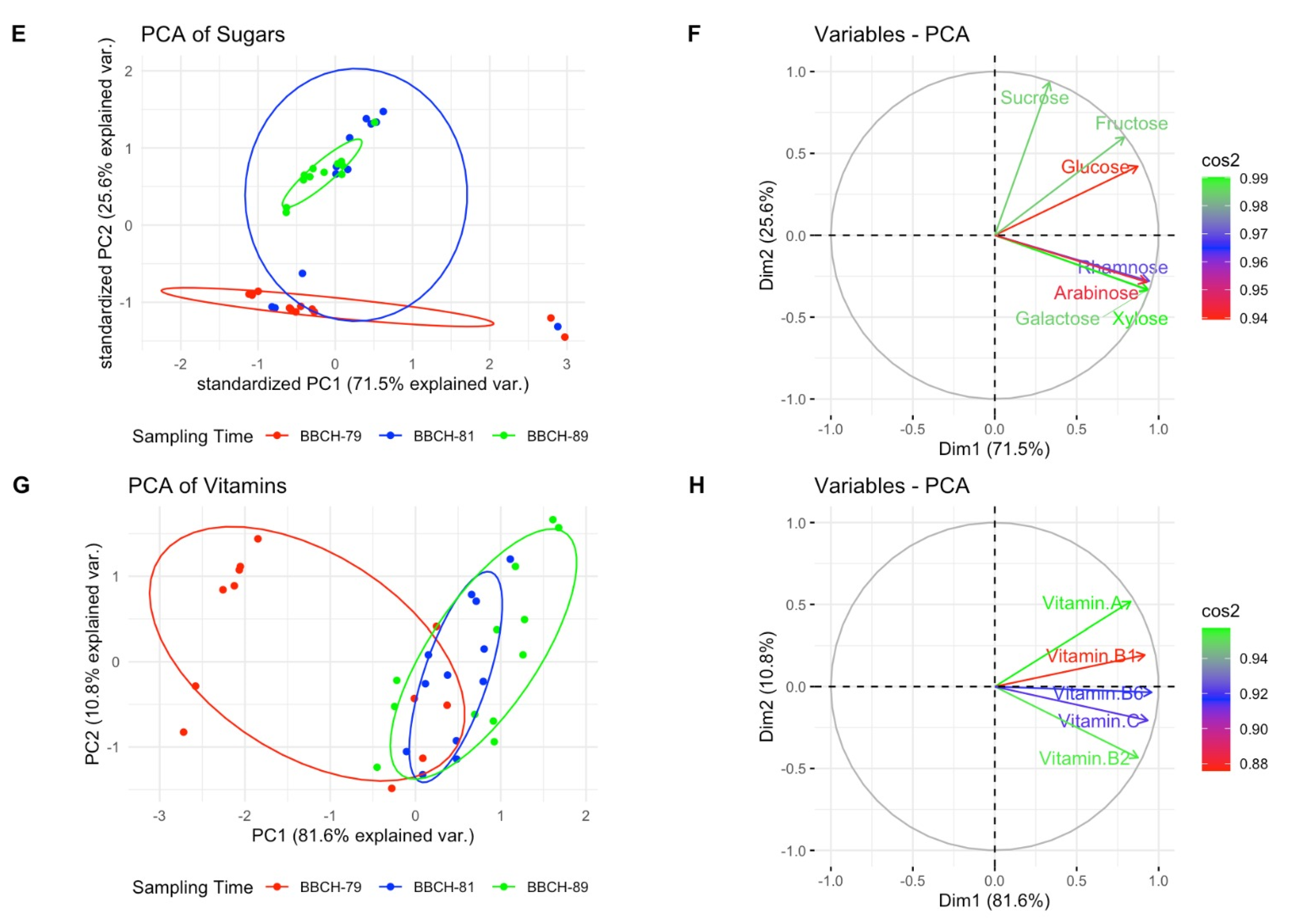
4.4. Effect of Sampling Time and Variety on Berry Sugar Content
4.5. Effect of Sampling Time and Variety on Berry Vitamin Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, M.W. Grapes. In The New York Times Natural Foods Cookbook; Random House: New York, NY, USA, 1957; pp. 497–506. [Google Scholar]
- Gupton, C. Grapes. In The Encyclopedia of Fruit and Nuts; CABI: Houston, TX, USA, 2000; pp. 287–312. [Google Scholar]
- Conner, P.J. Breeding table grapes. In Grapes; CABI: Houston, TX, USA, 2009; pp. 1–20. [Google Scholar]
- Conner, P.J. Seedlessness in grapes. In Grapes; CABI: Houston, TX, USA, 2014; pp. 213–233. [Google Scholar]
- Reimer, G.; Detjen, L.R. A study of the development of the flower and fruit of the grape. Bull. Torrey Bot. Club 1910, 37, 563–582. [Google Scholar]
- Krewer, G.; Perry, R.; Mullis, S.; NeSmith, D. Muscadine grape. Univ. Ga. Coop. Ext. Bull. 2000, 739, 1012. [Google Scholar]
- Ramos, M.J.; Coito, J.L.; Silva, H.G.; Cunha, J.; Costa, M.M.; Rocheta, M. Flower development and sex specification in wild grapevine. BMC Genom. 2014, 15, 1095. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.J.; Chaïb, J.; Martínez-Zapater, J.M.; Thomas, M.R. A molecular genetic perspective of reproductive development in grapevines. J. Exp. Bot. 2008, 59, 2579–2596. [Google Scholar] [CrossRef] [PubMed]
- Heazlewood, J.L.; Wilson, I.W. Acid phosphatases, phytases, and proteases in the vacuole. In The Plant Vacuole; Springer: Berlin/Heidelberg, Germany, 2004; pp. 271–315. [Google Scholar]
- Meneghetti, S.; Costacurta, A.; Battilana, J.; Moser, S.; Vecchione, A.; Grando, M.S. Linkage mapping and QTL analysis of aroma compounds in winegrapes. Theor. Appl. Genet. 2006, 112, 1658–1672. [Google Scholar]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar] [CrossRef]
- Bessis, J. Grapevine Breeding Programs for the Wine Industry: Structure and Economic Efficiency; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Dry, P.R.; Loveys, B.R. Grapevine moderate water-deficit and moderate light together induce summer dormancy and repress stress-related genes while activating protective ones. Aust. J. Grape Wine Res. 2010, 16, 270–283. [Google Scholar]
- Caporali, E.; Spada, A.; Marziani, G.; Nuti Ronchi, V.; Reale, L.; Scalabrelli, G.; Famiani, F.; Walker, R. Grape. In Genetic Improvement of Vegetable Crops; Elsevier Science: Pergamon, Turkey, 2003; pp. 269–290. [Google Scholar]
- Keskin, N.; Kunter, B.; Celik, H.; Kaya, O. Evaluation of Clonal Variability of Berry Phenolics in Vitis vinifera L. Cv. Kalecik Karası. Erwerbs-Obstbau 2022, 64 (Suppl. S1), 65–72. [Google Scholar]
- May, P. Winegrape quality response to short-duration water stress. Am. J. Enol. Vitic. 2000, 51, 115–123. [Google Scholar]
- Munoz-Rodriguez, A.F.; Oliveira, C.; Aguilar, J.F. Molecular systematics of the trnL-F region in Vitis (Vitaceae). Am. J. Bot. 2011, 98, 1515–1527. [Google Scholar]
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Tello, J.; Ibáñez, J.; Muñoz-Organero, G. Microsatellite markers in grapevine reveal DNA stability during processing and after six months of simulated shipping conditions. Sci. Rep. 2018, 8, 7771. [Google Scholar]
- Ağaoğlu, Y.S. Bilimsel ve Uygulamalı Bağcılık. Cilt 1 Asma Biyolojisi; Kavaklıdere Eğitim Yayınları No. 1: Ankara, Turkey, 1999. [Google Scholar]
- Oraman, M.N. Bağcılık Tekniği II; Ankara Üniversitesi Ziraat Fakültesi Yayınları: Ankara, Turkey, 1972. [Google Scholar]
- Dardeniz, A.; Şeker, M.; Yancar, A.; Gökbayrak, Z.; Bahar, E.; Kahraman, K.A. Çanakkale’de Bozcaada Çavuşu Üzüm Çeşidi Yetiştiriciliği ve Karşılaşılan Sorunlar. In Proceedings of the Uluslararası Katılımlı I, Ali Numan Kıraç Tarım Kongresi ve Fuarı, Eskişehir, Turkey, 27–30 April 2011. [Google Scholar]
- Kaya, O.; Incesu, M.; Ates, F.; Keskin, N.; Verdugo-Vásquez, N.; Gutiérrez-Gamboa, G. Study of volatile organic compounds of two table grapes (Cv. Italia and Bronx Seedless) along ripening in vines established in the Aegean region (Turkey). Plants 2022, 11, 1935. [Google Scholar] [CrossRef] [PubMed]
- Kaya, O. Effect of manual leaf removal and its timing on yield, the presence of lateral shoots and cluster characteristics with the grape variety ‘Karaerik’. Mitt Klosterneubg Rebe Wein Obstbau Früchteverwertung 2019, 69, 83–92. [Google Scholar]
- Kaya, O.; Ates, F.; Kara, Z.; Turan, M.; Gutiérrez-Gamboa, G. Study of Primary and Secondary Metabolites of Stenospermocarpic, Parthenocarpic, and Seeded Raisin Varieties. Horticulturae 2022, 8, 1030. [Google Scholar] [CrossRef]
- Keskin, N.; Kunter, B.; Çelik, H.; Kaya, Ö.; Keskin, S. ANOM approach for the statistical evaluation of organic acid contents of clones of the grape variety ‘Kalecik Karası. Mitt Klosterneubg. 2021, 71, 126–138. [Google Scholar]
- Keskin, N.; Kaya, O.; Ates, F.; Turan, M.; Gutiérrez-Gamboa, G. Drying grapes after the application of different dipping solutions: Effects on hormones, minerals, vitamins, and antioxidant enzymes in Gök Üzüm (Vitis vinifera L.) raisins. Plants 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.; Ates, F.; Keskin, N.; Turan, M.; Kaya, O. Comparison of contents of sugars, organic acids and free amino acids in raisins obtained from Gök Üzüm (Vitis vinifera L.). Mitt Klosterneubg. 2023, 73, 98–113. [Google Scholar]
- Incesu, M.; Karakus, S.; Seyed Hajizadeh, H.; Ates, F.; Turan, M.; Skalicky, M.; Kaya, O. Changes in Biogenic Amines of Two Table Grapes (cv. Bronx Seedless and Italia) during Berry Development and Ripening. Plants 2022, 11, 2845. [Google Scholar] [CrossRef]
- Karakus, S.; Kaya, O.; Hajizadeh, H.S.; Gutiérrez-Gamboa, G.; Ates, F.; Turan, M.; Araya-Alman, M. Characterization of volatile compounds of Gök Üzüm raisins produced from grapes pre-treated with different dipping solutions. Chem. Biol. Technol. Agric. 2023, 10, 55. [Google Scholar] [CrossRef]
- Pons, Z.; Guerrero, L.; Margalef, M.; Arola, L.; Arola-Arnal, A.; Muguerza, B. Effect of low molecular grape seed pro-anthocyanidins on blood pressure and lipid homeostasis in cafeteria diet-fed rats. J. Physiol. Biochem. 2014, 70, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological Growth Stages of the Grapevine (Vitis vinifera L. ssp. Vinifera) Codes and Descriptions According to the Extended BBCH Scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Food, C.; Kjeldahl, T.M.C. Changes in AOAC® Official Methods of Analysis. J. AOAC Int. 2020, 79, 1060–3271. [Google Scholar]
- Kojima, K.; Ikarashi, H.; Andou, D.; Matsumoto, T. Endogenous Plant Hormone Profiles in Growing Campbell Early Grape Berries. Hortic. J. 2020, 89, 509–515. [Google Scholar] [CrossRef]
- Ma, C.M.; Sun, Z.; Chen, C.B.; Zhang, L.L.; Zhu, S.H. Simultaneous separation and determination of fructose, sorbitol, glucose, and sucrose in fruits by HPLC-ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Samydurai, P.; Ramakrishnan, R.; Nagarajan, N. Polyphenols, vitamin-E estimation and in vitro antioxidant activity of Adiantum capillus-veneris. Int. J. Innov. Pharm. Res. 2013, 4, 258–262. [Google Scholar]
- Mozumder, N.R.; Akhter, M.J.; Khatun, A.A.; Rokibuzzaman, M.; Akhtaruzzaman, M. Estimation of water-soluble vitamin B-complex in selected leafy and non-leafy vegetables by HPLC method. Orient. J. Chem. 2019, 35, 1344. [Google Scholar] [CrossRef]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.4-0. 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 11 April 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 11 April 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 11 April 2024).
- Kolde, R. pheatmap: Pretty Heatmaps. R Package Version 1.0.12. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 11 April 2024).
- Coombe, B.G.; McCarthy, M.G. Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 2000, 6, 131–135. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Guillaumie, S.; Fouquet, R.; Kappel, C.; Camps, C.; Terrier, N.; Moncomble, D.; Delrot, S. Transcriptional analysis of late ripening stages of grapevine berry. BMC Plant Biol. 2011, 11, 165. [Google Scholar] [CrossRef]
- Peña-Neira, A.; Cortiella, M.G.I.; Ubeda, C.; Pastenes, C.; Villalobos, L.; Contador, L.; Gómez, C. Phenolic, Poly-saccharides Composition, and Texture Properties during Ripening and Storage Time of New Table Grape Cultivars in Chile. Plants 2023, 12, 2488. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Ferrara, G.; Malerba, A.D.; Matarrese, A.M.S.; Mondelli, D.; Mazzeo, A. Nitrogen distribution in annual growth of ‘Italia’ table grape vines. Front. Plant Sci. 2018, 9, 1374. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Cunha, A.; Gerós, H.; Hanana, M.; Blumwald, E. Mineral compounds in grape berry. Biochem. Grape Berry 2012, 23–43. [Google Scholar]
- Conradie, W.J. Seasonal uptake of nutrients by Chenin blanc in sand culture: II. Phosphorus, potassium, calcium and magnesium. S. Afr. J. Enol. Vitic. 1981, 2, 7–13. [Google Scholar] [CrossRef]
- Zhenming, N.; Xuefeng, X.; Yi, W.; Tianzhong, L.; Jin, K.; Zhenhai, H. Effects of leaf-applied potassium, gibberellin and source–sink ratio on potassium absorption and distribution in grape fruits. Sci. Hortic. 2008, 115, 164–167. [Google Scholar] [CrossRef]
- Cabanne, C.; Donèche, B. Calcium accumulation and redistribution during the development of grape berry. Vitis 2003, 42, 19–21. [Google Scholar]
- Schaller, K. Influence of different soil tillage systems on uptake of N, P, K, Mg, Ca, and organic N-compounds by grape berries during growth and development of the variety “white Riesling”. Bull. OIV 1999, 72, 603–629. [Google Scholar]
- Archbold, D.D.; Dennis, F.G. Quantification of free and conjugated IAA in strawberry achene and receptacle tissue during fruit development. J. Am. Soc. Hortic. Sci. 1984, 109, 330–335. [Google Scholar] [CrossRef]
- Purgatto, E.; Nascimento, J.R.O.; Lajolo, F.M.; Cordenunsi, B.R. The onset of starch degradation during banana ripening is concomitant to changes in the content of free and conjugated forms of indole-3-acetic acid. J. Plant. Physiol. 2002, 159, 1105–1111. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Delker, C.; Raschke, A.; Quint, M. Auxin dynamics: The dazzling complexity of a small molecule’s message. Planta 2008, 227, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Lee, K.H.; Piao, H.L.; Kim, H.Y.; Choi, S.M.; Jiang, F.; Hartung, W.; Hwang, I. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 2006, 126, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.; Loveys, B.; Ford, C. Grape berry ripening is partly regulated by ABA, which promotes berry softness. Vitis 2009, 48, 87–93. [Google Scholar]
- Teszlák, P.; Gaál, K.; Shahin, M.; Nikfardjam, M.S.P. Influence of grapevine flower treatment with gibberellic acid (GA3) on polyphenol content of Vitis vinifera L. wine. Anal. Chim. Acta 2005, 543, 275–281. [Google Scholar] [CrossRef]
- Rodriguez-Lovelle, B.; Gaudillère, J.P. Carbon and nitrogen partitioning in either fruiting or non-fruiting grapevines: Effects of nitrogen limitation before and after véraison. Aust. J. Grape Wine Res. 2002, 8, 86–94. [Google Scholar] [CrossRef]
- Dreier, L.P.; Hunter, J.L.; Ruffner, H.P. Invertase activity, grape berry development and cell compartmentation. Plant Physiol. Biochem. 1998, 36, 865–872. [Google Scholar] [CrossRef]
- Bari, B.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Rock, C.D. Abscisic Acid Biosynthesis and Response. In The Arabidopsis Book; Sommerville, C.R., Meyerowitz, E.M., Eds.; American Society of Plant Biologists: Rockville, MD, USA, 2002; pp. 1–48. [Google Scholar]
- Keskin, N.; Kunter, B.; Çelik, H. Clonal trans-Resveratrol Potential in the Ripened Grapes of Vitis vinifera L. cv ‘Kalecik Karası’. Erwerbs-Obstbau 2020, 62 (Suppl. S1), 81–85. [Google Scholar] [CrossRef]
- Zhang, X.R.; Luo, G.G.; Wang, R.H.; Wang, J.; Himelrick, D.G. Growth and developmental responses of seeded and seedless grape berries to shoot girdling. J. Am. Soc. Hort. Sci. 2003, 128, 316–323. [Google Scholar] [CrossRef]
- Kondo, S.; Fukuda, K. Changes in jasmonates in grape berries and their possible role in fruit development. Sci. Hortic. 2001, 91, 275–288. [Google Scholar] [CrossRef]
- Coombe, B.G. Influence of temperature on composition and quality of grapes. Acta Hortic. 1986, 206, 23–36. [Google Scholar] [CrossRef]
- Matthews, M.A.; Anderson, M.M. Fruit ripening in Vitis vinifera L.: Responses to seasonal water deficits. Am. J. Enol. Vitic. 1988, 39, 313. [Google Scholar] [CrossRef]
- Kliewer, W.M. Sugars and organic acids of Vitis vinifera. Plant Physiol. 1970, 46, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Cushman, J.C. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Tebib, K.; Rouanet, J.M.; Besançon, P. Antioxidant effects of dietary polymeric grape seed tannins in tissues of rats fed a high cholesterol-vitamin E-deficient diet. Food Chem. 1997, 59, 135–141. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Bavaresco, L.; Falginella, L.; Gonçalves, M.I.V.Z.; Di Gaspero, G.; Gerós, H.; Delrot, S. Phenolics in grape berry and key antioxidants. Biochem. Grape Berry 2012, 22, 89–110. [Google Scholar]
- Teissedre, P.L. Wine and health. In The Biochemistry of the Grape Berry; Gerós, M.-M.H., Chaves, S.D., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2012; pp. 269–285. [Google Scholar]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Monica, H.; Jansen, M.; Guisez, Y.; Horemans, N. Accumulation of tocopherols and tocotrienols during seed development of grape (Vitis vinifera L. cv. Albert Lavallee). Plant Physiol. Biochem. 2006, 44, 724–731. [Google Scholar] [CrossRef]
- Chervin, C.; Tira-Umphon, A.; Terrier, N.; Zouine, M. Stimulation of grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol. Plant. 2008, 134, 534–546. [Google Scholar] [CrossRef]
- Di Matteo, A.; Russo, R.; Catinella, G.; Scorzillo, G.; Greco, M.L.; Di Vaio, C.; Santamaria, P. Antioxidant properties of Vitis vinifera L. cv. Greco in relation to maturity. Food Res. Int. 2013, 54, 133–140. [Google Scholar]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The relationship between the expression of ABA biosynthesis genes, accumulation of ABA and the promotion of Vitis vinifera L. berry ripening by ABA. Aust. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- Ates, F.; Delavar, H.; Dardeniz, A.; Yilmaz, T.; Turan, M.; Kaya, O. Dynamics of berry characteristics, biochemical composition, and physiological responses across ripening stages: Investigating the impact of pollinizer varieties on physiological femaleness in Bozcaada Çavuşu (Vitis vinifera L. cv). J. Plant Growth Regul. 2024, 1–20. [Google Scholar]
- Kaya, O.; Delavar, H.; Ates, F.; Yilmaz, T.; Sahin, M.; Keskin, N. Fine-Tuning Grape Phytochemistry: Examining the Distinct Influence of Oak Ash and Potassium Carbonate Pre-Treatments on Essential Components. Horticulturae 2024, 10, 95. [Google Scholar] [CrossRef]
| Sampling Time (S) x | Berry Weight (g/Berry) | Berry Width (mm) | Berry Length (mm) | TSS (°Brix) | TA (g/L Tartaric Acid) | Maturity Index (MI-°Brix) |
|---|---|---|---|---|---|---|
| BBCH_79 | 1.17 ± 0.40 c | 4.40 ± 0.65 c | 4.60 ± 0.81 c | 4.30 ± 0.38 c | 28.16 ± 1.19 a | 1.52 ± 0.07 c |
| BBCH_81 | 3.23 ± 0.02 b | 13.17 ± 1.76 b | 14.02 ± 2.27 b | 11.25 ± 0.13 b | 18.89 ± 1.11 b | 5.98 ± 0.36 b |
| BBCH_89 | 4.58 ± 0.46 a | 18.62 ± 2.13 a | 19.91 ± 2.26 a | 19.30 ± 1.84 a | 6.11 ± 0.62 c | 31.62 ± 1.74 a |
| Cultivar (C) y | ||||||
| Kuntra | 2.66 ± 0.34 b | 11.96 ± 2.91 c | 12.24 ± 1.04 c | 12.75 ± 1.59 a | 18.73 ± 2.74 a | 13.06 ± 1.22 b |
| Bozcaada Çavuşu*Kuntra | 3.84 ± 0.78 a | 13.79 ± 2.88 a | 15.07 ± 1.59 a | 10.82 ± 0.93 d | 16.59 ± 2.91 d | 12.60 ± 1.23 c |
| Vasilâki | 1.67 ± 0.86 c | 9.87 ± 1.29 d | 9.90 ± 0.25 c | 11.60 ± 1.43 b | 18.39 ± 3.82 b | 12.49 ± 1.52 c |
| Bozcaada Çavuşu*Vasilâki | 3.79 ± 0.96 a | 12.65 ± 2.77 b | 14.15 ± 1.88 b | 11.30 ± 1.10 c | 17.17 ± 1.86 c | 14.00 ± 1.29 a |
| Significance | ||||||
| S | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** |
| C | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** |
| S x C | 0.9714 | 0.8727 | 0.7865 | 09243 | 0.9875 | 0.9658 |
| Sampling Time (S) x | N (%) | P (%) | K(%) | Ca | Mg | S | Mn | Fe | Zn | B | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BBCH_79 | 1.71 ± 0.12 b | 0.18 ± 0.00 | 1.38 ± 0.06 a | 0.79 ± 0.06 | 0.17 ± 0.02 b | 0.12 ± 0.03 b | 18.80 ± 2.24 b | 55.8 ± 3.48 a | 14.54 ± 3.41 a | 5.34 ± 0.69 a | 1.22 ± 0.41 b |
| BBCH_81 | 1.64 ± 0.10 b | 0.16 ± 0.00 | 1.24 ± 0.05 a | 0.75 ± 0.05 | 0.18 ± 0.03 b | 0.13 ± 0.01 b | 19.60 ± 2.64 b | 49.9 ± 2.58 a | 11.85 ± 5.42 a | 4.77 ± 0.03 a | 0.99 ± 0.39 b |
| BBCH_89 | 2.25 ± 0.15 a | 0.15 ± 0.00 | 0.60 ± 0.05 b | 0.65 ± 0.04 | 0.38 ± 0.02 a | 0.35 ± 0.02 a | 51.90 ± 2.37 a | 23.3 ± 2.69 b | 3.95 ± 0.52 b | 2.92 ± 0.16 b | 6.40 ± 0.45 a |
| Cultivar (C) y | |||||||||||
| Kuntra | 2.25 ± 0.13 a | 0.20 ± 0.00 a | 1.39 ± 0.06 a | 0.88 ± 0.07 | 0.28 ± 0.02 | 0.20 ± 0.02 ab | 33.70 ± 2.33 | 55.80 ± 3.18 a | 12.78 ± 6.88 a | 5.42 ± 0.69 | 2.67 ± 0.36 |
| Bozcaada Çavuşu*Kuntra | 1.76 ± 0.14 ab | 0.16 ± 0.00 ab | 1.06 ± 0.04 b | 0.70 ± 0.06 | 0.20 ± 0.03 | 0.18 ± 0.01 b | 27.00 ± 2.88 | 41.80 ± 2.96 b | 10.78 ± 6.09 ab | 4.04 ± 0.19 | 3.40 ± 0.85 |
| Vasilâki | 1.61 ± 0.17 b | 0.15 ± 0.00 b | 0.92 ± 0.07 b | 0.65 ± 0.05 | 0.21 ± 0.02 | 0.17 ± 0.03 b | 26.60 ± 2.43 | 37.90 ± 2.76 b | 8.22 ± 3.91 b | 3.60 ± 0.94 | 2.67 ± 0.60 |
| Bozcaada Çavuşu*Vasilâki | 1.87 ± 0.10 ab | 0.14 ± 0.00 b | 0.92 ± 0.05 b | 0.69 ± 0.04 | 0.28 ± 0.04 | 0.26 ± 0.01 a | 33.10 ± 3.74 | 36.60 ± 3.96 b | 8.66 ± 5.75 b | 4.30 ± 0.67 | 2.72 ± 0.74 |
| Significance | |||||||||||
| S | 0.0031 ** | 0.1111 | 0.0001 *** | 0.3094 | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0007 *** | 0.0001 *** |
| C | 0.0270 * | 0.0084 ** | 0.0003 *** | 0.1323 | 0.1134 | 0.0145 * | 0.1295 | 0.0001 *** | 0.0061 ** | 0.0628 | 0.6866 |
| S x C | 0.7054 | 0.6957 | 0.6238 | 0.5519 | 0.8765 | 0. 7858 | 0.7565 | 0.5998 | 0.8958 | 0.7699 | 0.8166 |
| Sampling Time (S) x | IAA | ABA | GA3 | SA | Cytokinin | Zeatin | Jasmonic Acid |
|---|---|---|---|---|---|---|---|
| BBCH_79 | 0.16 ± 0.01 b | 5842.60 ± 42.74 a | 0.21 ± 0.06 b | 0.14 ± 0.08 b | 0.22 ± 0.01 b | 0.04 ± 0.04 b | 0.96 ± 0.12 |
| BBCH_81 | 0.22 ± 0.02 b | 4773.06 ± 34.54 b | 0.32 ± 0.09 b | 0.20 ± 0.09 b | 0.20 ± 0.05 b | 0.09 ± 0.03 b | 1.19 ± 0.25 |
| BBCH_89 | 0.33 ± 0.02 a | 208.33 ± 26.31 c | 0.67 ± 0.07 a | 0.81 ± 0.08 a | 0.58 ± 0.04 a | 0.64 ± 0.07 a | 1.17 ± 0.14 |
| Cultivar (C) y | |||||||
| Kuntra | 0.33 ± 0.02 a | 2987.44 ± 67.76 c | 0.53 ± 0.08 | 0.39 ± 0.04 | 0.46 ± 0.04 a | 0.32 ± 0.03 a | 1.61 ± 0.15 a |
| Bozcaada Çavuşu*Kuntra | 0.21 ± 0.03 bc | 1880.03 ± 38.41 d | 0.33 ± 0.04 | 0.55 ± 0.09 | 0.23 ± 0.06 b | 0.24 ± 0.07 ab | 0.99 ± 0.13 b |
| Vasilâki | 0.11 ± 0.05 c | 5025.09 ± 42.95 a | 0.36 ± 0.03 | 0.36 ± 0.06 | 0.18 ± 0.07 b | 0.14 ± 0.03 b | 0.84 ± 0.11 b |
| Bozcaada Çavuşu*Vasilâki | 0.30 ± 0.04 ab | 4539.41 ± 34.68 b | 0.38 ± 0.04 | 0.24 ± 0.09 | 0.47 ± 0.05 a | 0.35 ± 0.05 a | 0.98 ± 0.18 b |
| Significance | |||||||
| S | 0.0002 *** | 0.0002 *** | 0.0002 *** | 0.0003 *** | 0.0001 *** | 0.0002 *** | 0.3725 |
| C | 0.0007 *** | 0.0002 *** | 0.3212 | 0.0682 | 0.0003 *** | 0.0001 *** | 0.0041 * |
| S x C | 0.9795 | 0.6983 | 0.7741 | 0.8565 | 0.8755 | 0.9875 | 0.8232 |
| Sampling Time (S) x | Sucrose | Glucose | Fructose | Rhamnose | Galactose | Xylose | Arabinose |
|---|---|---|---|---|---|---|---|
| BBCH_79 | 0.44 ± 0.24 b | 6.04 ± 1.59 b | 6.56 ± 1.09 b | 1.82 ± 0.19 | 1.20 ± 0.40 | 1.92 ± 0.80 | 1.33 ± 0.23 |
| BBCH_81 | 2.28 ± 1.26 a | 11.16 ± 1.37 a | 13.05 ± 1.24 a | 1.81 ± 0.34 | 1.12 ± 0.97 | 1.63 ± 0.23 | 1.17 ± 0.92 |
| BBCH_89 | 2.42 ± 0.21 a | 9.45 ± 1.04 ab | 12.48 ± 1.52 a | 0.75 ± 0.70 | 0.47 ± 0.37 | 1.05 ± 0.26 | 0.98 ± 0.18 |
| Cultivar (C) y | |||||||
| Kuntra | 1.21 ± 0.33 | 7.50 ± 1.97 | 9.02 ± 1.36 | 1.39 ± 0.83 | 0.93 ± 0.27 | 1.52 ± 0.50 | 1.12 ± 0.12 |
| Bozcaada Çavuşu*Kuntra | 1.79 ± 0.53 | 8.76 ± 1.11 | 9.78 ± 1.92 | 1.08 ± 0.77 | 0.67 ± 0.42 | 1.24 ± 0.33 | 0.98 ± 0.24 |
| Vasilâki | 1.65 ± 0.15 | 7.69 ± 1.41 | 9.85 ± 1.86 | 0.99 ± 0.69 | 0.65 ± 0.41 | 1.10 ± 0.31 | 0.98 ± 0.16 |
| Bozcaada Çavuşu*Vasilâki | 2.21 ± 0.08 | 11.60 ± 1.36 | 14.13 ± 1.59 | 2.39 ± 0.25 | 1.47 ± 0.48 | 2.28 ± 0.96 | 1.56 ± 0.34 |
| Significance | |||||||
| S | 0.0001 *** | 0.0068 ** | 0.0039 ** | 0.0715 | 0.0796 | 0.1519 | 0.5495 |
| C | 0.0579 | 0.0881 | 0.1137 | 0.0903 | 0.1475 | 0.1205 | 0.3551 |
| S x C | 0.9848 | 0.9598 | 0. 9287 | 0.9763 | 0.7658 | 0.9449 | 0.8762 |
| Sampling Time (S) x | Vitamin A | Vitamin B1 | Vitamin B2 | Vitamin B6 | Vitamin C |
|---|---|---|---|---|---|
| BBCH_79 | 4.57 ± 0.96 b | 8.19 ± 0.46 b | 10.10 ± 1.94 b | 26.80 ± 2.13 b | 27.30 ± 2.02 b |
| BBCH_81 | 5.81 ± 0.84 a | 12.03 ± 0.47 a | 21.80 ± 1.34 a | 63.70 ± 3.97 a | 58.40 ± 3.10 a |
| BBCH_89 | 6.24 ± 0.20 a | 13.24 ± 0.67 a | 23.00 ± 1.53 a | 68.10 ± 2.14 a | 62.00 ± 3.36 a |
| Cultivar (C) y | |||||
| Kuntra | 4.77 ± 0.36 | 9.88 ± 0.10 | 14.40 ± 1.69 b | 43.00 ± 3.23 c | 38.00 ± 2.56 b |
| Bozcaada Çavuşu*Kuntra | 5.56 ± 0.95 | 10.71 ± 0.32 | 17.30 ± 1.76 ab | 43.90 ± 3.19 bc | 39.60 ± 2.74 b |
| Vasilâki | 5.89 ± 0.43 | 11.58 ± 0.96 | 20.00 ± 1.10 ab | 59.60 ± 3.83 ab | 55.00 ± 3.95 a |
| Bozcaada Çavuşu*Vasilâki | 5.93 ± 0.82 | 12.45 ± 0.47 | 21.50 ± 1.14 a | 64.90 ± 4.27 a | 64.40 ± 3.98 a |
| Significance | |||||
| S | 0.0008 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** |
| C | 0.0603 | 0.0635 | 0.0181 * | 0.0011 ** | 0.0001 *** |
| S x C | 0.6257 | 0. 5907 | 0.9876 | 0.9218 | 0.8748 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, O.; Delavar, H.; Ates, F.; Sahin, M.; Keskin, N.; Yilmaz, T.; Turan, M.; Hatterman-Valenti, H. Pollinator Diversity and Phenological Interplay: Exploring Mineral, Hormonal, Sugar, and Vitamin Contents in Vitis vinifera L. cv Bozcaada Çavuşu. Plants 2024, 13, 1612. https://doi.org/10.3390/plants13121612
Kaya O, Delavar H, Ates F, Sahin M, Keskin N, Yilmaz T, Turan M, Hatterman-Valenti H. Pollinator Diversity and Phenological Interplay: Exploring Mineral, Hormonal, Sugar, and Vitamin Contents in Vitis vinifera L. cv Bozcaada Çavuşu. Plants. 2024; 13(12):1612. https://doi.org/10.3390/plants13121612
Chicago/Turabian StyleKaya, Ozkan, Hava Delavar, Fadime Ates, Muge Sahin, Nurhan Keskin, Turhan Yilmaz, Metin Turan, and Harlene Hatterman-Valenti. 2024. "Pollinator Diversity and Phenological Interplay: Exploring Mineral, Hormonal, Sugar, and Vitamin Contents in Vitis vinifera L. cv Bozcaada Çavuşu" Plants 13, no. 12: 1612. https://doi.org/10.3390/plants13121612
APA StyleKaya, O., Delavar, H., Ates, F., Sahin, M., Keskin, N., Yilmaz, T., Turan, M., & Hatterman-Valenti, H. (2024). Pollinator Diversity and Phenological Interplay: Exploring Mineral, Hormonal, Sugar, and Vitamin Contents in Vitis vinifera L. cv Bozcaada Çavuşu. Plants, 13(12), 1612. https://doi.org/10.3390/plants13121612











