A Transcriptomic and Metabolomic Study on the Biosynthesis of Iridoids in Phlomoides rotata from the Qinghai–Tibet Plateau
Abstract
1. Introduction
2. Results
2.1. Analysis of Leaf Metabolome Data of P. rotata Leaves in Different Regions
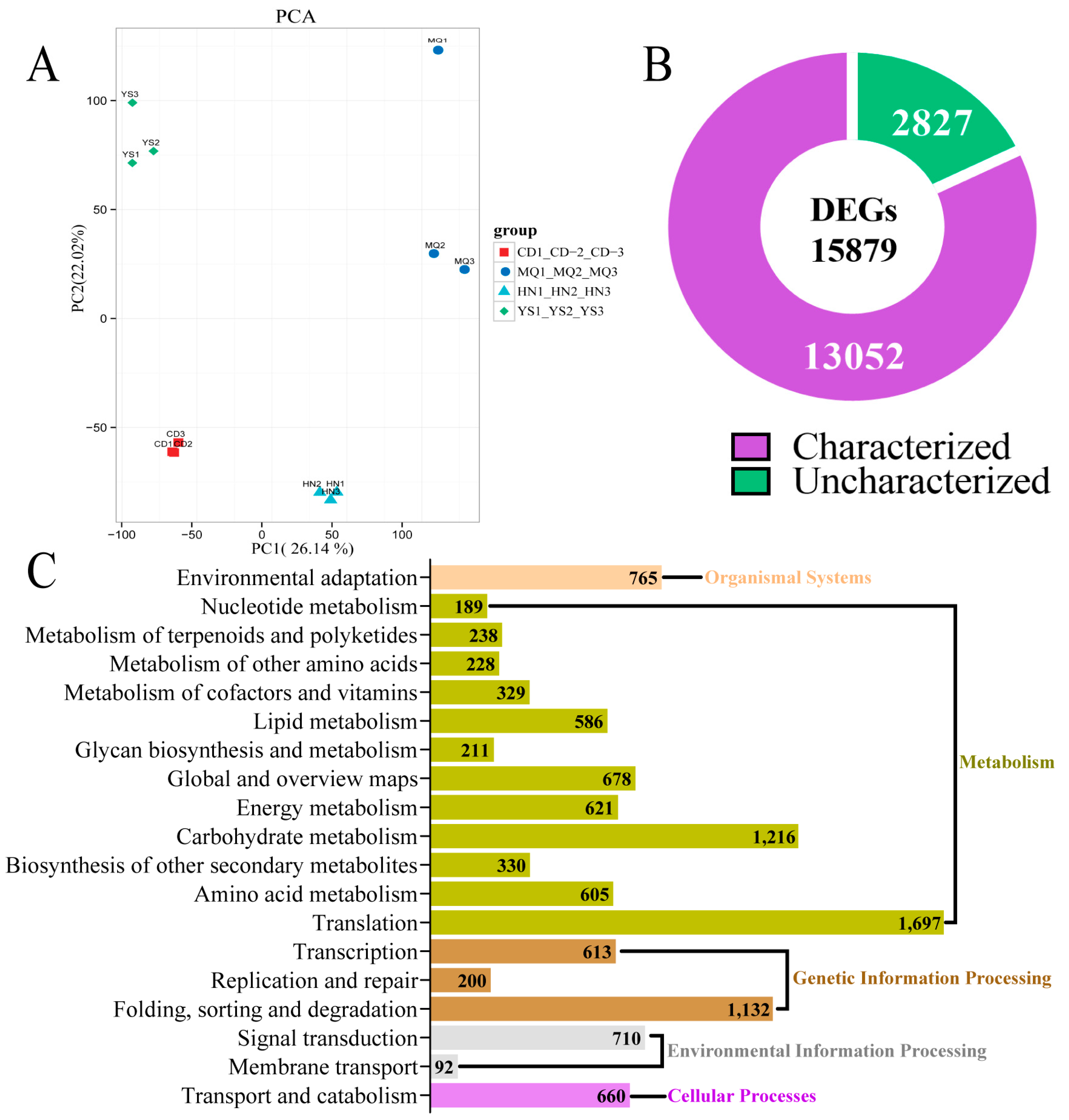
2.2. Overview of Transcriptome Sequencing Data of P. rotata Leaves from four Different Regions
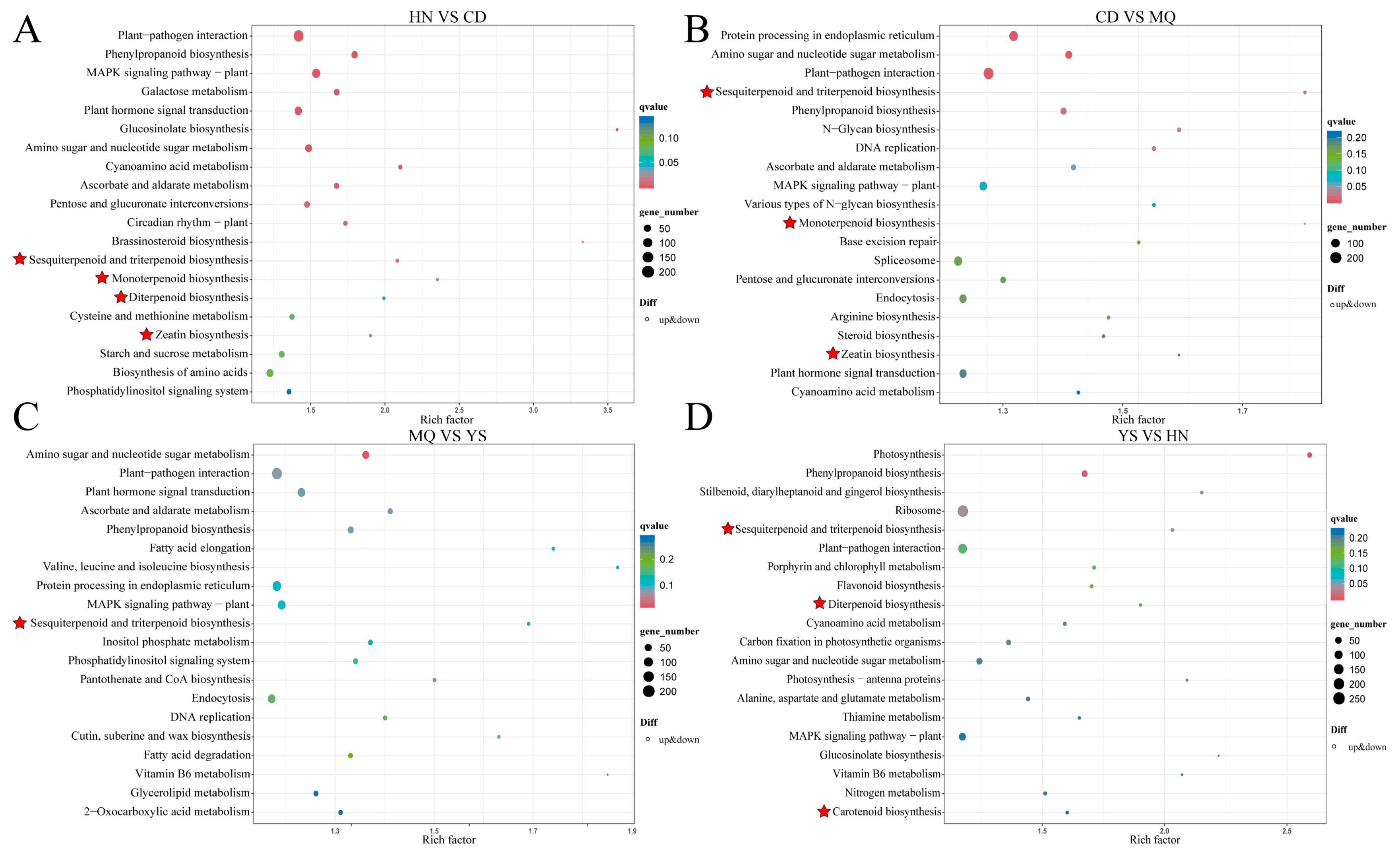
2.3. KEGG Enrichment of DEGs in Different Groups of Samples
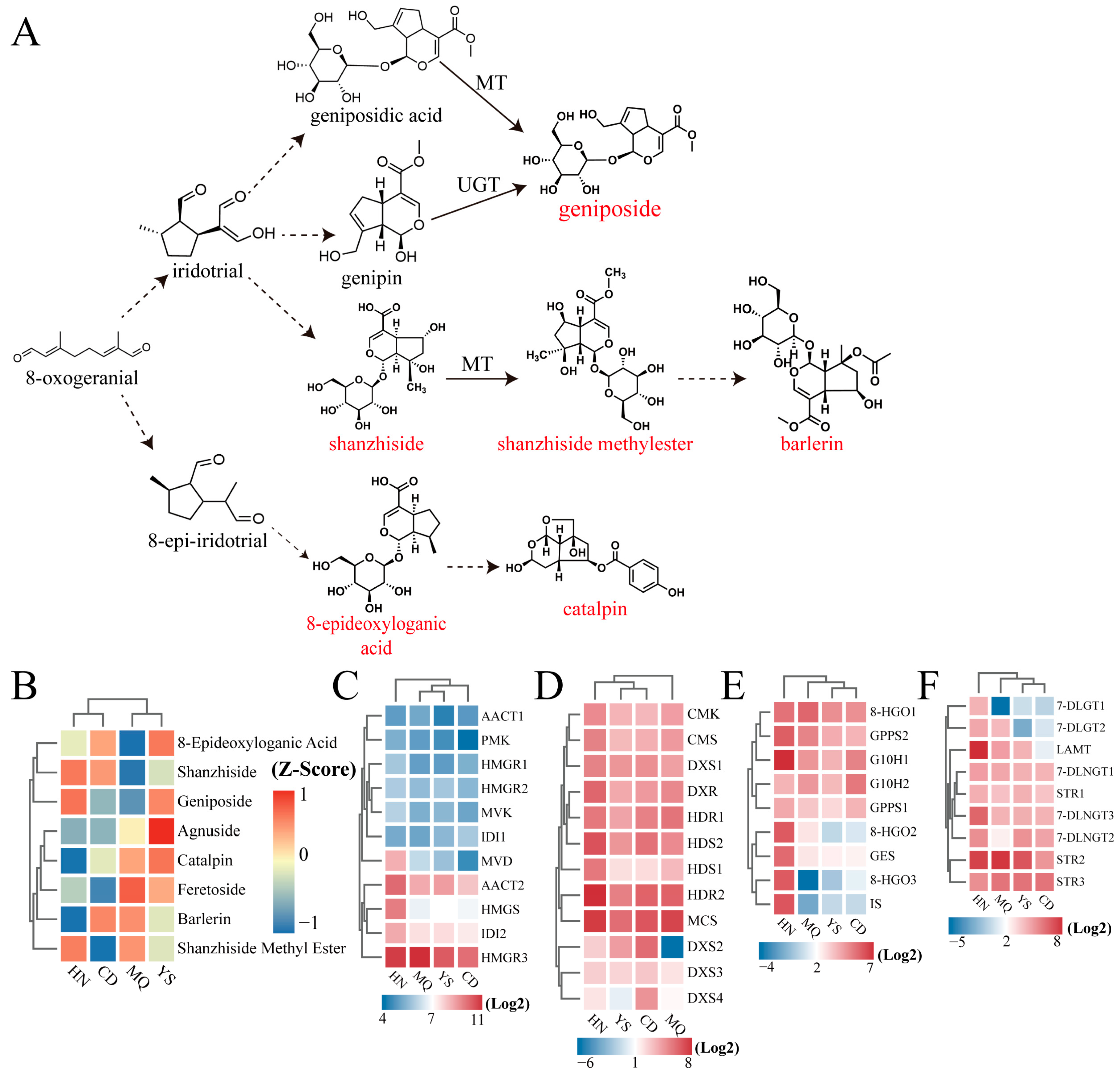
2.4. Regulation of MVA/MEP/Iridoid Pathway Genes in Different Regions
2.5. Analysis of Physicochemical Properties of P. rotata Rhizosphere Soil
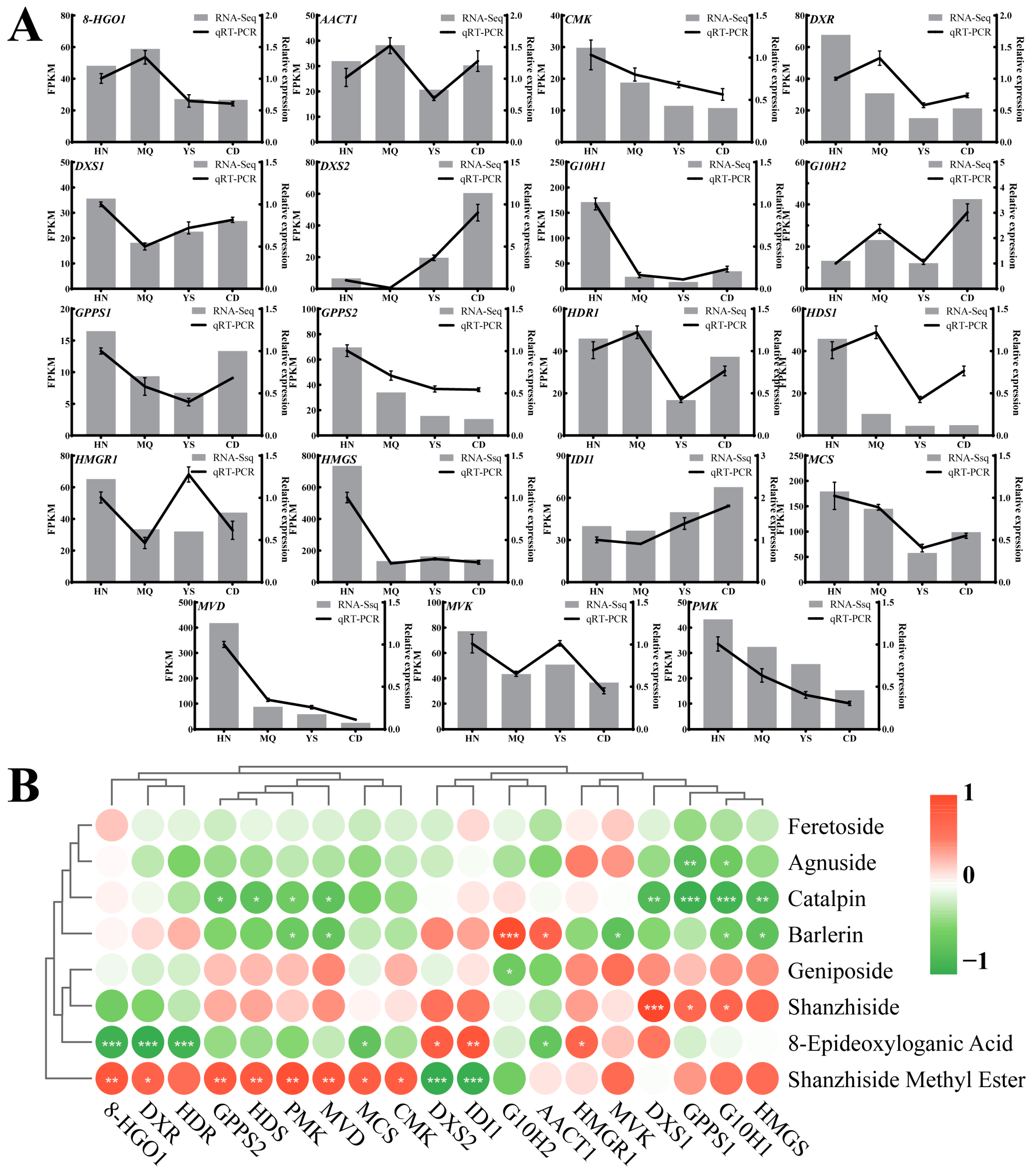
2.6. Validation of DEGs Related to the Metabolism of Iridoids by qRT-PCR
3. Discussion
3.1. Iridoid Compounds Found in P. rotata Leaves
3.2. Correlation Analysis between the Contents of Iridoid Metabolites and Soil Nutrients
3.3. Correlation Analysis between the Content of Iridoid Metabolites and the Expression of Related Genes
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of Samples from the Leaves of P. rotata
4.3. LC-MS/MS Analysis
4.4. Qualitative and Quantitative Analysis of Metabolites
4.5. Transcriptome Sequencing and Data Analyses
4.6. Soil Sample Collection and Physicochemical Property Measurements
4.7. qRT PCR Analysis
4.8. Data Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liu, M.; Chen, P.; Liu, H.; Wang, Y.; Yang, D.; Zhao, Z.; Ding, P. Iridoids with anti-inflammatory effect from the aerial parts of Morinda officinalis How. Fitoterapia 2021, 153, 104991. [Google Scholar] [CrossRef]
- Ma, Q.; Lu, Y.; Deng, Y.; Hu, X.; Li, W.; Jia, H.; Guo, Y.; Shi, X. Optimization for ultrasonic-microwave synergetic extraction of total iridoid glycosides and screening of analgesic and anti-inflammatory active fractions from patrinia scabra Bunge (Valerianaceae). BMC Complement. Med. Ther. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Kharb, A.; Chauhan, R.S. Complexity of gene paralogues resolved in biosynthetic pathway of hepatoprotective iridoid glycosides in a medicinal herb, Picrorhiza kurroa through differential NGS transcriptomes. Mol. Genet. Genom. 2021, 296, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Zhang, S.; Lu, Q.; Sun, Y.; Tan, S.; Chen, F.; Tang, L. Protective effects of n-Butanol extract and iridoid glycosides of Veronica ciliata Fisch. Against ANIT-induced cholestatic liver injury in mice. J. Ethnopharmacol. 2021, 266, 113432. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fan, L.; Feng, S.; Ding, X.; An, X.; Chen, J.; Wang, M.; Zhai, X.; Li, Y. Network pharmacology of iridoid glycosides from Eucommia ulmoides Oliver against osteoporosis. Sci. Rep. 2022, 12, 7430. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Li, Z.; Yang, T.; Shen, X.; Wang, X.; Li, H.; Zhou, K.; Li, L.; Xia, Z.; Zheng, X.; et al. Therapeutic potential of plant iridoids in depression: A review. Pharm. Biol. 2022, 60, 2167–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Liu, J.; Chen, K.; Li, Y. Iridoids and derivatives from Catalpa ovata with their antioxidant activities. Fitoterapia 2023, 169, 105599. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Sherden, N.H.; Lichman, B.; Caputi, L.; Zhao, D.; Kamileen, M.O.; Buell, C.R.; O’Connor, S.E. Identification of iridoid synthases from Nepeta species: Iridoid cyclization does not determine nepetalactone stereochemistry. Phytochemistry 2018, 145, 48–56. [Google Scholar] [CrossRef]
- Kharb, A.; Sharma, S.; Sharma, A.; Nirwal, N.; Pandey, R.; Bhattacharyya, D.; Chauhan, R.S. Capturing acyltransferase(s) transforming final step in the biosynthesis of a major Iridoid Glycoside, (Picroside-II) in a Himalayan Medicinal Herb, Picrorhiza kurroa. Mol. Biol. Rep. 2022, 49, 5567–5576. [Google Scholar] [CrossRef]
- Rodríguez-López, C.E.; Jiang, Y.; Kamileen, M.O.; Lichman, B.R.; Hong, B.; Vaillancourt, B.; Buell, C.R.; O’Connor, S.E. Phylogeny-Aware Chemoinformatic Analysis of Chemical Diversity in Lamiaceae Enables Iridoid Pathway Assembly and Discovery of Aucubin Synthase. Mol. Biol. Evol. 2022, 39, msac057. [Google Scholar] [CrossRef] [PubMed]
- Kouda, R.; Yakushiji, F. Recent Advances in Iridoid Chemistry: Biosynthesis and Chemical Synthesis. Chem. Asian J. 2020, 15, 3771–3783. [Google Scholar] [CrossRef]
- Jin, C.; Li, Z.; Li, Y.; Wang, S.; Li, L.; Liu, M.; Ye, J. Transcriptome analysis of terpenoid biosynthetic genes and simple sequence repeat marker screening in Eucommia ulmoides. Mol. Biol. Rep. 2020, 47, 1979–1990. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, Y.; Zhou, W.H.; Feng, R.Z.; Shuai, Y.K.; Yang, L.; Liu, M.J.; He, X.Y.; Wei, Q. Transcriptome and metabolome reveal the accumulation of secondary metabolites in different varieties of Cinnamomum longepaniculatum. BMC Plant Biol. 2022, 22, 243. [Google Scholar] [CrossRef]
- Jia, Y.; Yin, X.; Yang, H.; Xiang, Y.; Ding, K.; Pan, Y.; Jiang, B.; Yong, X. Transcriptome Analyses Reveal the Aroma Terpeniods Biosynthesis Pathways of Primula forbesii Franch. and the Functional Characterization of the PfDXS2 Gene. Int. J. Mol. Sci. 2023, 24, 12730. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Yi, X.; Li, Z.; Ai, Y.; Ma, S.; Chen, Q. A Comparative Transcriptomic with UPLC-Q-Exactive MS Reveals Differences in Gene Expression and Components of Iridoid Biosynthesis in Various Parts of Gentiana macrophylla. Genes 2022, 13, 2372. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Shi, R.; Tao, R.; Fang, Q.; Jiang, X.; Ji, H.; Feng, L.; Huang, L. Cloning, molecular characterization and functional analysis of 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase (HDR) gene for diterpenoid tanshinone biosynthesis in Salvia miltiorrhiza Bge. f. alba. Plant Physiol. Biochem. 2013, 70, 21–32. [Google Scholar] [CrossRef]
- Li, R.; Chen, P.; Zhu, L.; Wu, F.; Chen, Y.; Zhu, P.; Ji, K. Characterization and Function of the 1-Deoxy-D-xylose-5-Phosphate Synthase (DXS) Gene Related to Terpenoid Synthesis in Pinus massoniana. Int. J. Mol. Sci. 2021, 22, 848. [Google Scholar] [CrossRef]
- Xing, B.; Yang, D.; Yu, H.; Zhang, B.; Yan, K.; Zhang, X.; Han, R.; Liang, Z. Overexpression of SmbHLH10 enhances tanshinones biosynthesis in Salvia miltiorrhiza hairy roots. Plant Sci. 2018, 276, 229–238. [Google Scholar] [CrossRef]
- Wang, C.; Hao, X.; Wang, Y.; Maoz, I.; Zhou, W.; Zhou, Z.; Kai, G. Identification of WRKY transcription factors involved in regulating the biosynthesis of the anti-cancer drug camptothecin in Ophiorrhiza pumila. Hortic. Res. 2022, 9, uhac099. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Patra, B.; Paul, P.; Liu, Y.; Pattanaik, S.; Yuan, L. BHLH IRIDOID SYNTHESIS 3 is a member of a bHLH gene cluster regulating terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Direct 2021, 5, e00305. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.H.; Qin, S.S.; Zang, E.H.; Li, C.; Gao, L.; Li, Q.C.; Wang, Y.L.; Huang, X.Z.; Zhang, Z.Y.; Li, M.-H. Traditional uses, phytochemistry, pharmacology and toxicology of Lamiophlomis rotata (Benth.) Kudo: A review. RSC Adv. 2020, 10, 11463–11474. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Fan, G.; Yang, R.P.; Luo, W.Z.; Zhou, X.D.; Zhang, Y. Discriminating Lamiophlomis rotata According to Geographical Origin by (1)H-NMR Spectroscopy and Multivariate Analysis. Phytochem. Anal. 2015, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Zheng, T.T.; Shi, L.; Zhang, Z.G.; Niu, T.M.; Wang, Q.Y.; Zhao, D.S.; Li, W.; Zhao, P. Lamiophlomis herba: A comprehensive overview of its chemical constituents, pharmacology, clinical applications, and quality control. Biomed. Pharmacother. 2021, 144, 112299. [Google Scholar] [CrossRef] [PubMed]
- La, M.; Zhang, F.; Gao, S.; Liu, X.; Wu, Z.; Sun, L.; Tao, X.; Chen, W. Constituent analysis and quality control of Lamiophlomis rotata by LC-TOF/MS and HPLC-UV. J. Pharm. Biomed. Anal. 2015, 102, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, Y.; Jiang, S.; Chen, Y.; Zhang, Y.; Pan, Z. The similarity and variability of the iridoid glycoside profile and antioxidant capacity of aerial and underground parts of Lamiophlomis rotata according to UPLC-TOF-MS and multivariate analyses. RSC Adv. 2018, 8, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Chen, R.; Zhong, M.; Wang, G.; Jiang, G.; Tao, X.; Chen, M.; Jiang, Y. Exploring the pharmacological mechanisms and key active ingredients of total flavonoids from Lamiophlomis Rotata (Benth.) Kudo against rheumatoid arthritis based on multi-technology integrated network pharmacology. J. Ethnopharmacol. 2023, 317, 116850. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Chen, Z.; Lei, L.; Geng, X.; Zhang, Y.; Yang, C.; Cao, W.; Pan, Z. The total polyphenolic glycoside extract of Lamiophlomis rotata ameliorates hepatic fibrosis through apoptosis by TGF-β/Smad signaling pathway. Chin. Med. 2023, 18, 20. [Google Scholar] [CrossRef]
- Yang, C.; Geng, X.; Wan, G.; Song, L.; Wang, Y.; Zhou, G.; Wang, J.; Pan, Z. Transcriptomic and proteomic investigation of the ameliorative effect of total polyphenolic glycoside extract on hepatic fibrosis in Lamiophlomis rotata Kudo via the AGE/RAGE pathway. J. Ethnopharmacol. 2024, 324, 117720. [Google Scholar] [CrossRef]
- Lei, L.; Wan, G.; Geng, X.; Sun, J.; Zhang, Y.; Wang, J.; Yang, C.; Pan, Z. The total iridoid glycoside extract of Lamiophlomis rotata Kudo induces M2 macrophage polarization to accelerate wound healing by RAS/ p38 MAPK/NF-κB pathway. J. Ethnopharmacol. 2023, 307, 116193. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.C.; Ma, H.P.; Hao, Y.; He, X.R.; Sun, A.J.; Jiang, W.; Li, M.X.; Jing, L.L.; He, L.; Ma, J.; et al. A new anti-fibrinolytic hemostatic compound 8-O-acetyl shanzhiside methylester extracted from Lamiophlomis rotata. J. Ethnopharmacol. 2016, 187, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shang, X.; Zhang, R.; Jia, Z.; Fan, P.; Ying, Q.; Wei, L. Antinociceptive and anti-inflammatory activities of iridoid glycosides extract of Lamiophlomis rotata (Benth.) Kudo. Fitoterapia 2010, 81, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, S.; Dong, Q.; Dang, J.; Liu, Z.; Han, H.; Tao, Y.; Yue, H. Anti-rheumatoid arthritis effects of iridoid glucosides from Lamiophlomis rotata (Benth.) kudo on adjuvant-induced arthritis in rats by OPG/RANKL/NF-κB signaling pathways. J. Ethnopharmacol. 2021, 266, 113402. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Lu, Y.; Geng, G.; Zhou, L.; Chen, Z.; Wang, L.; Xie, H.; Qiu, Q.S. Flavonoid synthesis in Lamiophlomis rotata from Qinghai-Tibet Plateau is influenced by soil properties, microbial community, and gene expression. J. Plant Physiol. 2023, 287, 154043. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Liang, S.; Wang, X.; Duan, L.; Jiang-Yan, F.; Yang, J.; Zhan, R.; Ma, D. Transcriptome analysis and targeted metabolic profiling for pathway elucidation and identification of a geraniol synthase involved in iridoid biosynthesis from Gardenia jasminoides. Ind. Crops Prod. 2019, 132, 48–58. [Google Scholar] [CrossRef]
- Li, M.; Zhang, R.; Jia, Z.; Sheng, J.; Qiu, J.; Wang, J. Isolation and identification of hemostatic ingredients from lamiophlomis rotata (Benth.) kudo. Phytother. Res. 2009, 23, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gong, N.; Fan, H.; Peng, C.S.; Ding, X.J.; Jiang, Y.; Wang, Y.X. Lamiophlomis rotata, an orally available Tibetan herbal painkiller, specifically reduces pain hypersensitivity states through the activation of spinal glucagon-like peptide-1 receptors. Anesthesiology 2014, 121, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Sogame, M.; Naraki, Y.; Sasaki, T.; Seki, M.; Yokota, K.; Masada, S.; Hakamatsuka, T. Quality Assessment of Medicinal Product and Dietary Supplements Containing Vitex agnus-castus by HPLC Fingerprint and Quantitative Analyses. Chem. Pharm. Bull. 2019, 67, 527–533. [Google Scholar] [CrossRef]
- Shah, S.; Dhanani, T.; Kumar, S. Validated HPLC method for identification and quantification of p-hydroxy benzoic acid and agnuside in Vitex negundo and Vitex trifolia. J. Pharm. Anal. 2013, 3, 500–508. [Google Scholar] [CrossRef]
- Pandey, A.; Bani, S.; Satti, N.K.; Gupta, B.D.; Suri, K.A. Anti-arthritic activity of agnuside mediated through the down-regulation of inflammatory mediators and cytokines. Inflamm. Res. 2012, 61, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, S.; Zhang, X.; Xing, J.; Liu, Z.; Song, F. A strategy for identification and structural characterization of compounds from Gardenia jasminoides by integrating macroporous resin column chromatography and liquid chromatography-tandem mass spectrometry combined with ion-mobility spectrometry. J. Chromatogr. A 2016, 1452, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.W.; Kim, S.Y.; Yoon, T.; Lee, Y.J.; Kil, Y.S.; Lee, Y.S.; Seo, E.K. Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents. Chem. Biodivers. 2013, 10, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, F.; Lu, S.; Ren, L.; Bian, S.; Liu, M.; Zhao, D.; Wang, S.; Wang, J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol. 2022, 283, 114739. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lu, X.; Wei, T.; Dong, Y.; Cai, Z.; Tang, L.; Liu, M. Asperuloside and Asperulosidic Acid Exert an Anti-Inflammatory Effect via Suppression of the NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages. Int. J. Mol. Sci. 2018, 19, 2027. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, S.; Lee, C.; Park, J.; Yang, G.; Yook, T. Verbenalin Reduces Amyloid-Beta Peptide Generation in Cellular and Animal Models of Alzheimer’s Disease. Molecules 2022, 27, 8678. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Du, C.; Xu, C.; Wang, Q.; Wang, Z.; Zhu, Q.; Lv, X.; Zhang, L.; Li, J.; Huang, C.; et al. Verbenalin attenuates hepatic damage and mitochondrial dysfunction in alcohol-associated steatohepatitis by regulating MDMX/PPARα-mediated ferroptosis. J. Ethnopharmacol. 2023, 307, 116227. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Guo, L.; Yan, X.; Wang, T.; Li, Z. Effects of verbenalin on prostatitis mouse model. Saudi J. Biol. Sci. 2016, 23, S148–S157. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, X.; Liu, Z.; Liu, K.; Huang, Y. Spatial distribution of soil nutrients and evaluation of cultivated land in Xuwen county. PeerJ 2022, 10, e13239. [Google Scholar] [CrossRef]
- Duan, M.; Lu, M.; Lu, J.; Yang, W.; Li, B.; Ma, L.; Wang, L. Soil Chemical Properties, Metabolome, and Metabarcoding Give the New Insights into the Soil Transforming Process of Fairy Ring Fungi Leucocalocybe mongolica. J. Fungi 2022, 8, 680. [Google Scholar] [CrossRef]
- Fan, D.; Zhao, Z.; Wang, Y.; Ma, J.; Wang, X. Crop-type-driven changes in polyphenols regulate soil nutrient availability and soil microbiota. Front. Microbiol. 2022, 13, 964039. [Google Scholar] [CrossRef] [PubMed]
- Chludil, H.D.; Corbino, G.B.; Leicach, S.R. Soil quality effects on Chenopodium album flavonoid content and antioxidant potential. J. Agric. Food Chem. 2008, 56, 5050–5056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chen, Y.; Zeng, L.; Cui, Y.; Li, J.; Tang, H.; Liu, J.; Tang, J. Soil nutrient deficiency decreases the postharvest quality-related metabolite contents of tea (Camellia sinensis (L.) Kuntze) leaves. Food Chem. 2022, 377, 132003. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Baldy, V.; Ballini, C.; Fernandez, C. Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: Effect of soil nutrients. J. Chem. Ecol. 2008, 34, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.A.; Seastedt, T.R.; Bowers, M.D. Nitrogen enrichment differentially affects above- and belowground plant defense. Am. J. Bot. 2012, 99, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Su, P.; Zhao, Y.; Zhang, M.; Wang, X.; Liu, Y.; Zhang, X.; Gao, W.; Huang, L. Molecular Cloning and Characterization of DXS and DXR Genes in the Terpenoid Biosynthetic Pathway of Tripterygium wilfordii. Int. J. Mol. Sci. 2015, 16, 25516–25535. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Patra, B.; Singleton, J.J.; Liu, Y.; Paul, P.; Sui, X.; Suttipanta, N.; Pattanaik, S.; Yuan, L. Identification and Characterization of Transcription Factors Regulating Terpenoid Indole Alkaloid Biosynthesis in Catharanthus roseus. Methods Mol. Biol. 2022, 2505, 203–221. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, F.; Li, S.; Wang, Y.; Zhou, C.; Shi, M.; Wang, J.; Chen, Y.; Wang, Y.; Wang, H.; et al. Molecular cloning and characterization of two 1-deoxy-D-xylulose-5-phosphate synthase genes involved in tanshinone biosynthesis in Salvia miltiorrhiza. Mol. Breed. 2016, 36, 124. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Zhang, B.; Jin, X.; Zhang, H.; Jin, Z. Transcriptional Analysis of Metabolic Pathways and Regulatory Mechanisms of Essential Oil Biosynthesis in the Leaves of Cinnamomum camphora (L.) Presl. Front. Genet. 2020, 11, 598714. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Pan, G.; Yang, A. Bioinformatics study of 1-deoxy-d-xylulose-5-phosphate synthase (DXS) genes in Solanaceae. Mol. Biol. Rep. 2019, 46, 5175–5184. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Wang, J.; Hu, T.; Tong, Y.; Zhou, J.; Gao, J.; Huang, L.; Gao, W. The expression of TwDXS in the MEP pathway specifically affects the accumulation of triptolide. Physiol. Plant. 2020, 169, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, Z.; Wiberley-Bradford, A.E.; Weise, S.E.; Sharkey, T.D. Isopentenyl diphosphate and dimethylallyl diphosphate/isopentenyl diphosphate ratio measured with recombinant isopentenyl diphosphate isomerase and isoprene synthase. Anal. Biochem. 2013, 440, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, C.; Zhang, F.; Guo, B.; Miao, Z.; Sun, X.; Tang, K. Molecular cloning, expression profiling and functional analyses of a cDNA encoding isopentenyl diphosphate isomerase from Gossypium barbadense. Biosci. Rep. 2009, 29, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Cai, Y.; Zhang, F.; Xia, G.; Xiang, F. Cloning and Functional Analysis of Geraniol 10-Hydroxylase, a Cytochrome P450 from Swertia mussotii Franch. Biosci. Biotechnol. Biochem. 2010, 74, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Collu, G.; Unver, N.; Peltenburg-Looman, A.M.; van der Heijden, R.; Verpoorte, R.; Memelink, J. Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett. 2001, 508, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Valletta, A.; Trainotti, L.; Santamaria, A.R.; Pasqua, G. Cell-specific expression of tryptophan decarboxylase and 10-hydroxygeraniol oxidoreductase, key genes involved in camptothecin biosynthesis in Camptotheca acuminata Decne (Nyssaceae). BMC Plant Biol. 2010, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yang, B.; Komatsu, S.; Lu, X.; Li, X.; Tian, J. Binary stress induces an increase in indole alkaloid biosynthesis in Catharanthus roseus. Front. Plant Sci. 2015, 6, 582. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Roberts, T.L.; Ross, W.J.; Norman, R.J.; Slaton, N.A.; Wilson, C.E., Jr. Predicting Nitrogen Fertilizer Needs for Rice in Arkansas Using Alkaline Hydrolyzable-Nitrogen. Soil. Sci. Soc. Am. J. 2011, 75, 1161–1171. [Google Scholar] [CrossRef]
- Yoo, J.C.; Beiyuan, J.; Wang, L.; Tsang, D.C.W.; Baek, K.; Bolan, N.S.; Ok, Y.S.; Li, X.D. A combination of ferric nitrate/EDDS-enhanced washing and sludgederived biochar stabilization of metal-contaminated soils. Sci. Total Environ. 2018, 616–617, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiao, F.; Geng, G.; Lu, Y. Evaluation of Candidate Reference Genes for Gene Expression Analysis in Wild Lamiophlomis rotata. Genes 2023, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




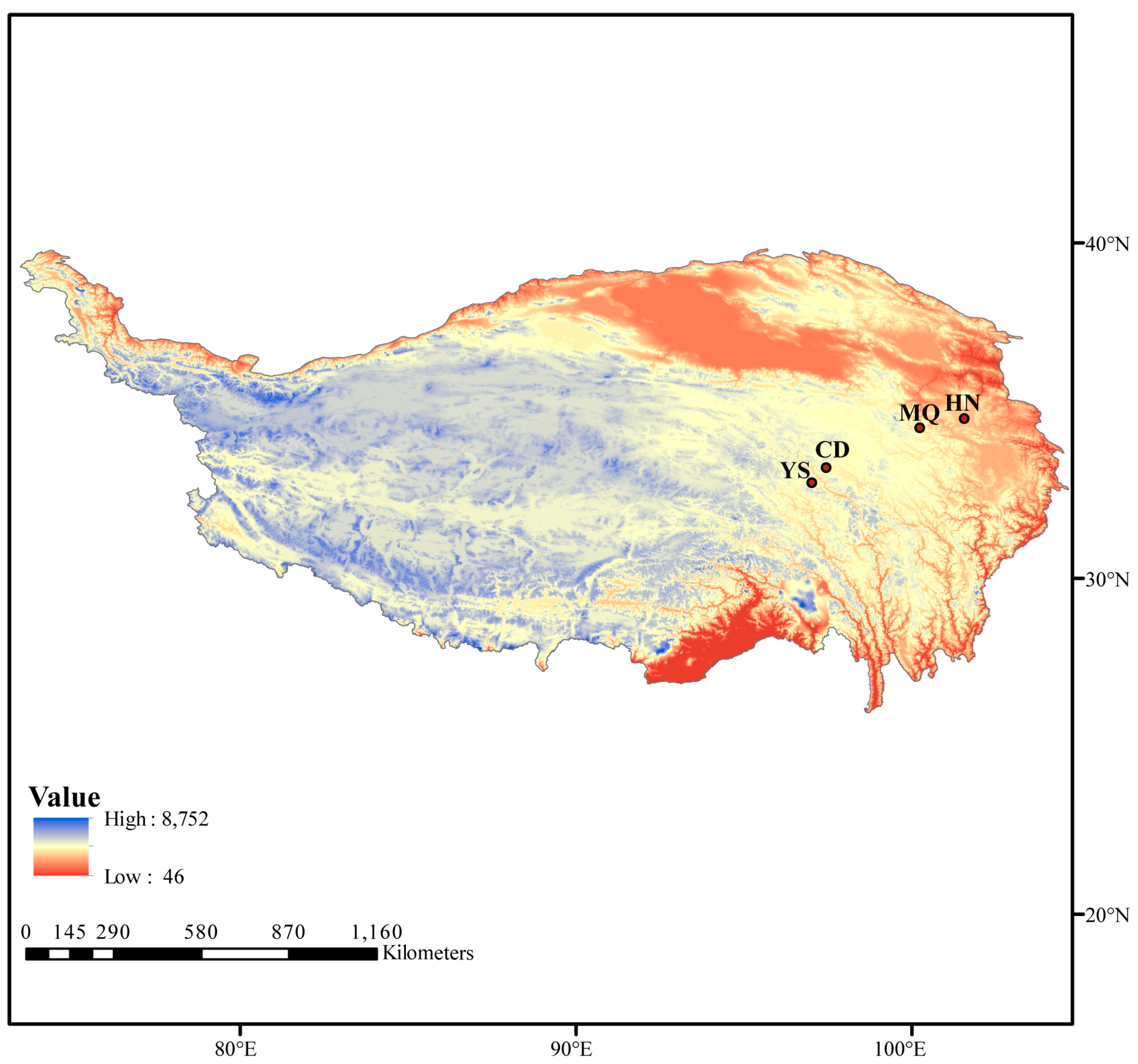
| Sample ID | Sampling Location | Altitude | East Longitude | North Latitude |
|---|---|---|---|---|
| HN | Henan County, Qinghai Province | 3540 m | 101°33′36″ | 34°45′36″ |
| MQ | Maqin County, Qinghai Province | 3750 m | 100°14′38″ | 34°29′10″ |
| YS | Yushu City, Qinghai Province | 3880 m | 97°1′23″ | 32°51′4″ |
| CD | Chengduo County, Qinghai Province | 4270 m | 97°27′16″ | 33°18′2″ |
| Group | DAM Numbers | Up Number | Down Number |
|---|---|---|---|
| CD vs. YS | 299 | 158 | 141 |
| HN vs. MQ | 297 | 174 | 123 |
| HN vs. YS | 282 | 148 | 134 |
| HN vs. CD | 299 | 144 | 155 |
| MQ vs. CD | 270 | 104 | 166 |
| MQ vs. YS | 319 | 132 | 187 |
| Sample | Clean Reads (M) | Clean Bases (G) | GC Content (%) | Q30 (%) |
|---|---|---|---|---|
| CD1 | 20.82 | 6.22 | 46.27 | 94.13 |
| CD2 | 20.61 | 6.16 | 46.32 | 94.95 |
| CD3 | 21.15 | 6.32 | 46.22 | 94.53 |
| MQ1 | 20.43 | 6.10 | 49.12 | 93.59 |
| MQ2 | 23.18 | 6.92 | 47.32 | 95.11 |
| MQ3 | 19.26 | 5.75 | 46.72 | 95.11 |
| HN1 | 22.16 | 6.63 | 45.44 | 95.72 |
| HN2 | 20.58 | 6.16 | 45.40 | 95.25 |
| HN3 | 19.10 | 5.71 | 45.35 | 94.98 |
| YS1 | 21.26 | 6.28 | 46.13 | 93.22 |
| YS2 | 21.39 | 6.32 | 47.20 | 94.23 |
| YS3 | 19.80 | 5.90 | 47.82 | 94.31 |
| Transcript | Unigene | |
|---|---|---|
| Total Number | 73,584 | 29,833 |
| Total Length | 154,415,732 | 48,842,910 |
| 300–500 (bp) | 12,718 | 8543 |
| 500–1000 (bp) | 11,026 | 5933 |
| 1000–2000 (bp) | 11,409 | 5025 |
| 2000+ (bp) | 38,431 | 10,332 |
| Mean Length | 2098.50 | 1637.21 |
| N50 Length | 2940 | 2786 |
| Database Type | Number of Unigenes | Length ≥ 1000 |
|---|---|---|
| COG | 7099 | 5111 |
| GO | 18,406 | 12,329 |
| KEGG | 15,207 | 10,501 |
| KOG | 12,901 | 8894 |
| Pfam | 17,074 | 12,143 |
| Swissprot | 14,647 | 10,377 |
| TrEMBL | 21,454 | 14,062 |
| eggNOG | 17,911 | 12,236 |
| NR | 23,041 | 14,625 |
| Total number of Unigenes | 23,540 | 14,723 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Geng, G.; Xie, H.; Zhou, L.; He, Y.; Li, Z.; Qiao, F. A Transcriptomic and Metabolomic Study on the Biosynthesis of Iridoids in Phlomoides rotata from the Qinghai–Tibet Plateau. Plants 2024, 13, 1627. https://doi.org/10.3390/plants13121627
Wang L, Geng G, Xie H, Zhou L, He Y, Li Z, Qiao F. A Transcriptomic and Metabolomic Study on the Biosynthesis of Iridoids in Phlomoides rotata from the Qinghai–Tibet Plateau. Plants. 2024; 13(12):1627. https://doi.org/10.3390/plants13121627
Chicago/Turabian StyleWang, Luhao, Guigong Geng, Huichun Xie, Lianyu Zhou, Yujiao He, Zuxia Li, and Feng Qiao. 2024. "A Transcriptomic and Metabolomic Study on the Biosynthesis of Iridoids in Phlomoides rotata from the Qinghai–Tibet Plateau" Plants 13, no. 12: 1627. https://doi.org/10.3390/plants13121627
APA StyleWang, L., Geng, G., Xie, H., Zhou, L., He, Y., Li, Z., & Qiao, F. (2024). A Transcriptomic and Metabolomic Study on the Biosynthesis of Iridoids in Phlomoides rotata from the Qinghai–Tibet Plateau. Plants, 13(12), 1627. https://doi.org/10.3390/plants13121627





