Abstract
The nitrogen-stable isotopes of plants can be used to verify the source of fertilizers, but the fertilizer uptake patterns in tea (Camellia sinensis) plants are unclear. In this study, potted tea plants were treated with three types of organic fertilizers (OFs), urea, and a control. The tea leaves were sampled over seven months from the top, middle, and base of the plants and analyzed for the δ15N and nitrogen content, along with the corresponding soil samples. The top tea leaves treated with the rapeseed cake OF had the highest δ15N values (up to 6.6‰), followed by the chicken manure, the cow manure, the control, and the urea fertilizer (6.5‰, 4.1‰, 2.2‰, and 0.6‰, respectively). The soil treated with cow manure had the highest δ15N values (6.0‰), followed by the chicken manure, rapeseed cake, control, and urea fertilizer (4.8‰, 4.0‰, 2.5‰, and 1.9‰, respectively). The tea leaves fertilized with rapeseed cake showed only slight δ15N value changes in autumn but increased significantly in early spring and then decreased in late spring, consistent with the delivery of a slow-release fertilizer. Meanwhile, the δ15N values of the top, middle, and basal leaves from the tea plants treated with the rapeseed cake treatment were consistently higher in early spring and lower in autumn and late spring, respectively. The urea and control samples had lower tea leaf δ15N values than the rapeseed cake-treated tea and showed a generalized decrease in the tea leaf δ15N values over time. The results clarify the temporal nitrogen patterns and isotope compositions of tea leaves treated with different fertilizer types and ensure that the δ15N tea leaf values can be used to authenticate the organic fertilizer methods across different harvest periods and leaf locations. The present results based on a pot experiment require further exploration in open agricultural soils in terms of the various potential fertilizer effects on the different variations of nitrogen isotope ratios in tea plants.
1. Introduction
Tea (Camellia sinensis (L.) Kuntze) is one of the most widely consumed non-alcoholic beverages in the world [1,2]. Tea is well-known for its bioactive compound content, with reported antioxidant, anti-cancer, anti-diabetes, and anti-obesity functions [3,4,5]. In recent years, consumers have become increasingly drawn towards organic tea products as they believe that organic tea is safer and healthier than conventional tea [6,7,8]. However, the superior market prices of organic tea relative to conventional tea increase the risk of adulteration or mislabeling [9]. Previous studies have shown that nitrogen-stable isotopes (δ15N) are useful to identify organic products from their conventional counterparts [10,11], but the temporal change in the nitrogen isotopes in tea plants by different fertilizers is still unclear.
Nitrogen-stable isotopes (δ15N) are mainly related to the nitrogen fixation patterns in plant-microbial symbiosis, soil cultivation, and fertilization methods [12,13]. They have been successfully used to identify organic crops from their conventional counterparts, such as rice, yams, and vegetables [14,15,16]. A key difference between organic and conventional crop cultivation is whether the applied nutrients are derived from organic or chemical fertilizers [17,18]. Studies have shown that the δ15N values of chemical fertilizers are close to air (~0‰), while the δ15N values of organic fertilizers from different sources are between +0.6‰ and 16.2‰ [19,20,21]. The fertilizers commonly applied in China during organic tea cultivation include organic plant fertilizers such as rapeseed cake, and animal wastes such as chicken, pig, or cow manure. Previous studies have used different fertilizer types (including fish meal, rapeseed cake, and chemical fertilizer) to grow tea plants and found that the nitrogen isotope differences among the tea leaves correlated with the planting year and tea variety [22]. There are only a few tea studies that investigate the nitrogen isotope effects from the commonly used fertilizers in China.
Plants assimilate, metabolize, and reflect the nitrogen sources from fertilizers. Different parts of the plant can also vary in N content and 15N abundance according to the δ15N values of the applied fertilizer [23]. The previous studies found that the newly formed leaves on sweet pepper plants had the highest δ15N values, followed by the stems and older leaves, and the lowest values were found in the roots [24]. A few studies discuss the temporal isotopic changes for different parts of tea plants. A previous study showed that the δ15N values of the young tea leaves had more positive δ15N values than the older leaves [25]. Tea is usually harvested in spring, summer, and autumn, and the nitrogen isotopes of tea plants at different collecting periods are also different. Other studies found that, for field-grown tea plants, the δ15N values of the young leaves picked on 20 March 2023 (3.6‰) had lower δ15N values than the young leaves picked on 30 May 2023 (4.2‰) [26]. Accordingly, these dynamic δ15N leaf value changes across different tea collection periods need to be studied in more detail. Most of the previous nitrogen isotope tea studies consist of field trials conducted in tea gardens that are traditionally perched on steep slopes, with highly variable nitrogen leaching, surface runoff, and nitrogen-fixing microbial activities that affect the nutrient input to the tea plant [27]. Researchers have reported that the plant δ15N values in plant–soil systems are closely related to the soil nitrogen content, nitrogen cycling rate, and plant nitrogen uptake strategy [28]. It is necessary to design pot experimental conditions to minimize these external influences, ensuring that only the fertilizer applied to the tea plant is the main nitrogen isotope variable.
In this study, organic fertilizers (OFs, including rapeseed cake, chicken manure, and cow manure), a chemical fertilizer (CF, urea), and no fertilizer (control) were used to carry out pot experiments on tea plants. The nitrogen isotopes were determined for the tea leaves in different spatial positions on the plant during different collecting periods. This study contributes towards understanding the temporal dynamic changes in the nitrogen-stable isotopes in tea plants from different fertilizer types and provides a fundamental basis for the study of the nitrogen isotope composition and transfer mechanism for organic tea.
2. Materials and Methods
2.1. Pot Experiments and Sampling
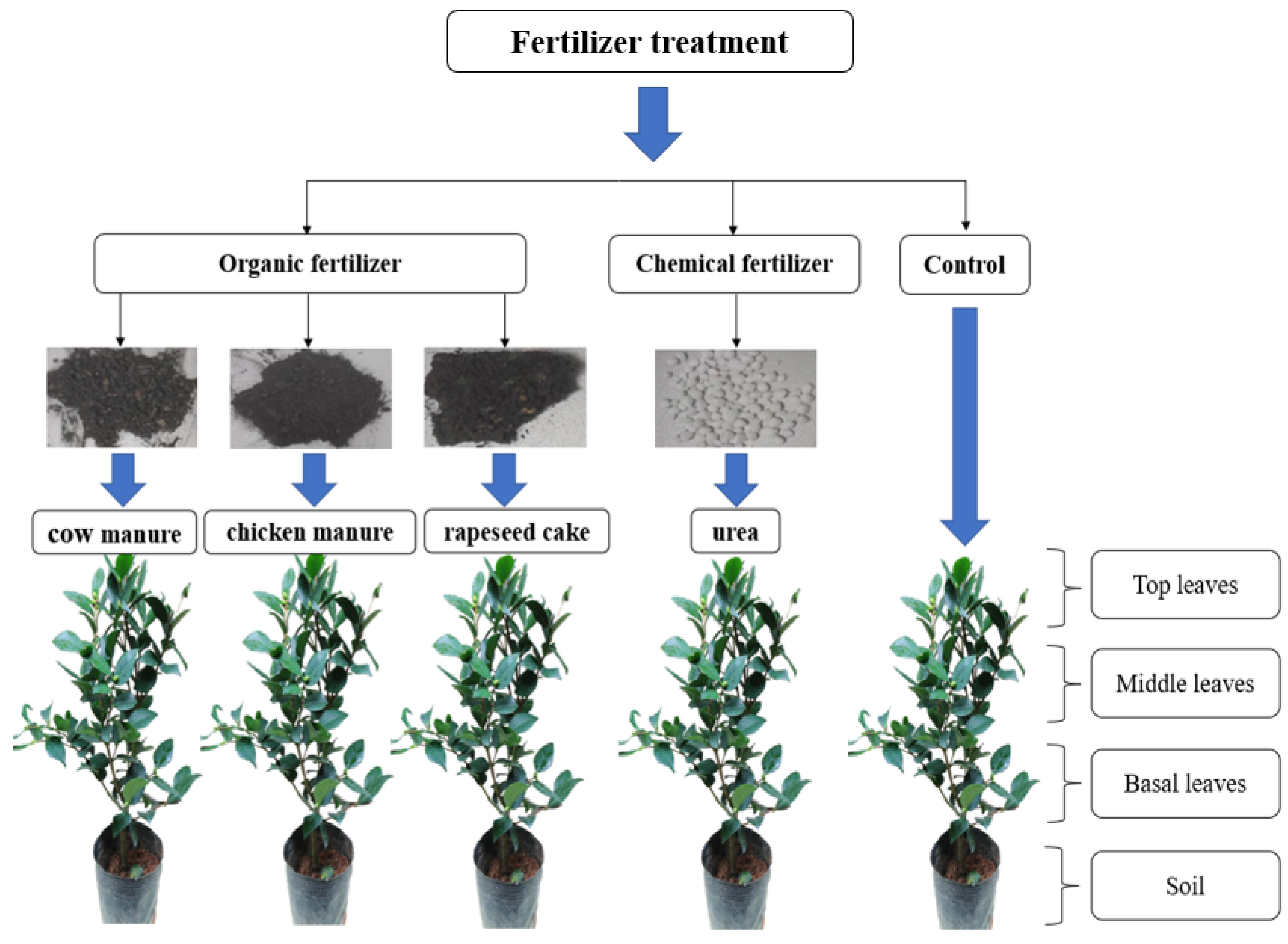
Three-year-old potted tea plants (Longjing 43 variety) were selected for experimental fertilizer trials at the Tea Research Institute, Chinese Academy of Agricultural Sciences, China (latitude 30°17′99.62″ N; longitude 120°09′39.20″ E) at an altitude of 16 m. Five different fertilizer treatments were selected: organic fertilizers (rapeseed cake, chicken manure, and cow manure), a chemical fertilizer (urea), and a control (no fertilizer). Each fertilizer treatment had 3 replicate pots, with a total of 15 pots containing a single tea plant around 60 cm tall. The size of each experimental pot was 20 cm in diameter at the base, 22 cm in diameter at the top, and 20 cm in height and contained around 3 kg of soil in each pot. The initial %N and δ15N values of the tea plants and soil are represented by the control samples. Potted tea plants were fertilized only once at the start of the study (5 October 2022), and cultivation conditions (temperature, irrigation water, humidity, sunshine hours, etc.) were the same as those used by local tea gardens except for the different fertilizer treatments. Fertilizer type, nitrogen content, dosage, and δ15N values used in the experiment are shown in Table 1, with around 1.6 g of nitrogen delivered to each pot at the start of the experiment, apart from the control samples.

Table 1.
Nitrogen content, fertilizer dosage, and δ15N values of fertilizers.
Eight sampling events from each pot were undertaken during the study, covering the period of autumn through to late spring growth. In Hangzhou, tea leaf samples from the same growth phase collected on 20 October and 18 November 2022 were classified as autumn; 17 March, 21 April, and 10 May 2023 as early spring; and 20 May and 30 May 2023 as late spring. Top leaves were collected from the top 10 cm of the plant, middle leaves were collected between 20 and 40 cm above the soil, and basal leaves were collected between 0 and 20 cm above the soil. Around 2–3 g of soil and tea leaves were collected from each pot during each sampling event. Soil samples were air-dried at 25 °C and then ground into fine powder (<100 mesh). The freshly picked tea leaves were vacuum-dried for 48 h, crushed and finely ground (<80 mesh, with an aperture of 0.180 mm), and stored over silica gel in a desiccator prior to analysis. Experimental design of the tea plants is shown in Figure 1.

Figure 1.
Experimental design of the tea plant study.
2.2. Stable Isotope Analysis
Nitrogen content (%N) and isotope values (δ15N) of tea leaves and soil samples were determined using an elemental analyzer (Isotope PYRO cube, Elementar, Langenselbold, Germany) coupled with an isotope ratio mass spectrometer (Isoprime 100, Elementar, Handforth, UK). Multi-point isotope calibration was applied for more accurate measurement using primary reference standard materials provided by IAEA (International Atomic Energy Agency, Vienna, Austria), including B2155 (protein, δ15N = 5.94‰), USGS40 (L-glutamic acid, δ15N = −4.52‰), USGS64 (glycine, δ15N = 1.76‰), and IAEA-N2 ((NH4)2SO4, δ15N = 20.3‰). High-purity N2 gas served as a reference gas, and helium (He) gas was used as the carrier gas during the analytical process. Elemental analyzer oxidation and reduction furnace temperatures were set at 1020 °C and 650 °C, respectively.
Tea leaves (4 mg) and soil (10 mg) were weighed in duplicate (Mettler Toledo, XP6, d = 1 μg, Greifensee, Switzerland) and packed into 6 × 4 mm2 tin capsules for δ15N analysis. Stable isotope ratios are expressed by the following Equation (1):
where δX is the d15N value, and Rsample and Rstandard denote the abundance of the ‘heavy’ to ‘light’ isotope ratio of unknown samples and reference materials [29]. The analytical precision and reproducibility are ±0.2‰ for δ15N.
δX = (Rsample − Rreference)/Rreference
The formulas used to calculate the isotope composition differences between leaves from different parts of the tea plant were as follows in Equation (2):
where δ15N values are from the basal, middle, and top leaves and Δ15N is the difference between two locations.
Δ15Nbasal-middle = δ15Nmiddle − δ15Nbasal or Δ15Nmiddle-top = δ15Ntop − δ 15Nmiddle
2.3. Statistical Analysis
One-way analysis of variance (ANOVA) was performed using SPSS 22.0 software (IBM, Armonk, NY, USA) to calculate the mean and standard deviation (SD) of nitrogen isotope values (δ15N) for leaves of different positions and soil of tea plants. Least significant difference method (LSD) was used to evaluate significant differences between different sets of data, where a significant difference is defined as p < 0.05. Origin 2019b (OriginLab, Northampton, MA, USA) software was used for mapping the sample locations.
3. Results and Discussion
3.1. Fertilizer Effect on Nitrogen Content and δ15N Values of Top Tea Leaves and Soil
A comparison of the top tea leaf %N and δ15N values was conducted after different fertilizer treatments, and the results are shown in Table S1 and Table 2 and Figure 2. Initially, the nitrogen content of the top leaves was similar for all the fertilizer treatments and ranged between 2.5 and 3.0%. During the cooler autumn months (October to November 2022), the N content rose slightly as the tea plant was more dormant, and it stored nitrogen in the leaves. As early spring arrived, a rapid increase was noted in the %N due to the rapid growth of early spring leaves (March to 10 May 2023), and then a further %N decrease (potentially due to cold weather) occurred during late spring (20 May to 30 May 2023), and then, as the weather warmed, the leaf growth resumed.

Table 2.
Nitrogen-stable isotopes (δ15N) of top tea leaves treated with different fertilizers.
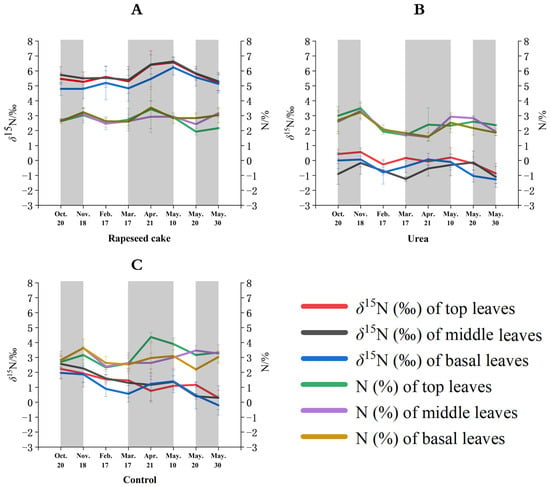
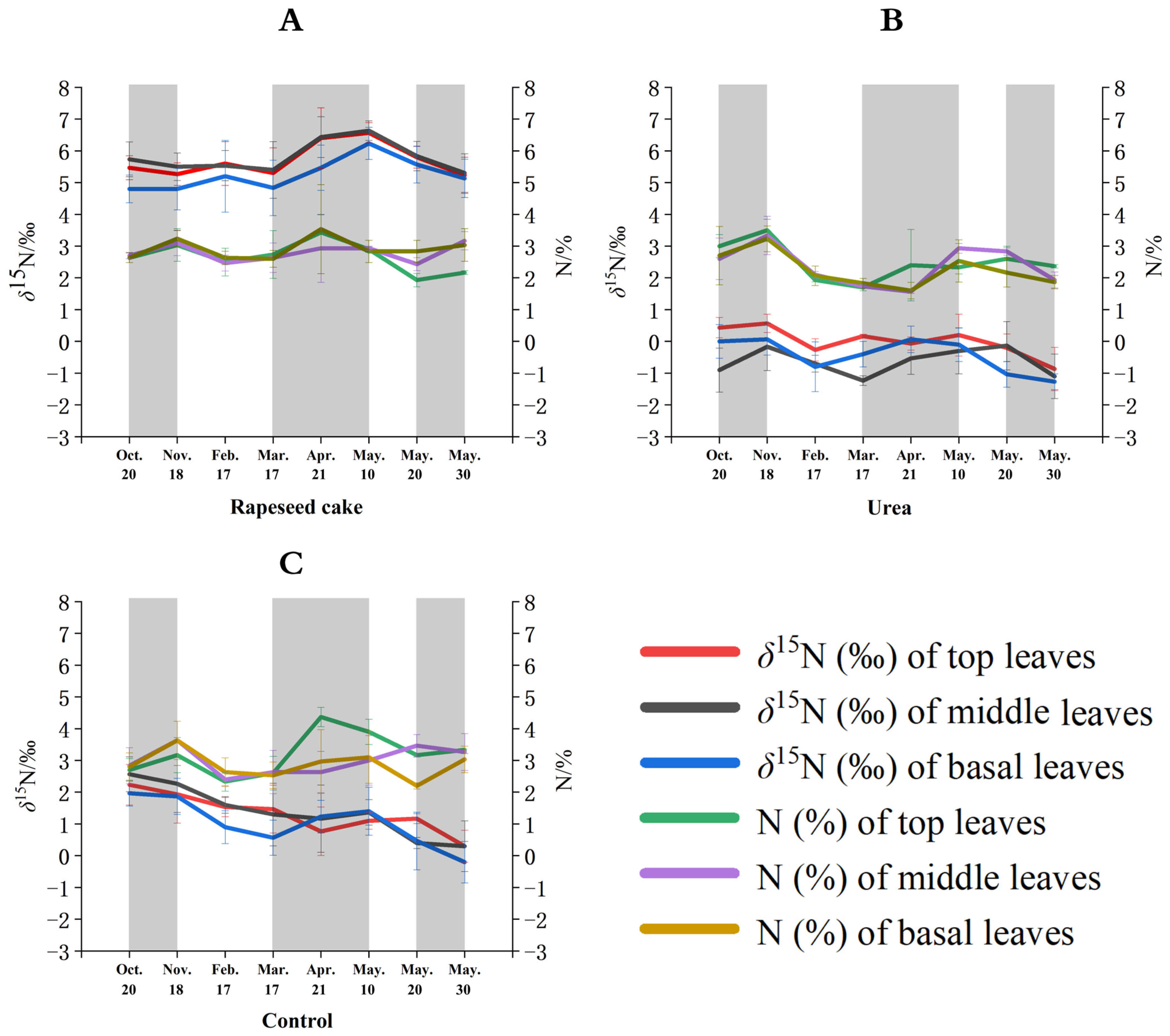
Figure 2.
Temporal %N and δ15N values of different leaves on tea plants using (A) rapeseed cake, (B) urea, and (C) no fertilizer (control) treatments.
Overall, the N content of the tea leaves was similar under different fertilizer treatments. Initially, in autumn, the organically fertilized top tea leaves had slightly lower N content than the tea leaves fertilized using urea or the control pots. The nitrogen from the organic fertilizers is not as chemically available to the plant as the soluble urea fertilizer, but, over the duration of the study, the N content of the urea-treated tea plants decreased more rapidly than that of the organic-treated plants. The organic fertilizers appear to provide a more sustained and continuous delivery of plant nutrients over time compared to chemical fertilizers.
The δ15N values of the top leaves treated with the OFs (chicken manure, rapeseed cake, and cow manure) from each sampling period were significantly more positive (3.4‰ to 6.6‰) than those treated with the CF (−0.9‰ to 0.6‰) and the control samples (0.3‰ to 2.2‰). The δ15N values of the top leaves treated with the CF had the lowest δ15N values of all five treatments. On 20 October and 18 November 2022 and 17 February, 17 March, and 21 April 2023, the δ15N values of the top leaves treated with chicken manure had the highest δ15N values, ranging from 5.4‰ to 6.5‰. However, on 10, 20, and 30 May 2023, the δ15N values of the top leaves treated with the rapeseed cake were higher and ranged from 5.2‰ to 6.6‰. The application of the OF systematically increased the δ15N values of the tea leaves, similar to previous studies of other crops [30,31]. OF is derived from organically grown plant debris or animal waste, providing a comprehensive fertilizer for tea plants with a longer-term nutrient release effect [32,33]. Meanwhile, OFs can improve the net photosynthetic rate, stomatal conductance, transpiration rate, and other photosynthetic characteristics of tea plants, increasing the dry matter accumulation and the δ15N values of tea leaves [34].
The urea application resulted in a decrease in the tea leaves’ δ15N values, which may be related to the preferential discrimination of 15N by nitrate reductase and glutamine synthetase during nutrient uptake and assimilation, as well as the factors including the nutrients provided by the urea being rapidly absorbed by the tea plants, nitrogen leaching, and poor microbial activity [35,36,37]. While the OF δ15N values of the chicken manure and cattle manure were similar to each other, and significantly higher than those of the rapeseed cake (Table 1), the resulting δ15N values of the tea leaves treated with chicken manure and rapeseed cake were higher than those of the tea plants treated with cow manure (Table 2). This may be attributed to the urea or ammonia content found in fresh manure, which may cause a temporary decrease in tea leaf δ15N values due to mineralization. Cow manure (as a fertilizer) is also prone to nitrogen leaching and results in lower microbial activity than chicken manure or rapeseed cake fertilizer, resulting in lower δ15N values. Previous studies have also shown that the content of ammonia nitrogen in cow manure is low, and tea trees preferentially absorb ammonia nitrogen [38,39]. In previous ecology studies, some papers reported that the soil nitrogen composition is the main factor affecting plant δ15N values, and the plant leaf δ15N values are strongly related to the nitrogen absorption strategies regarding different nitrogen forms [40,41].
Different pot fertilizer treatments not only changed the nitrogen content and isotope characteristics of the tea leaves but also those of the pot soil (Table S2 and Table 3). The soil nitrogen content slowly increased in autumn (October to November 2022) for the OF (1.4% to 2.2%), CF (0.8% to 1.4%), and control treatments (1.3% to 1.4%). Although no further fertilizer was applied to the soil in early spring (March to 10 May 2023), the soil N content also slowly increased for the OF (1.4% to 2.3%) and control treatments (1.1% to 1.3%). All the treatment soils demonstrated a gradual decrease in %N during late spring (20 May to 30 May 2023). The soil values show similar trends to the tea leaves’ %N, suggesting that warmer spring conditions and root exudates may be conducive to symbiotic N-producing soil microbes [42].

Table 3.
Nitrogen-stable isotopes (δ15N) of soils treated with different fertilizers.
The soil δ15N values showed similar trends to the δ15N values of the top leaves, where the highest δ15N values were found in the OF-treated soils and the lowest δ15N values in the CF-treated soils. The δ15N values of the soil treated with the OF (3.3‰ to 6.0‰) became significantly more positive than the control soil (2.0‰ to 2.5‰), and the soils treated with the CF (1.2‰ to 1.9‰) became less positive than the control soil. During the study period, the cattle manure-treated soil δ15N sampled on November 18 had the highest soil δ15N value (6.0‰), and the CF urea-treated soil sampled on 30 May had the lowest δ15N value (1.2‰). The OF was shown to effectively improve the activity of the soil microorganisms and soil enzymes and can promote the growth and reproduction of microorganisms, enabling more nitrogen to be assimilated, absorbed, and utilized by the soil microorganisms, thereby increasing the soil nitrogen storage [43,44]. Some previous studies have shown that CF urea can lead to soil compaction acidification and a damaged soil ecological environment [45]. Among the three organic fertilizer treatments, the cow manure-treated soils had the highest δ15N values, followed by the chicken manure-treated soils, and the rapeseed cake-treated soils had the lowest δ15N values. This trend is most likely due to the rapid denitrification of animal manure relative to the degradation of plant organic matter, resulting in higher soil δ15N values [46].
3.2. Temporal δ15N Variations in Tea Leaves Treated with Different Fertilizers
The temporal δ15N variations in the tea leaves (sampled from the top, middle, and basal leaves) treated with rapeseed cake, urea, and the control treatments are shown in Figure 2A, Figure 2B, and Figure 2C, respectively. The δ15N trends changed according to the different types of fertilizer applied. Rapeseed cake is the most widely used organic fertilizer in Chinese tea gardens, so this study provides a deeper investigation of the temporal effects from this fertilizer instead of the traditional animal manures. The control samples (no fertilizer added) equate to the tea plants growing in their natural state without fertilizer treatment. The δ15N values of the control top, middle, and basal leaves show an overall temporal downward trend over the study period, ranging from 2.6‰ to 0.2‰, although there was a slight increase from 17 March to 10 May (Figure 2C). The δ15N values of the top, middle, and basal tea leaves treated with rapeseed cake showed minor fluctuations between 20 October and 17 February (4.8‰ to 5.6‰) and a significant increase from 17 March to 10 May (4.8‰ to 6.6‰) until 30 May, and then the δ15N values rapidly decreased to 5.1‰ (Figure 2A). The urea treatment showed an upward δ15N trend from 20 October to 18 November and from 17 March to 10 May, and a downward trend for the rest of the study period (Figure 2B).
Overall, the temporal tea leaf δ15N changes for the three fertilizer treatments appear to have a strong association with the temperature and annual growth patterns of the tea plants in this region. The δ15N values of the top, middle, and basal leaves in autumn showed minor fluctuations as the temperature slowly decreased in autumn. At this time, the tea plants gradually entered a metabolic dormancy state, and the leaf nitrogen metabolism was relatively slow. During the dormancy state, most of the photosynthetic products (such as proteins) produced by leaf photosynthesis are stored in the rhizomes [47]. The δ15N values of the top, middle, and basal leaves increased in early spring as the warmer temperatures were more suitable for photosynthesis [48]. During early spring, most of the photosynthetic products were transferred to the axillary buds, while the nutrients stored in the roots and stems were also rapidly remobilized and transferred to the buds, encouraging new leaf growth [49]. This sharp nitrogen metabolic reaction in the leaves results in an increase in the δ15N values. Finally, the δ15N values of the top, middle, and basal leaves decreased in late spring, mainly due to a reduction in the leaf photosynthetic efficiency with increasing temperatures as the tea plants entered the summer metabolic resting period. The top leaf δ15N values (red line) were generally more positive than the basal leaves (blue line) for the different treatments and sampling events. This may be due to a higher 15N fractionation effect caused by more light exposure, photosynthesis, and growth within the top leaves compared to the basal leaves. Plant studies have shown that the lighter 14N isotope will be preferentially metabolized (fractionated) and allocated under higher photosynthetic rates, thus enriching 15N [50].
3.3. δ15 N Differences between the Top, Middle, and Basal Tea Leaves
The temporal tea leaf δ 15N differences (Δ15N) between the top, middle, and basal leaf positions under three fertilizer treatments (rapeseed cake, urea, and control treatments) are shown in Table 4. The results show that, during autumn and early spring, the tea leaves treated with rapeseed cake showed a δ15N pattern similar to the control samples: an 15N enrichment from the basal to middle leaves and a depletion in 15N from the middle to top leaves. The tea plants treated with CF urea showed an opposite trend, with a depletion from the basal to middle leaves and then an enrichment from the middle to top leaves. In autumn, the Δ15N isotopic differences between the basal to middle and middle to top (δ15Nbasal-middle and δ15Nmiddle-top) of the potted tea plants treated with rapeseed cake were 0.6‰ and −0.2‰, the control treatments were 0.5‰ and −0.3‰, and the urea treatments were −0.5‰ and 1.0‰. In early spring, the Δ15N isotopic differences between the basal to middle and middle to top of potted tea plants treated with rapeseed cake were 0.7‰ and −0.1‰, the control treatments were 0.2‰ and −0.2‰, and the urea treatments were −0.6‰ and 0.8‰. In late spring, the tea plants treated with rapeseed cake showed isotopic enrichment from the basal to middle leaves and depletion from the middle to top leaves. The tea plants treated with urea and the control samples were enriched in 15N from the basal to middle leaves and middle to top leaves, with Δ15N isotopic differences for the urea treatment of 0.6‰ and 0.1‰, and the control differences were 0.3‰ and 0.3‰.

Table 4.
Δ15N differences between top, middle, and basal leaves during different seasons.
The tea leaf δ15N isotopic values are associated with the metabolism and transport efficiency of nitrogen to different tissue sites in tea plants. The roots absorb nitrogen from the soil and convert it into amino acids, which the tea plant requires for growth [51]. When tea plants grow rapidly, e.g., in spring, amino acids are transported through the stem to the leaves to provide essential nutrients for ongoing cell division and multiplication, which is accompanied by nitrogen isotope fractionation [52,53]. The Δ15N isotopic differences from the basal to middle and top leaves of the control plants first show enrichment and then depletion in autumn and early spring, and continuous enrichment in late spring. The results showed that the nitrogen metabolism of the control plants was more vigorous in the middle leaves than in the basal and top leaves in autumn and early spring, while the nitrogen metabolism of the top leaves was more vigorous in late spring.
The Δ15N isotopic leaf position differences of the potted tea plants treated with rapeseed cake were the same across all the seasons. Firstly, the tea plants were enriched from the basal to middle leaves and then depleted from the middle to top leaves. Rapeseed cake continuously provides nitrogen nutrients to tea plants from autumn to late spring, providing a sustained long-term effect on the nitrogen isotope metabolism of tea plants. The middle leaves from each sampling period treated with the rapeseed cake fertilizer showed strong nitrogen metabolic activities, such as amino acid synthesis and transport [54]. The Δ15N isotopic leaf position differences of the tea plants treated with urea and the control treatment showed an opposite trend in autumn and early spring and the same trend in late spring. It is speculated that urea changed the nitrogen metabolism of tea plants in autumn and early spring and that urea is absorbed by tea plants in late spring [36].
4. Conclusions
This study demonstrates that the OF significantly increases the δ15N values of potted tea plants compared with the CF and control treatments. Compared to the urea and control treatments, the δ15N values of the basal, middle, and top leaves sampled from the rapeseed cake-treated tea plants showed a significant increase in early spring. The application of the rapeseed cake fertilizer changed the δ15N values of the tea leaves sampled from different locations on the plant, first showing enrichment from the basal to middle leaves and then depletion from the middle to top leaves in autumn, early spring, and late spring. Δ15N isotopic differences were noted between the basal, middle, and top leaves and were clearly affected by the fertilizer type and sampling period. Overall, the δ15N signatures of the OF- and CF-treated tea plants were sustained during the early and late spring harvest period, providing clear evidence that the δ15N values of the harvested tea from different locations on the tea plant can be directly related to the fertilizer type and farming methods. The outcomes of this research show that the top and middle tea leaves typically collected during a tea harvest can be reliably used to authenticate organic production claims, increasing the tea production verification tools and consumer confidence. While our research does not identify the precise internal mechanisms that give rise to these results, further studies using labelled fertilizers will be undertaken to establish the kinetic mechanisms and confirm our observations in more detail. Meanwhile, this study is only a report from a pot experiment for variations in the nitrogen isotope ratios in tea plants and soil and may not accurately reflect the fertilizer effect on the nitrogen isotope composition in open agricultural soils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13121628/s1, Table S1: Nitrogen content (%) of top tea leaves treated with different fertilizers; Table S2: Nitrogen content (%) of soil treated with different fertilizers.
Author Contributions
Conceptualization, Z.G.; Methodology, S.S. and D.L.; Software, C.L.; Validation, X.L.; Formal analysis, K.M.R. and Q.L.; Resources, T.H.; Data curation, Z.G. and S.S.; Writing—original draft, Z.G. and C.L.; Writing—review & editing, C.L., K.M.R., H.G. and Y.Y.; Visualization, X.L.; Supervision, T.H. and Y.Y.; Project administration, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding-Tea Plant] grant number [2021C02067-7], [Zhejiang Province Science and Technology Cooperation Project for Nine Affiliations of Agriculture, Rural affairs and Farmers] grant number [2024SNJF031], [Hangzhou Agricultural and Social Development Research Project] grant number [2020ZDSJ0632] and [the National Natural Science Foundation of China] grant number [Project No. 32102438], And The APC was funded by [2021C02067-7].
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, S.; Nie, Q.; Tai, H.; Song, X.; Tong, X.; Zhang, L.; Wu, X.; Lin, Z.; Zhang, Y.; Ye, D.; et al. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 1–40. [Google Scholar] [CrossRef]
- Engelhardt, U.H. Tea chemistry—What do and what don’t we know?—A micro review. Food Res. Int. 2020, 132, 109120. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, G.; Cao, S.; Xu, X.; Gan, R.; Liu, Q.; Mao, Q.; Shang, A.; Li, H. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Ho, C.; Huang, Q. Chemistry and health effect of tea polyphenol (−)-epigallocatechin 3-O-(3-O-methyl) gallate. J. Agric. Food Chem. 2018, 67, 5374–5378. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, L.; Chen, W.; Wang, Y. Green tea catechin epigallocatechin gallate alleviates high-fat diet-induced obesity in mice by regulating the gut–brain axis. Food Front. 2023, 4, 1413–1425. [Google Scholar] [CrossRef]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J.M.; Regolo, L.; Sánchez-González, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, B. Organic vs. conventional plant-based foods: A review. Food Chem. 2022, 383, 132352. [Google Scholar] [CrossRef]
- Hoefkens, C.; Vandekinderen, I.; Meulenaer, D.B.; Devlieghere, F.; Baert, K.; Sioen, I.; Henauw, S.D.; Verbeke, W.; Camp, J.V. A literature-based comparison of nutrient and contaminant contents between organic and conventional vegetables and potatoes. Br. Food J. 2009, 111, 1078–1097. [Google Scholar] [CrossRef]
- Muñoz-Redondo, J.M.; Montenegro, J.C.; Moreno-Rojas, J.M. Using Nitrogen Stable Isotopes to Authenticate Organically and Conventionally Grown Vegetables: A New Tracking Framework. Agronomy 2022, 13, 131. [Google Scholar] [CrossRef]
- Li, C.; Kang, X.; Nie, J.; Li, A.; Farag, M.A.; Liu, C.; Rogers, K.M.; Xiao, J.; Yuan, Y. Recent advances in Chinese food authentication and origin verification using isotope ratio mass spectrometry. Food Chem. 2023, 398, 133896. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D.; Woolfe, M. Nitrogen isotope composition of organically and conventionally grown crops. J. Agric. Food Chem. 2007, 55, 2664–2670. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Kelly, S.D.; Douence, C.; Islam, M.; Monteiro, L.; Abrahim, A.; Rinke, P. Assessment of rapid low-cost isotope (δ15N, δ18O) analyses of nitrate in fruit extracts by Ti (III) reduction to differentiate organic from conventional production. Rapid Commun. Mass Spectrom. 2022, 36, e9259. [Google Scholar] [CrossRef]
- Rogers, K.M.; Turnbull, R.E.; Martin, A.P.; Baisden, W.T.; Rattenbury, M.S. Stable isotopes reveal human influences on southern New Zealand soils. Appl. Geochem. 2017, 82, 15–24. [Google Scholar] [CrossRef]
- Busari, M.A.; Salako, F.K.; Tuniz, C. Stable isotope technique in the evaluation of tillage and fertilizer effects on soil carbon and nitrogen sequestration and water use efficiency. Eur. J. Agron. 2016, 73, 98–106. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, W.; Zhang, Y.; Liu, Z.; Shao, S.; Zhou, L.; Rogers, K.M. Differentiating organically farmed rice from conventional and green rice harvested from an experimental field trial using stable isotopes and multi-element chemometrics. J. Agric. Food Chem. 2018, 66, 2607–2615. [Google Scholar] [CrossRef]
- Lyu, C.; Yang, J.; Wang, T.; Kang, C.; Wang, S.; Wang, H.; Wan, X.; Zhou, L.; Zhang, W.; Huang, L.; et al. A field trials-based authentication study of conventionally and organically grown Chinese yams using light stable isotopes and multi-elemental analysis combined with machine learning algorithms. Food Chem. 2021, 33, 128506. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Qian, Q.; Song, W.; Rogers, K.M.; Rao, Q.; Wang, S.; Zhang, Q.; Shao, S.; Tian, M.; et al. Isotope chemometrics determines farming methods and geographical origin of vegetables from Yangtze River Delta Region, China. Food Chem. 2021, 342, 128379. [Google Scholar] [CrossRef]
- Choi, W.J.; Kwak, J.H.; Lim, S.S.; Park, H.; Chang, S.; Lee, S.; Arshad, M.; Yun, S.; Kim, H. Synthetic fertilizer and livestock manure differently affect δ15N in the agricultural landscape: A review. Agric. Ecosyst. Environ. 2017, 237, 1–15. [Google Scholar] [CrossRef]
- Shin, W.J.; Ryu, J.S.; Mayer, B.; Lee, K.S.; Kim, I. Nitrogen, Sulfur, and Oxygen Isotope Ratios of Animal- and Plant-Based Organic Fertilizers Used in South Korea. J. Environ. Qual. 2017, 237, 559–567. [Google Scholar] [CrossRef]
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable isotope techniques for verifying the declared geographical origin of food in legal cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Fabroni, S.; Bontempo, L.; Campanelli, G.; Canali, S.; Montemurro, F. Innovative Tools for the Nitrogen Fertilization Traceability of Organic Farming Products. Horticulturae 2023, 9, 723. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. 2007, 43, 237–247. [Google Scholar] [CrossRef]
- Hayashi, N.; Ujihara, T.; Tanaka, E.; Kishi, Y.; Ogawa, H.; Matsuo, H. Annual variation of natural 15N abundance in tea leaves and its practicality as an organic tea indicator. J. Agric. Food Chem. 2011, 59, 10317–10321. [Google Scholar] [CrossRef]
- Trapp, T.; Inácio, C.T.; Ciotta, M.N.; Hindersmann, J.; Lima, A.P.; Santos, T.S.; Ferreira, G.W.; Morais, G.P.; Conti, L.; Comin, J.J.; et al. Natural abundance analysis of the role played by 15N as indicator for the certification of organic-system deriving food. J. Sci. Food Agric. 2022, 102, 330–340. [Google Scholar] [CrossRef]
- del Amor, F.M.; Navarro, J.; Aparicio, P.M. Isotopic discrimination as a tool for organic farming certification in sweet pepper. J. Environ. 2008, 37, 182–185. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Zhang, Y.; Yang, G.; Shao, S.; Nie, J.; Yuan, Y.; Rogers, K.M. Influence of leaf age, species and soil depth on the authenticity and geographical origin assignment of green tea. Rapid Commun. Mass Spectrom. 2019, 33, 625–634. [Google Scholar] [CrossRef]
- Xia, W.; Li, Z.; Yu, C.; Liu, Z.; Nie, J.; Li, C.; Shao, S.; Zhang, Y.; Rogers, K.M.; Yuan, Y. Understanding processing, maturity and harvest period effects to authenticate early-spring Longjing tea using stable isotopes and chemometric analyses. Food Control 2021, 124, 107907. [Google Scholar] [CrossRef]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 143059. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, G.; Wang, R.; Rousk, K.; Li, A.; Hasi, M.; Wang, C.; Xue, J.; Yang, G.; Lv, X.; et al. Enhanced foliar 15N enrichment with increasing nitrogen addition rates: Role of plant species and nitrogen compounds. Glob. Change Biol. 2023, 29, 1591–1605. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef]
- Magdas, D.A.; Dehelean, A.; Feher, I.; Radu, S. Isotopic and multielemental fingerprinting of organically and conventionally grown potatoes. Isot. Environ. Health Stud. 2017, 53, 610–619. [Google Scholar] [CrossRef]
- Chi, H.Y.; Kim, W.R.; Kim, J.Y.; Kim, S.H. Improved organic and pesticide-free rice (Oryza sativa L.) authentication based on multiple stable isotope ratio analysis and rice milling state. Heliyon 2024, 10, E26725. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Kang, J.; Chen, Y.; Hong, L.; Li, M.; Jia, Y.; Wang, Y.; Jia, X.; Wu, Z.; et al. Effects of long-term use of organic fertilizer with different dosages on soil improvement, nitrogen transformation, tea yield and quality in acidified tea plantations. Plants 2022, 12, 122. [Google Scholar] [CrossRef]
- Lee, J.; Jo, N.Y.; Shim, S.Y.; Linh, L.T.Y.; Kim, S.; Lee, M.; Hwang, S. Effects of Hanwoo (Korean cattle) manure as organic fertilizer on plant growth, feed quality, and soil bacterial community. Front. Plant Sci. 2023, 14, 1135947. [Google Scholar] [CrossRef]
- Kubar, M.S.; Zhang, Q.; Feng, M.; Wang, C.; Yang, W.; Kubar, K.A.; Riaz, S.; Gul, H.; Samoon, H.Z.; Sun, H.; et al. Growth, Yield and Photosynthetic Performance of Winter Wheat as Affected by Co-Application of Nitrogen Fertilizer and Organic Manures. Life 2022, 12, 1000. [Google Scholar] [CrossRef]
- Kalcsits, L.A.; Buschhaus, H.A.; Guy, R.D. Nitrogen isotope discrimination as an integrated measure of nitrogen fluxes, assimilation and allocation in plants. Physiol. Plant. 2014, 151, 293–304. [Google Scholar] [CrossRef]
- Trandel, M.A.; Vigardt, A.; Walters, S.A.; Lefticariu, M.; Kinsel, M. Nitrogen isotope composition, nitrogen amount, and fruit yield of tomato plants affected by the soil–fertilizer types. ACS Omega 2018, 3, 6419–6426. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Glob. Biogeochem. Cycles 2002, 16, 8-1–8-14. [Google Scholar] [CrossRef]
- Dadrasnia, A.; de Bona Muñoz, I.; Yáñez, E.H.; Lamkaddam, I.U.; Mora, M.; Ponsá, S.; Ahmed, M.; Argelaguet, L.L.; Williams, P.M.; Oatley-Radcliffe, D.L. Sustainable nutrient recovery from animal manure: A review of current best practice technology and the potential for freeze concentration. J. Clean. Prod. 2021, 315, 128106. [Google Scholar] [CrossRef]
- Ruan, L.; Wei, K.; Wang, L.; Cheng, H.; Zhang, F.; Wu, L.; Bai, P.; Zhang, C. Characteristics of NH4+ and NO3− fluxes in tea (Camellia sinensis) roots measured by scanning ion-selective electrode technique. Sci. Rep. 2016, 6, 38370. [Google Scholar] [CrossRef]
- Gurmesa, G.; Hobbie, E.; Zhang, S.; Wang, A.; Zhu, W.; Koba, K.; Yoh, M.; Wang, C.; Zhang, Q.; Fang, Y. Natural 15N abundance of ammonium and nitrate in soil profiles: New insights into forest ecosystem nitrogen saturation. Ecosphere 2022, 13, e3998. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Koba, K.; Sasaki, Y.; Fang, Y.; Yoh, M. The natural abundance of 15N in plant and soil-available N indicates a shift of main plant N resources to NO from NH along the N leaching gradient. Rapid Commun. Mass Spectrom. 2010, 24, 1001–1008. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Yu, Y.; Tian, Y.; Li, H.; Chen, X.; Li, W.; Liu, Y.; Lu, T.; He, B.; et al. Root microbiota of tea plants regulate nitrogen homeostasis and theanine synthesis to influence tea quality. Curr. Biol. 2024, 34, 868–880.e6. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Chernov, T.I.; Semenov, M.V. Management of soil microbial communities: Opportunities and prospects (a review). Eurasian Soil Sci. 2021, 54, 1888–1902. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Huo, X.; Salim, R.; Bloch, H.; Zhang, H. Assessing fertilizer use efficiency and its determinants for apple production in China. Ecol. Indic. 2019, 104, 268–278. [Google Scholar] [CrossRef]
- Yan, R.; Wu, H.; Yang, X.; Yang, C.; Lyu, H.; Zhang, H.; Li, S.; Liu, T.; Li, R.; Yao, Y. Soil decreases N2O emission and increases TN content during combined composting of wheat straw and cow manure by inhibiting denitrification. Chem. Eng. J. 2023, 477, 147306. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Z.; Chen, T.; Deng, W.; Wan, X.; Zhang, Z. Theanine metabolism and transport in tea plants (Camellia sinensis L.): Advances and perspectives. Crit. Rev. Biotechnol. 2023, 43, 327–341. [Google Scholar] [CrossRef]
- Xia, W.; Li, C.; Nie, J.; Shao, S.; Rogers, K.M.; Zhang, Y.; Li, Z.; Yuan, Y. Stable isotope and photosynthetic response of tea grown under different temperature and light conditions. Food Chem. 2022, 368, 130771. [Google Scholar] [CrossRef]
- Li, F.; Dong, C.; Yang, T.; Ma, J.; Zhang, S.; Wei, S.; Wan, X.; Zhang, Z. Seasonal theanine accumulation and related gene expression in the roots and leaf buds of tea plants (Camellia sinensis L.). Front. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Zhang, W.; Ni, K.; Long, L.; Ruan, J. Nitrogen transport and assimilation in tea plant (Camellia sinensis): A review. Front. Plant Sci. 2023, 14, 1249202. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, J.; Dong, C.; Li, F.; Qiao, S.; Lin, S.; Yang, T.; Wu, Y.; Bao, S.; Lucas, W.J.; et al. CsAlaDC and CsTSI work coordinately to determine theanine biosynthesis in tea plants (Camellia sinensis L.) and confer high levels of theanine accumulation in a non-tea plant. Plant Biotechnol. 2021, 19, 2395. [Google Scholar] [CrossRef]
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef]
- Liu, W.; Cui, S.; Wu, L.; Qi, W.; Chen, J.; Ye, Z.; Ma, J.; Liu, D. Effects of Bio-organic Fertilizer on Soil Fertility, Yield, and Quality of Tea. J. Soil Sci. Plant Nutr. 2023, 23, 5109–5121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).