Salicylic Acid Priming Improves Cotton Seedling Heat Tolerance through Photosynthetic Pigment Preservation, Enhanced Antioxidant Activity, and Osmoprotectant Levels
Abstract
1. Introduction
2. Results
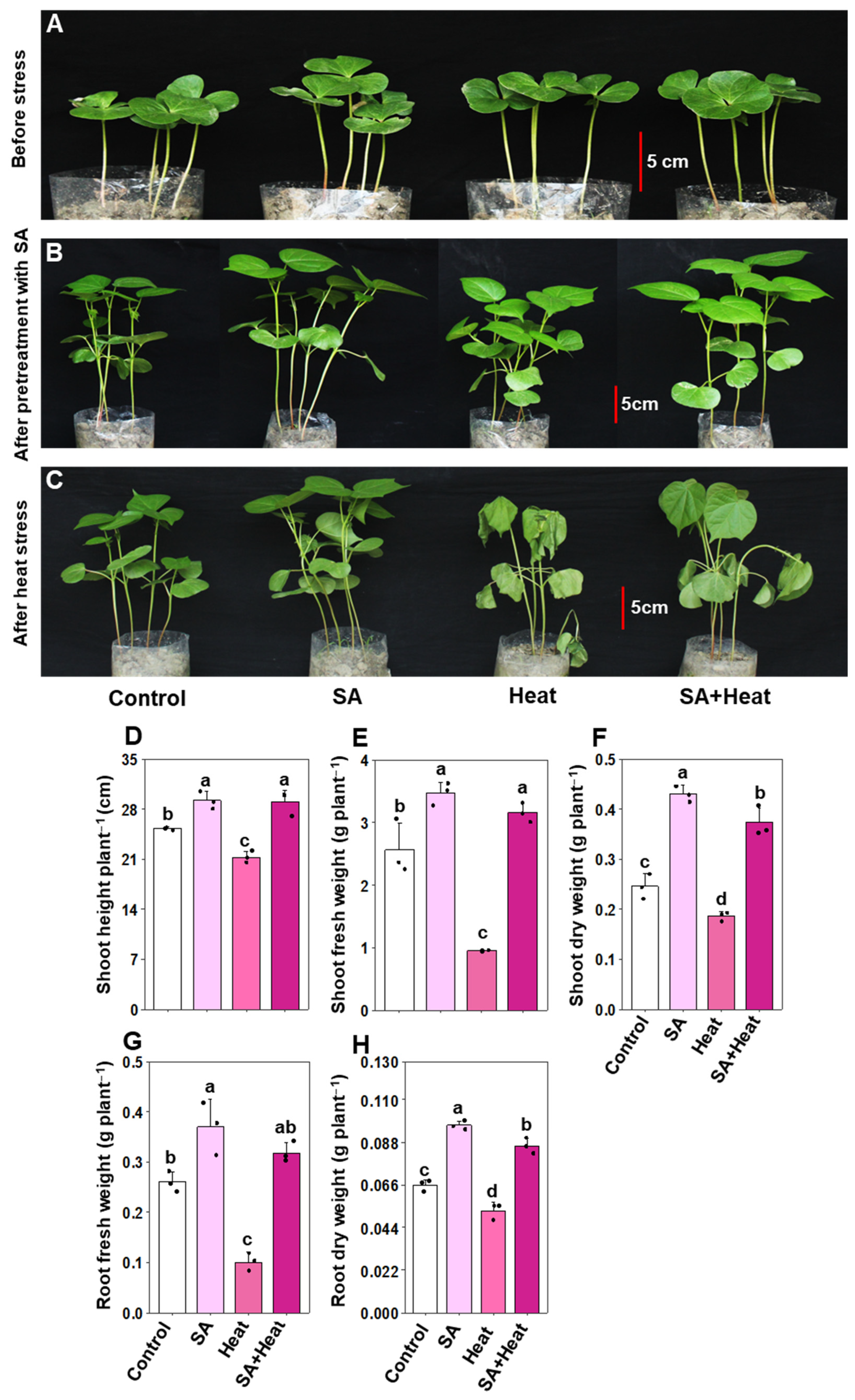
2.1. Salicylic Acid Positively Modulated the Growth and Phenotype of Heat-Stressed Cotton Plants
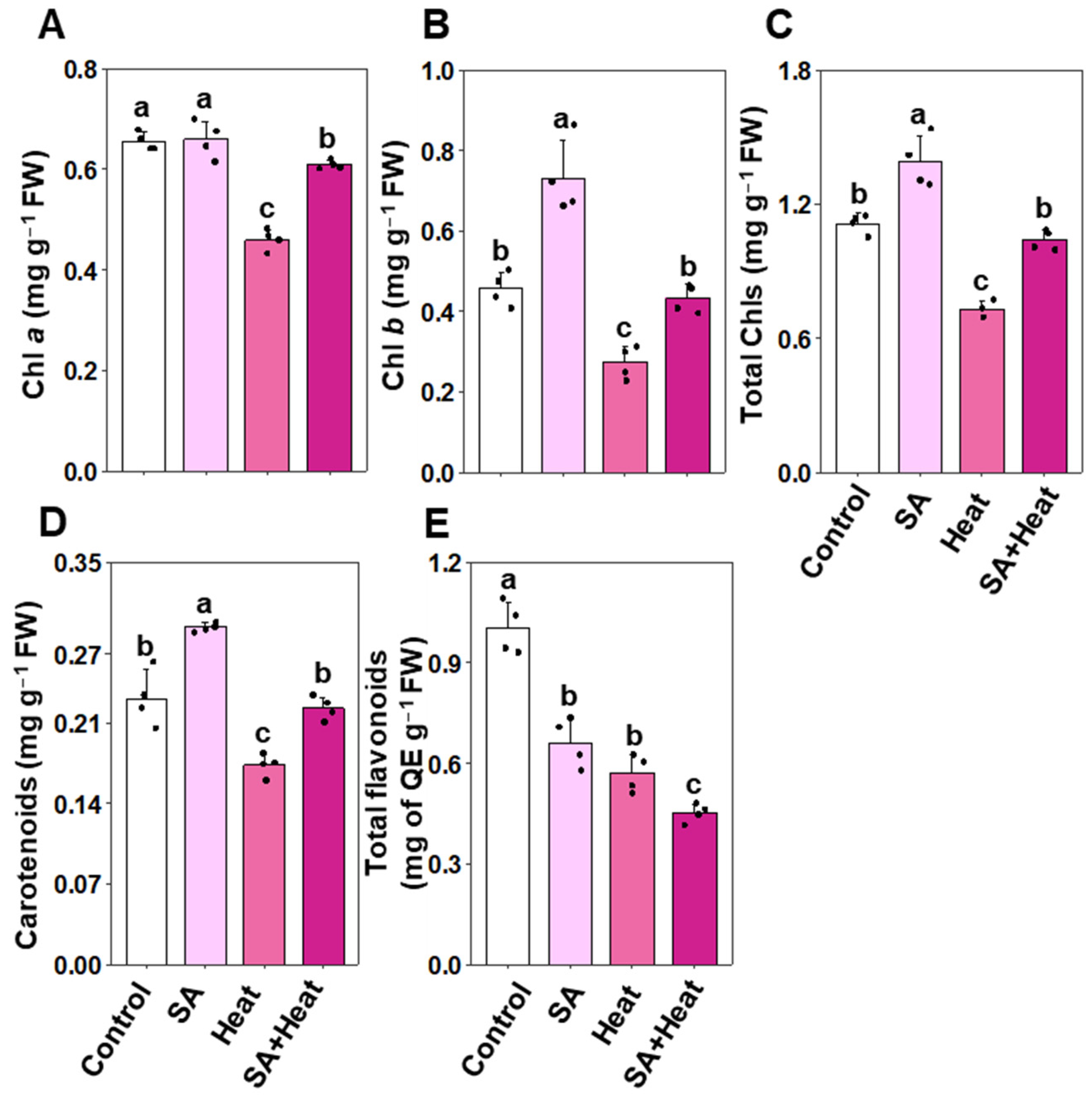
2.2. Comparative Analysis of Photosynthetic Pigments and Flavonoids in Salicylic Acid-Treated and Heat-Stressed Cotton
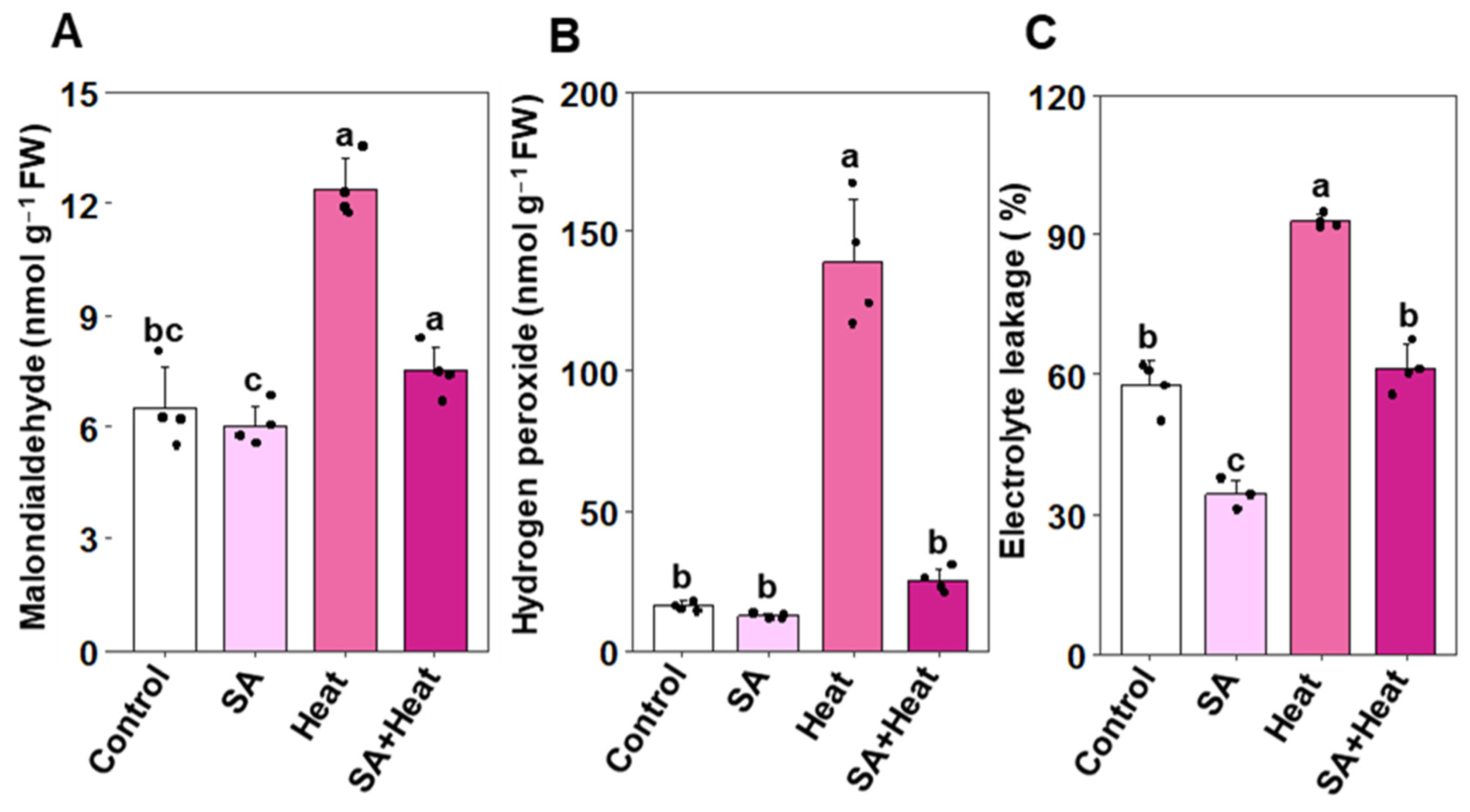
2.3. Salicylic Acid Reduced Heat-Induced Oxidative Stress in Cotton Plants
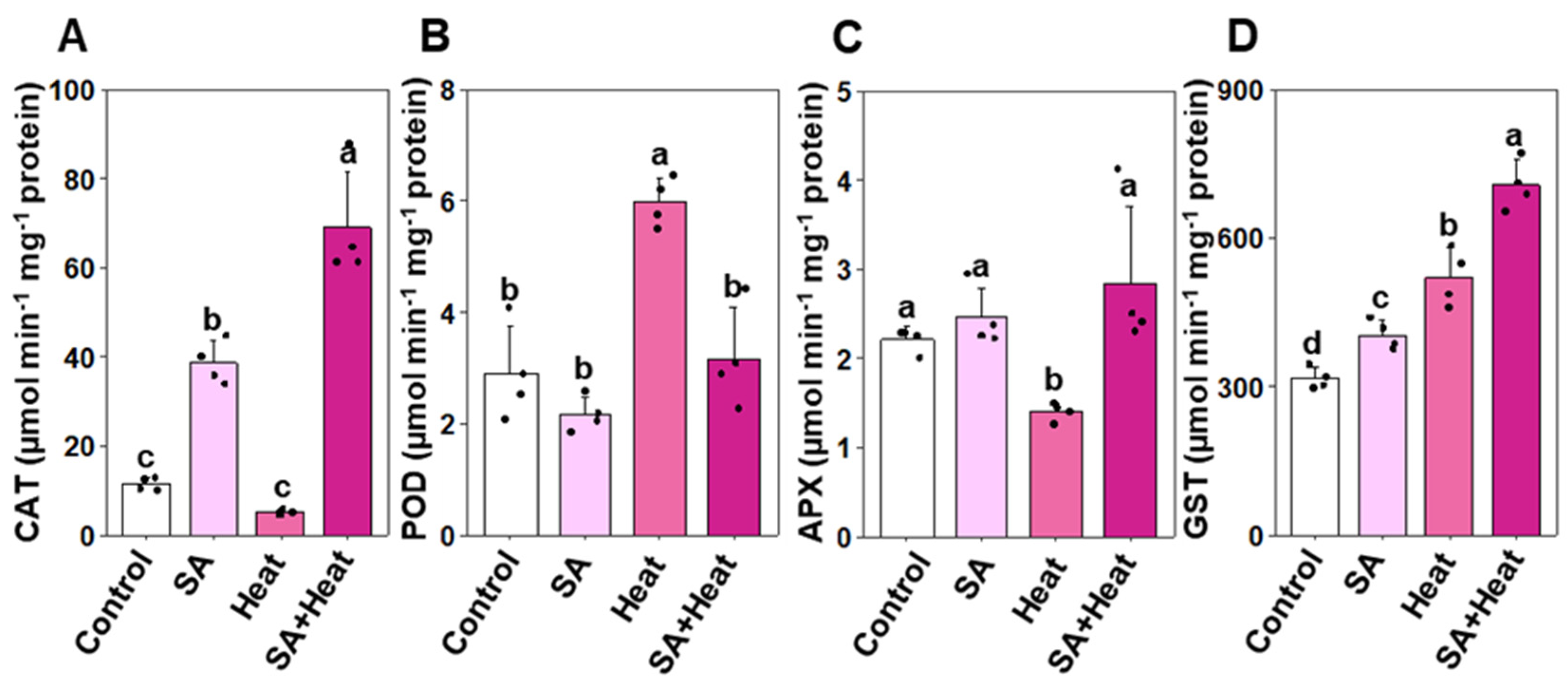
2.4. Differential Antioxidant Enzyme Responses to Salicylic Acid and Heat Stress in Cotton
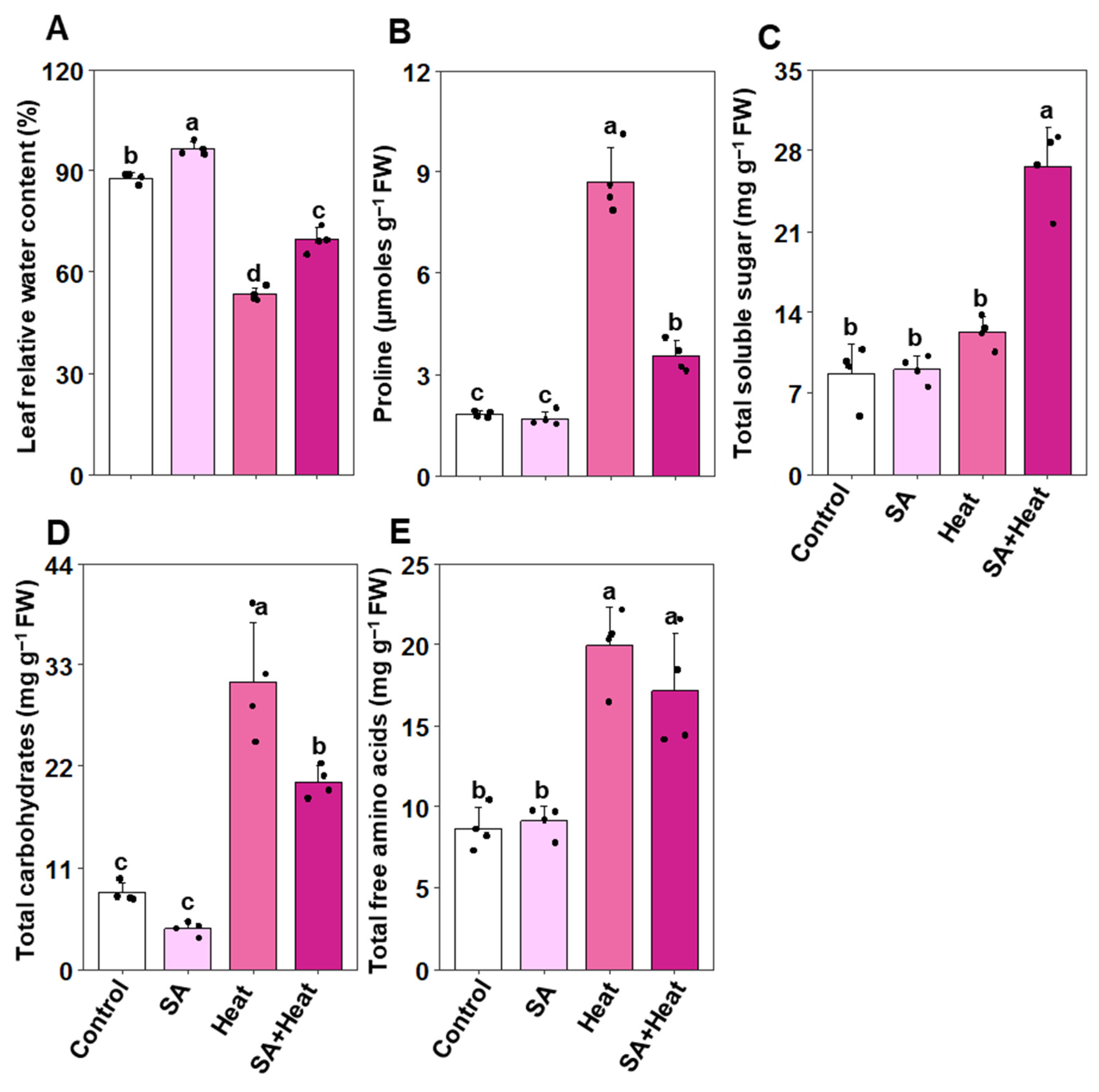
2.5. Salicylic Acid’s Role in Enhancing Water Status and Osmoprotectant Balance in Cotton Plants under Heat Stress
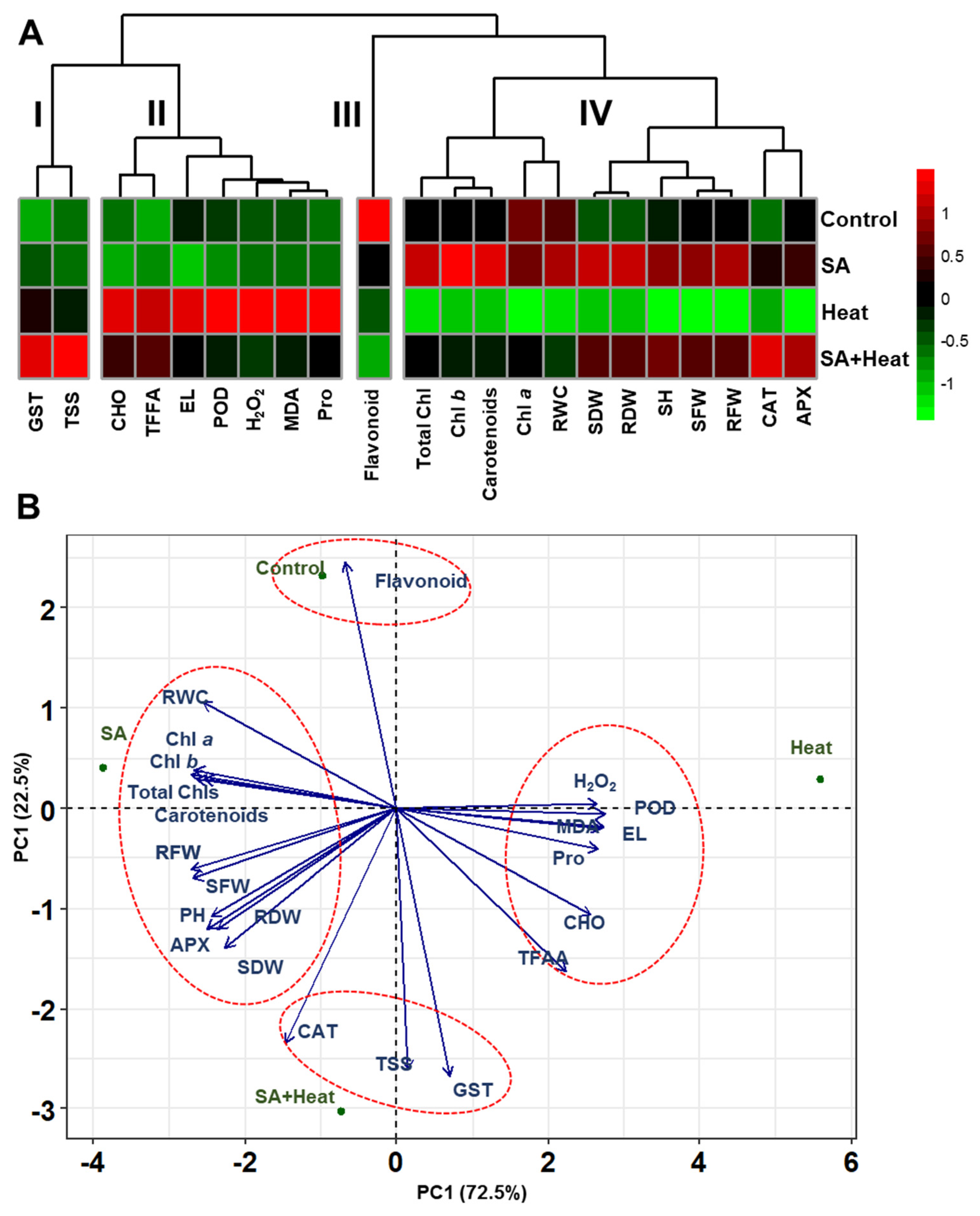
2.6. Visualizing the Differential Impact of Heat and Salicylic Acid Treatments on Cotton Plants: A Detailed Heatmap and Principal Component Analysis Approach
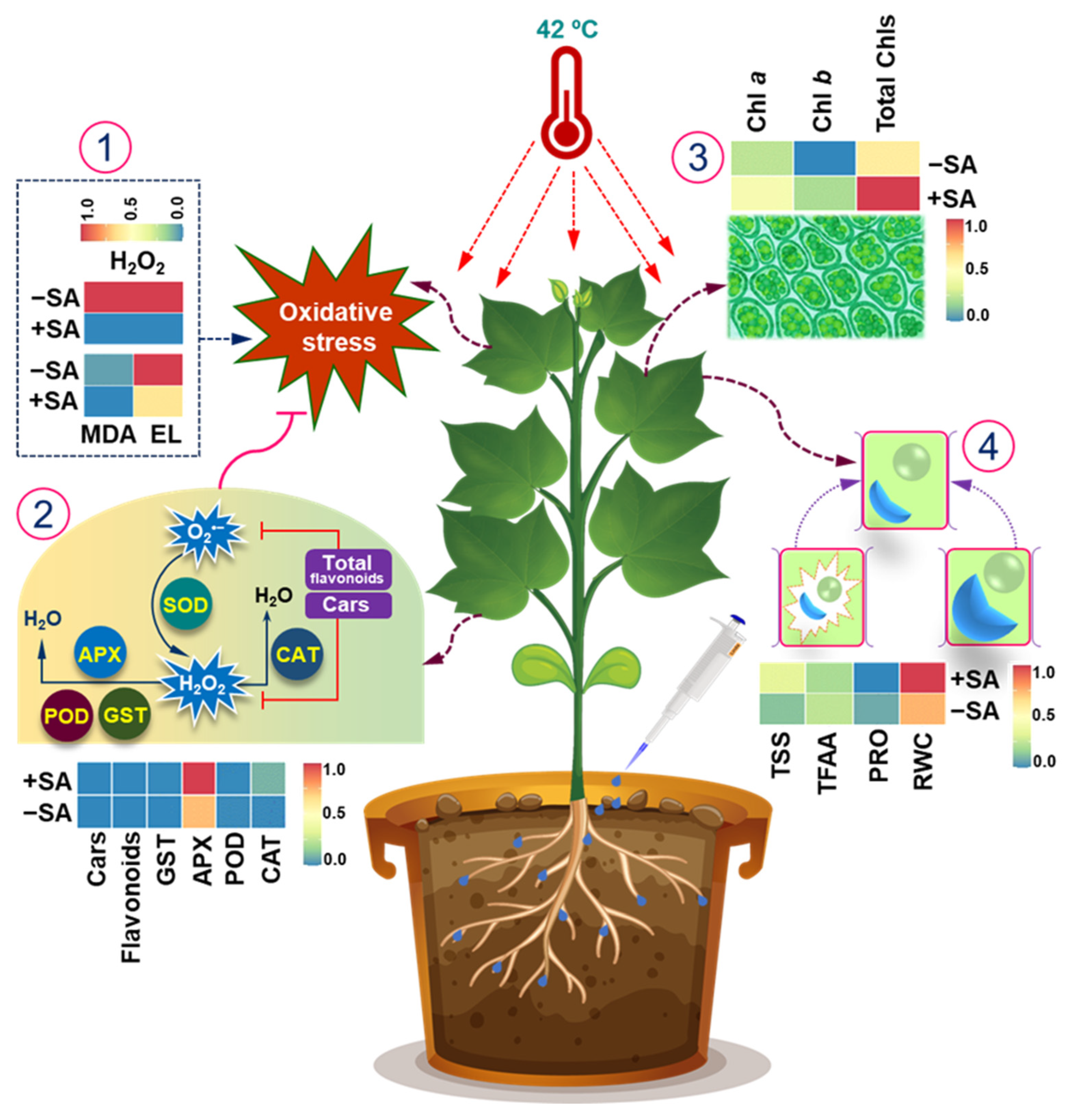
3. Discussion
4. Materials and Methods
4.1. Plant Species and Treatment Compositions
4.2. Growth Parameter Assessment
4.3. Methodical Approach to Evaluating Water Content and Cellular Integrity in Cotton Leaves
4.4. Assessment of Photosynthetic Pigments and Flavonoid Contents
4.5. Assessment of Hydrogen Peroxide, Malondialdehyde, and Proline
4.6. Extraction of Antioxidant Enzymes and Quantification of Enzyme Activities
4.7. Quantification Techniques for Soluble Sugars, Amino Acids, and Carbohydrate Levels
4.8. Statistical Methodologies and Visualization Tools for Data Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Desaint, H.; Aoun, N.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Fight hard or die trying: When plants face pathogens under heat stress. New Phytol. 2021, 229, 712–734. [Google Scholar] [CrossRef] [PubMed]
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Intergovernmental Panel on Climate Change (IPCC). Technical summary. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023; pp. 35–144. [Google Scholar]
- Zenda, T.; Wang, N.; Dong, A.; Zhou, Y.; Duan, H. Reproductive-stage heat stress in cereals: Impact, plant responses and strategies for tolerance improvement. Int. J. Mol. Sci. 2022, 23, 6929. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 520328. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.K.; Pandey, N.; Rai, S.P. Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 2020, 168, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Waraich, E.A.; Zulfiqar, U.; Ullah, A.; Farooq, M. Thiourea application improves heat tolerance in camelina (Camelina sativa L. Crantz) by modulating gas exchange, antioxidant defense and osmoprotection. Ind. Crop. Prod. 2021, 170, 113826. [Google Scholar]
- Alam, M.N.; Yang, L.; Yi, X.; Wang, Q.; Robin, A.H.K. Role of melatonin in inducing the physiological and biochemical processes associated with heat stress tolerance in tall fescue (Festuca arundinaceous). J. Plant Growth Regul. 2022, 41, 2759–2768. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.; Li, W.; Hu, T.; Wang, Q.; Yin, Y.; Liu, X.; He, S.; Zhang, M.; Liang, Y. Overexpression of SlBBX17 affects plant growth and enhances heat tolerance in tomato. Int. J. Biol. Macromol. 2022, 206, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ming, F.; Liang, Y.; Wang, Y.; Gan, Y.; Qiu, Z.; Yan, S.; Cao, B. Heat stress resistance mechanisms of two cucumber varieties from different regions. Int. J. Mol. Sci. 2022, 23, 1817. [Google Scholar] [CrossRef] [PubMed]
- Asthir, B. Mechanisms of heat tolerance in crop plants. Biol. Plant. 2015, 59, 620–628. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crop. Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Hou, S.; Zhu, G.; Li, Y.; Li, W.; Fu, J.; Niu, E.; Li, L.; Zhang, D.; Guo, W. Genome-wide association studies reveal genetic variation and candidate genes of drought stress related traits in cotton (Gossypium hirsutum L.). Front. Plant Sci. 2018, 9, 1276. [Google Scholar] [CrossRef]
- An, M.; Wang, X.; Chang, D.; Wang, S.; Hong, D.; Fan, H.; Wang, K. Application of compound material alleviates saline and alkaline stress in cotton leaves through regulation of the transcriptome. BMC Plant Biol. 2020, 20, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Dong, Y.; Zhang, F.; He, Q.; Chen, J.; Zhu, S.; Zhao, T. Seed priming with melatonin improves salt tolerance in cotton through regulating photosynthesis, scavenging reactive oxygen species and coordinating with phytohormone signal pathways. Ind. Crop. Prod. 2021, 169, 113671. [Google Scholar] [CrossRef]
- Majeed, S.; Rana, I.A.; Mubarik, M.S.; Atif, R.M.; Yang, S.-H.; Chung, G.; Jia, Y.; Du, X.; Hinze, L.; Azhar, M.T. Heat stress in cotton: A review on predicted and unpredicted growth-yield anomalies and mitigating breeding strategies. Agronomy 2021, 11, 1825. [Google Scholar] [CrossRef]
- Mercado Alvarez, K.; Bertero, H.D.; Paytas, M.J.; Ploschuk, E.L. Mesophyll conductance modulates photosynthetic rate in cotton crops exposed to heat stress under field conditions. J. Agron. Crop Sci. 2022, 208, 53–64. [Google Scholar] [CrossRef]
- Anwar, A.; Kim, J.-K. Transgenic breeding approaches for improving abiotic stress tolerance: Recent progress and future perspectives. Int. J. Mol. Sci. 2020, 21, 2695. [Google Scholar] [CrossRef]
- Pan, Q.; Zhan, J.; Liu, H.; Zhang, J.; Chen, J.; Wen, P.; Huang, W. Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci. 2006, 171, 226–233. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Nayyar, H.; Siddique, K.H. Role of phytohormones in regulating heat stress acclimation in agricultural crops. J. Plant Growth Regul. 2022, 41, 1041–1064. [Google Scholar] [CrossRef]
- Keya, S.S.; Mostofa, M.G.; Rahman, M.M.; Das, A.K.; Sultana, S.; Ghosh, P.K.; Anik, T.R.; Ahsan, S.; Rahman, M.A.; Jahan, N.; et al. Salicylic acid application improves photosynthetic performance and biochemical responses to mitigate saline stress in cotton. J. Plant Growth Regul. 2023, 42, 5881–5894. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, L.; He, H.; Li, Y.; Li, X.; Liu, D.; Wang, J.; Jin, G.; Huang, S. Application of exogenous KH2PO4 and salicylic acid and optimization of the sowing date enhance rice yield under high-temperature conditions. J. Plant Growth Regul. 2022, 41, 1532–1546. [Google Scholar] [CrossRef]
- Tanveer, S.; Akhtar, N.; Ilyas, N.; Sayyed, R.; Fitriatin, B.N.; Perveen, K.; Bukhari, N.A. Interactive effects of Pseudomonas putida and salicylic acid for mitigating drought tolerance in canola (Brassica napus L.). Heliyon 2023, 9, e14193. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Ren, H.; Zhao, B.; Zhang, J.; Ren, B.; Gao, H.; Liu, P. Exogenous SA or 6-BA maintains photosynthetic activity in maize leaves under high temperature stress. Crop J. 2023, 11, 605–617. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Lv, Z.; Khanzada, A.; Huang, M.; Cai, J.; Zhou, Q.; Huo, Z.; Jiang, D. Effects of cold and salicylic acid priming on free proline and sucrose accumulation in winter wheat under freezing stress. J. Plant Growth Regul. 2022, 41, 2171–2184. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, R.; Zhang, Y.; Yan, Z.; Chen, H.; Shao, G. Salicylic acid ameliorates cadmium toxicity by increasing nutrients uptake and upregulating antioxidant enzyme activity and uptake/transport-related genes in Oryza sativa L. indica. J. Plant Growth Regul. 2023, 42, 1158–1170. [Google Scholar] [CrossRef]
- Jangra, M.; Devi, S.; Satpal; Kumar, N.; Goyal, V.; Mehrotra, S. Amelioration effect of salicylic acid under salt stress in Sorghum bicolor L. Appl. Biochem. Biotechnol. 2022, 194, 4400–4423. [Google Scholar] [PubMed]
- Zafar, M.M.; Jia, X.; Shakeel, A.; Sarfraz, Z.; Manan, A.; Imran, A.; Mo, H.; Ali, A.; Youlu, Y.; Razzaq, A. Unraveling heat tolerance in upland cotton (Gossypium hirsutum L.) using univariate and multivariate analysis. Front. Plant Sci. 2022, 12, 727835. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.-S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Khan, M.S.; Akther, T.; Ali, D.M.; Hemalatha, S. An investigation on the role of salicylic acid alleviate the saline stress in rice crop (Oryza sativa (L.)). Biocatal. Agric. Biotechnol. 2019, 18, 101027. [Google Scholar] [CrossRef]
- Loutfy, N.; Sakuma, Y.; Gupta, D.K.; Inouhe, M. Modifications of water status, growth rate and antioxidant system in two wheat cultivars as affected by salinity stress and salicylic acid. J. Plant Res. 2020, 133, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Sotelo, J.; Martínez, L.M.; Ponce, G.; Cassab, G.I.; Alagón, A.; Meeley, R.B.; Ribaut, J.-M.; Yang, R. Maize HSP101 plays important roles in both induced and basal thermotolerance and primary root growth. Plant Cell 2002, 14, 1621–1633. [Google Scholar] [CrossRef]
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Ali, S.; Rizwan, M.; Shahid, M.R.; Alyemeni, M.N.; Alamri, S.A.; Ahmad, P. Role of mineral nutrition in alleviation of heat stress in cotton plants grown in glasshouse and field conditions. Sci. Rep. 2019, 9, 13022. [Google Scholar] [CrossRef]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Habib-ur-Rahman, M.; Noor, M.A.; Sher, A.; Fahad, S. Linking plants functioning to adaptive responses under heat stress conditions: A mechanistic review. J. Plant Growth Regul. 2022, 41, 2596–2613. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Hossain, A.; Islam, M.S.; Barutcular, C.; Ratnasekera, D.; Gormus, O.; Amanet, K.; Mubeen, M.; Nasim, W.; Fahad, S.; et al. Drought and Heat Stress in Cotton (Gossypium hirsutum L.): Consequences and Their Possible Mitigation Strategies. In Agronomic Crops: Volume 3: Stress Responses and Tolerance; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 613–634. [Google Scholar]
- Ahmad, F.; Perveen, A.; Mohammad, N.; Ali, M.A.; Akhtar, M.N.; Shahzad, K.; Danish, S.; Ahmed, N. Heat Stress in Cotton: Responses and Adaptive Mechanisms. In Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies; Ahmad, S., Hasanuzzaman, M., Eds.; Springer: Singapore, 2020; pp. 393–428. [Google Scholar]
- Azhar, M.T.; Wani, S.H.; Chaudhary, M.T.; Jameel, T.; Kaur, P.; Du, X. Heat Tolerance in Cotton. In Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; Wani, S.H., Kumar, V., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2020; pp. 1–22. [Google Scholar]
- Qamer, Z.; Chaudhary, M.; Du, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 9. [Google Scholar] [CrossRef]
- Ahanger, M.; Mir, R.; Alyemeni, M.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2019, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Lejmel, M.; Molnar, A.; Majláth, I.; Pál, M.; Quang Trung, N.; Nguyen, N.; Le, V.N.; Szalai, G. Interaction between elevated temperature and different types of Na-salicylate treatment in Brachypodium dystachion. PLoS ONE 2020, 15, e0227608. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Islam, M.R.; Keya, S.S.; Das, A.K.; Miah, M.G.; Kawser, A.Q.M.R.; Ahsan, S.; Hashem, A.; et al. Acetic acid: A cost-effective agent for mitigation of seawater-induced salt toxicity in mung bean. Sci. Rep. 2019, 9, 15186. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Touhidur Rahman, A.; Rahman, M.M.; Sanjida Sultana, K.; Islam, M.R.; Rahman, M.A.; Sultana, S.; Ghosh, P.K.; Khan, S.; Ahamed, T.; et al. Ethanol treatment enhances physiological and biochemical responses to mitigate saline toxicity in soybean. Plants 2022, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Das, A.K.; Anik, T.R.; Keya, S.S.; Ahsan, S.M.; Khan, M.A.R.; Ahmed, M.; Rahman, M.A.; Hossain, M.M.; et al. Ethanol positively modulates photosynthetic traits, antioxidant defense and osmoprotectant levels to enhance drought acclimatization in soybean. Antioxidants 2022, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Keya, S.S.; Mostofa, M.G.; Mezanur Rahman, M.; Das, A.K.; Abiar Rahman, M.; Anik, T.R.; Sultana, S.; Arifur Rahman Khan, M.; Robyul Islam, M.; Watanabe, Y.; et al. Effects of glutathione on waterlogging-induced damage in sesame crop. Ind. Crop. Prod. 2022, 185, 115092. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Miah, M.G.; Saha, S.R.; Karim, M.A.; Keya, S.S.; Akter, M.; Islam, M.; Tran, L.S.P. Insight into salt tolerance mechanisms of the halophyte Achras sapota: An important fruit tree for agriculture in coastal areas. Protoplasma 2018, 256, 181–191. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Zhang, X.F.; Tao, L.X.; Fu, G.F. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Inductive responses of some organic metabolites for osmotic homeostasis in peanut (Arachis hypogaea L.) seedlings during salt stress. Acta Physiol. Plant. 2013, 35, 2821–2832. [Google Scholar] [CrossRef]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016, 7, 184129. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Ku, H.; Lee, S.-Y. Crop enhancement of cucumber plants under heat stress by shungite carbon. Int. J. Mol. Sci. 2020, 21, 4858. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Smogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-P.; Takahashi, T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Sultana, S.; Keya, S.S.; Nihad, S.A.I.; Shams, S.-N.-U.; Hossain, M.S.; Tahiat, T.; Rahman, M.A.; Rahman, M.M.; Raza, A. Ethanol-mediated cold stress tolerance in sorghum seedlings through photosynthetic adaptation, antioxidant defense, and osmoprotectant enhancement. Plant Stress 2024, 11, 100401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.K.; Ghosh, P.K.; Nihad, S.A.I.; Sultana, S.; Keya, S.S.; Rahman, M.A.; Ghosh, T.K.; Akter, M.; Hasan, M.; Salma, U.; et al. Salicylic Acid Priming Improves Cotton Seedling Heat Tolerance through Photosynthetic Pigment Preservation, Enhanced Antioxidant Activity, and Osmoprotectant Levels. Plants 2024, 13, 1639. https://doi.org/10.3390/plants13121639
Das AK, Ghosh PK, Nihad SAI, Sultana S, Keya SS, Rahman MA, Ghosh TK, Akter M, Hasan M, Salma U, et al. Salicylic Acid Priming Improves Cotton Seedling Heat Tolerance through Photosynthetic Pigment Preservation, Enhanced Antioxidant Activity, and Osmoprotectant Levels. Plants. 2024; 13(12):1639. https://doi.org/10.3390/plants13121639
Chicago/Turabian StyleDas, Ashim Kumar, Protik Kumar Ghosh, Sheikh Arafat Islam Nihad, Sharmin Sultana, Sanjida Sultana Keya, Md. Abiar Rahman, Totan Kumar Ghosh, Munny Akter, Mehedi Hasan, Umme Salma, and et al. 2024. "Salicylic Acid Priming Improves Cotton Seedling Heat Tolerance through Photosynthetic Pigment Preservation, Enhanced Antioxidant Activity, and Osmoprotectant Levels" Plants 13, no. 12: 1639. https://doi.org/10.3390/plants13121639
APA StyleDas, A. K., Ghosh, P. K., Nihad, S. A. I., Sultana, S., Keya, S. S., Rahman, M. A., Ghosh, T. K., Akter, M., Hasan, M., Salma, U., Hasan, M. M., & Rahman, M. M. (2024). Salicylic Acid Priming Improves Cotton Seedling Heat Tolerance through Photosynthetic Pigment Preservation, Enhanced Antioxidant Activity, and Osmoprotectant Levels. Plants, 13(12), 1639. https://doi.org/10.3390/plants13121639







