Maize Autophagy-Related Protein ZmATG3 Confers Tolerance to Multiple Abiotic Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Sequence Analysis of ZmATG3
2.3. GUS Activity Analysis
2.4. Expression of ZmATG3 in Yeast and Stress Tolerance Assays
2.5. Generation of Transgenic Plants and Phenotypic Analysis
2.6. Phenotypic Analysis of Transgenic Plants under Low-Nitrogen Stress
2.7. Determination of Stress-Associated Physiological Indicators
2.8. RNA Extraction and Quantitative RT-qPCR Analysis
3. Results
3.1. Sequence Analysis of ZmATG3
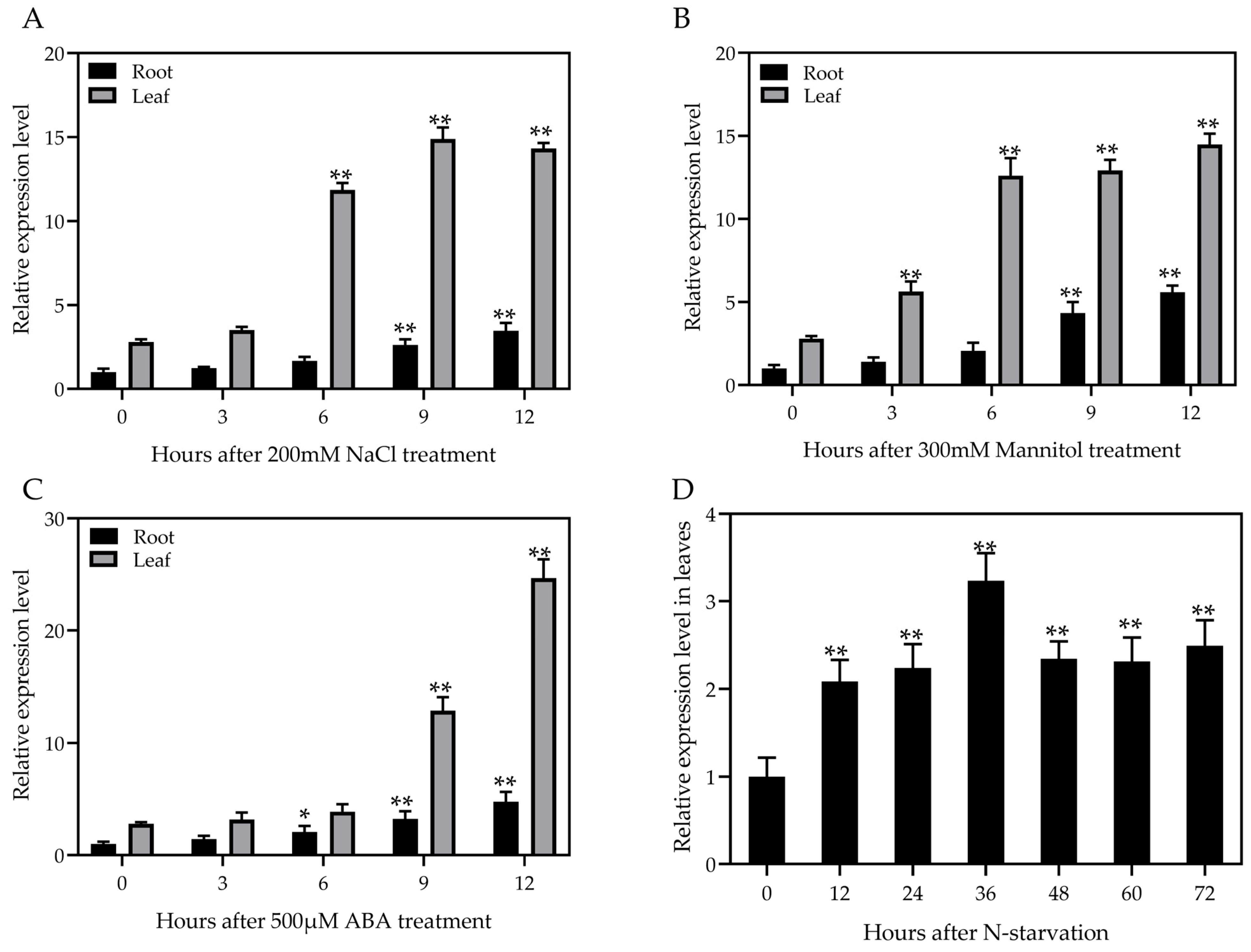
3.2. Expression Analysis of ZmATG3 under Various Abiotic Stresses
3.3. ZmATG3 Enhances the Tolerance of Yeast to Abiotic Stress
3.4. Overexpression of ZmATG3 Enhances Tolerance to NaCl and Mannitol Stresses in Arabidopsis
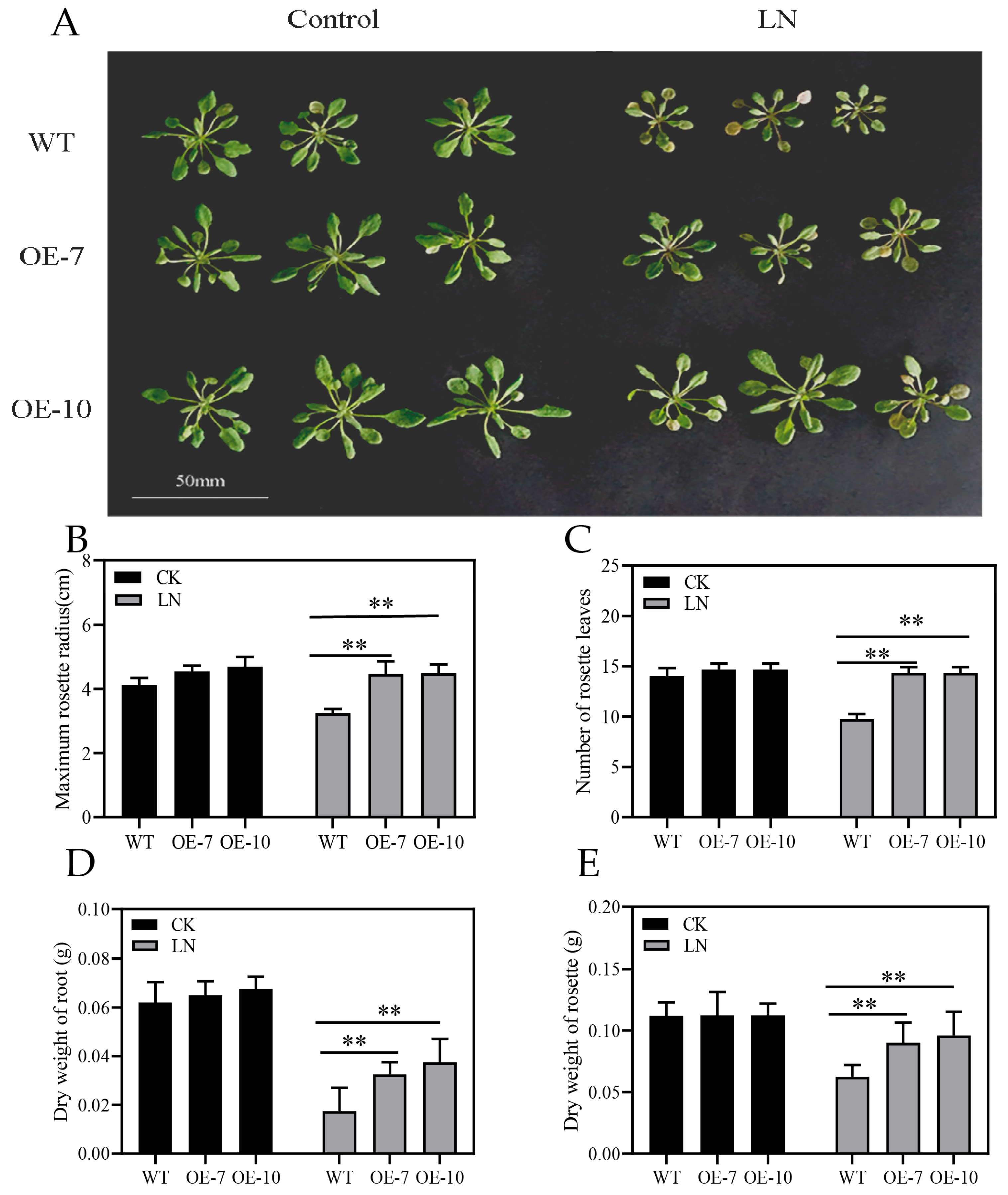
3.5. Overexpression of ZmATG3 Enhances Tolerance to LN Stress in Arabidopsis
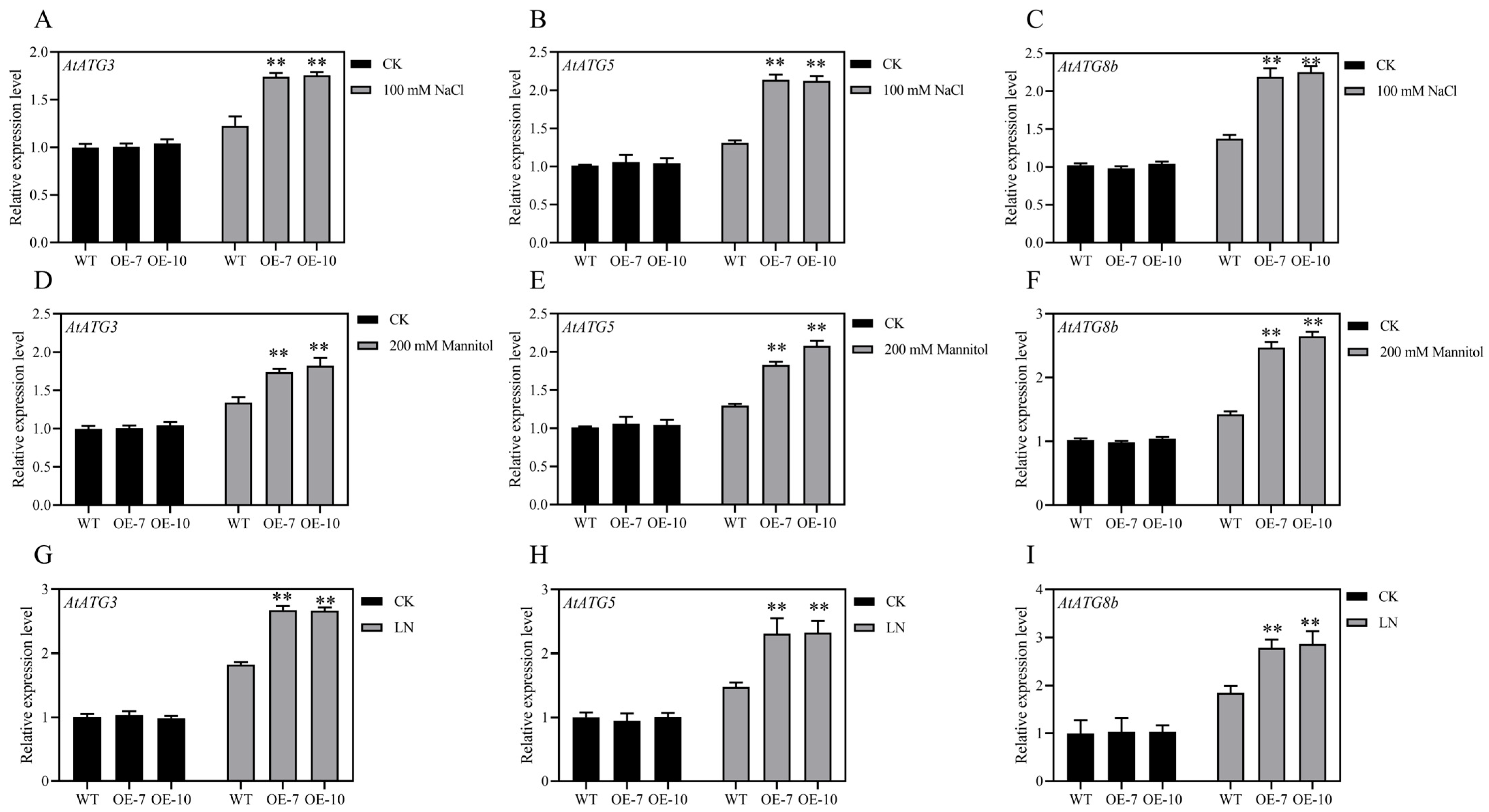
3.6. Overexpression of ZmATG3 Up-Regulated the Expression of Other AtATGs in Arabidopsis under Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, M.H.; Yang, Z.; Liu, L.; Duan, L. DNA Methylation in Plant Responses and Adaption to Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.Z.; Xiong, L.M.; Shi, H.Z.; Yang, S.H.; Herrera-Estrella, L.R.; Xu, G.H.; Chao, D.Y.; Li, J.R.; Wang, P.Y.; Qin, F. Plant abiotic stress response and nutrient use efficiency. Sci. China-Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, W.; Liu, F. Autophagy in the Lifetime of Plants: From Seed to Seed. Int. J. Mol. Sci. 2022, 23, 11410. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Vierstra, R.D. Autophagy: The Master of Bulk and Selective Recycling. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews; Palo Alto: Santa Clara, CA, USA, 2018; Volume 69, pp. 173–208. [Google Scholar]
- Yoshimoto, K. Beginning to Understand Autophagy, an Intracellular Self-Degradation System in Plants. Plant Cell Physiol. 2012, 53, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, J.; Wang, T. Autophagy in Plant Abiotic Stress Management. Int. J. Mol. Sci. 2021, 22, 4075. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, P.; Jia, X.; Huo, L.Q.; Che, R.M.; Ma, F.W. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018, 16, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.D.; Song, J.Y.; Wang, M.J.; Chen, X.X.; Du, B.H.; An, Y.M.; Zhang, L.S.; Wang, D.; Guo, C.H. Alfalfa MsATG13 Confers Cold Stress Tolerance to Plants by Promoting Autophagy. Int. J. Mol. Sci. 2023, 24, 2033. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.M.; Zhang, P.P.; Zhu, R.H.; Fu, J.; Su, J.; Zheng, J.; Wang, Z.Y.; Wang, D.; Gong, Q.Q. Autophagy Is Rapidly Induced by Salt Stress and Is Required for Salt Tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Chen, M.; Zhong, L.; Liu, J.M.; Xu, Z.S.; Li, L.C.; Zhou, Y.B.; Guo, C.H.; Ma, Y.Z. Overexpression of the autophagy-related gene SiATG8a from foxtail millet (Setaria italica L.) confers tolerance to both nitrogen starvation and drought stress in Arabidopsis. Biochem. Biophys. Res. Commun. 2015, 468, 800–806. [Google Scholar] [CrossRef]
- Hashimi, S.M.; Wu, N.N.; Ran, J.; Liu, J.Z. Silencing Autophagy-Related Gene 2 (ATG2) Results in Accelerated Senescence and Enhanced Immunity in Soybean. Int. J. Mol. Sci. 2021, 22, 11749. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ma, D.N.; Hao, X.L.; Li, J.; Xia, L.; Zhang, E.; Wang, P.; Wang, M.L.; Guo, F.; Wang, Y. CsATG101 Delays Growth and Accelerates Senescence Response to Low Nitrogen Stress in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Honig, A.; Avin-Wittenberg, T.; Ufaz, S.; Galili, G. A New Type of Compartment, Defined by Plant-Specific Atg8-Interacting Proteins, Is Induced upon Exposure of Arabidopsis Plants to Carbon Starvation. Plant Cell 2012, 24, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Koyano, T.; Hanamata, S.; Kubo, T.; Noguchi, Y.; Yagi, C.; Nagata, N.; Yamamoto, T.; Ohnishi, T.; Okazaki, Y. OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy 2014, 10, 878–888. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, X.M.; Zhao, L.L.; Cheung, A.Y.; Sun, M.X. Autophagy-mediated compartmental cytoplasmic deletion is essential for tobacco pollen germination and male fertility. Autophagy 2020, 16, 2180–2192. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, R.; Marion, J.; Le Borgne, R.; Satiat-Jeunemaitre, B.; Bianchi, M.W. ATG5 defines a phagophore domain connected to the endoplasmic reticulum during autophagosome formation in plants. Nat. Commun. 2014, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Czymmek, K.; Tallóczy, Z.; Levine, B.; Dinesh-Kumar, S.P. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005, 121, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Jiang, Y.T.; Lin, D.L.; Lin, W.H.; Xue, H.W. Phosphatidic acid suppresses autophagy through competitive inhibition by binding GAPC (glyceraldehyde-3-phosphate dehydrogenase) and PGK (phosphoglycerate kinase) proteins. Autophagy 2022, 18, 2656–2670. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Jia, X.; Ma, F.W. Apple autophagy-related protein MdATG3s afford tolerance to multiple abiotic stresses. Plant Sci. 2017, 256, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.Q. Regulation of Mammalian Autophagy in Physiology and Pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Suzuki, N.N.; Hanada, T.; Ichimura, Y.; Kumeta, H.; Fujioka, Y.; Ohsumi, Y.; Inagaki, F. The crystal structure of Atg3, an autophagy-related ubiquitin carrier protein (E2) enzyme that mediates Atg8 lipidation. J. Biol. Chem. 2007, 282, 8036–8043. [Google Scholar] [CrossRef] [PubMed]
- Taherbhoy, A.M.; Tait, S.W.; Kaiser, S.E.; Williams, A.H.; Deng, A.; Nourse, A.; Hammel, M.; Kurinov, I.; Rock, C.O.; Green, D.R. Atg8 Transfer from Atg7 to Atg3: A Distinctive E1–E2 Architecture and Mechanism in the Autophagy Pathway. Mol. Cell 2011, 44, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Kirisako, T.; Takao, T.; Satomi, Y.; Shimonishi, Y.; Ishihara, N.; Mizushima, N.; Tanida, I.; Kominami, E.; Ohsumi, M. A ubiquitin-like system mediates protein lipidation. Nature 2000, 408, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Murrow, L.; Malhotra, R.; Debnath, J. ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 2015, 17, 300–310. [Google Scholar] [CrossRef]
- Huang, W.; Ma, D.; Xia, L.; Zhang, E.; Wang, P.; Wang, M.; Guo, F.; Wang, Y.; Ni, D.; Zhao, H. Overexpression of CsATG3a improves tolerance to nitrogen deficiency and increases nitrogen use efficiency in arabidopsis. Plant Physiol. Biochem. 2023, 196, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.P.; Zhao, B.G.; Chao, Q.; Wang, B.C.; Zhang, Q.; Zhang, C.X.; Li, S.F.; Jin, F.X.; Yang, D.G.; Li, X.H. The Maize AP2/EREBP Transcription Factor ZmEREB160 Enhances Drought Tolerance in Arabidopsis. Trop. Plant Biol. 2020, 13, 251–261. [Google Scholar] [CrossRef]
- Wang, X.M.; Li, Z.G.; Yan, F.; Khalil, R.; Ren, Z.X.; Yang, C.W.; Yang, Y.W.; Deng, W. ZmSKIP, a homologue of SKIP in maize, is involved in response to abiotic stress in tobacco. Plant Cell Tissue Organ Cult. 2013, 112, 203–216. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, K.; Jha, A.K.; Yadava, P.; Pal, M.; Rakshit, S.; Singh, I. Global gene expression profiling under nitrogen stress identifies key genes involved in nitrogen stress adaptation in maize (Zea mays L.). Sci. Rep. 2022, 12, 4211. [Google Scholar]
- Chen, J.; Li, Q.; Zhang, P.M.; Lu, H.R.; Bian, Y.; Jian, Y.; Wang, Y.Z.; Ding, X.D.; Xiao, J.L. Cloning and functional characterization of two Gs SnRK1 gene promoters from wild soybean. Plant Biotechnol. Rep. 2021, 15, 627–639. [Google Scholar] [CrossRef]
- Srinath, T.; Reddy, V.D.; Rao, K.V. Isolation and functional characterization of a novel stress inducible promoter from pigeonpea (Cajanus cajan L). Plant Cell Tissue Organ Cult. (PCTOC) 2017, 128, 457–468. [Google Scholar] [CrossRef]
- Yong, Z.; Zhonghu, H.; Ye, G.; Aimin, Z.; Van Ginkel, M. Effect of environment and genotype on bread-making quality of spring-sown spring wheat cultivars in China. Euphytica 2004, 139, 75–83. [Google Scholar] [CrossRef]
- Jefferson, R.A. The GUS reporter gene system. Nature 1989, 342, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.-M.; Wang, J.-F.; Huang, J.; Zhang, H.-S. Cloning and characterization of three genes encoding Qb-SNARE proteins in rice. Mol. Genet. Genom. 2008, 279, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves 1. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Activities of antioxidant enzymes during senescence of Prunus armeniaca leaves. Biol. Plant 2001, 44, 41–46. [Google Scholar] [CrossRef]
- Guiboileau, A.; Masclaux-Daubresse, C. L’autophagie chez les plantes: Mécanismes, régulations et fonctions. Comptes Rendus Biol. 2012, 335, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Yang, M.K.; Guo, X.; Huang, W.; Chen, L. Multiple Functions of ATG8 Family Proteins in Plant Autophagy. Front. Cell Dev. Biol. 2020, 8, 13. [Google Scholar] [CrossRef]
- Suzuki, N.N.; Yoshimoto, K.; Fujioka, Y.; Ohsumi, Y.; Inagaki, F. The crystal structure of plant ATG12 and its biological implication in autophagy. Autophagy 2005, 1, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Vierstra, R.D. Autophagy: A multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 2012, 17, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chung, T.; Pennington, J.G.; Federico, M.L.; Kaeppler, H.F.; Kaeppler, S.M.; Otegui, M.S.; Vierstra, R.D. Autophagic Recycling Plays a Central Role in Maize Nitrogen Remobilization. Plant Cell 2015, 27, 1389–1408. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.Q.; Guo, Z.J.; Zhang, Z.J.; Jia, X.; Sun, Y.M.; Sun, X.; Wang, P.; Gong, X.Q.; Ma, F.W. The Apple Autophagy-Related Gene MdATG9 Confers Tolerance to Low Nitrogen in Transgenic Apple Callus. Front. Plant Sci. 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Q.; Su, W.; Zhang, H.; Niu, M.; Liu, X.; Li, Z.; Liu, C.; Wang, H.-L.; Yin, W.; Xia, X. Genome-wide analysis of autophagy-related gene family and PagATG18a enhances salt tolerance by regulating ROS homeostasis in poplar. Int. J. Biol. Macromol. 2023, 224, 1524–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Sun, X.; Liu, B.; Zhang, X.; Liang, W.; Huo, L.; Wang, P.; Ma, F.; Li, C. Overexpression of MdATG18a enhances alkaline tolerance and GABA shunt in apple through increased autophagy under alkaline conditions. Tree Physiol. 2020, 40, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.Q.; Sun, X.; Guo, Z.J.; Jia, X.; Che, R.M.; Sun, Y.M.; Zhu, Y.F.; Wang, P.; Gong, X.Q.; Ma, F.W. MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic. Res. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.Q.; Guo, Z.J.; Jia, X.; Sun, X.; Wang, P.; Gong, X.Q.; Ma, F.W. Increased autophagic activity in roots caused by overexpression of the autophagy-related gene MdATG10 in apple enhances salt tolerance. Plant Sci. 2020, 294, 11. [Google Scholar] [CrossRef]
- Huo, L.Q.; Guo, Z.J.; Wang, Q.; Jia, X.; Sun, X.P.; Ma, F.W. The protective role of MdATG10-mediated autophagy in apple plant under cadmium stress. Ecotoxicol. Environ. Saf. 2022, 234, 13. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, M.; Ling, B.Q.; Cao, T.; Wang, C.X.; Li, W.W.; Tang, W.S.; Chen, K.; Zhou, Y.B.; Chen, J. Overexpression of the autophagy-related gene SiATG8a from foxtail millet (Setaria italica L.) in transgenic wheat confers tolerance to phosphorus starvation. Plant Physiol. Biochem. 2023, 196, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Jia, X.; Li, T.; Wang, Y.; Sun, X.; Huo, L.; Wang, P.; Che, R.; Gong, X.; Ma, F. MdATG5a induces drought tolerance by improving the antioxidant defenses and promoting starch degradation in apple. Plant Sci. Int. J. Exp. Plant Biol. 2021, 312, 111052. [Google Scholar] [CrossRef]
- Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Meng, Z.; Zhang, G.; Qi, M.; Sun, Z.; Liu, Y.; Li, T. Sub-high Temperature and High Light Intensity Induced Irreversible Inhibition on Photosynthesis System of Tomato Plant (Solanum lycopersicum L.). Front Plant Sci. 2017, 8, 365. [Google Scholar]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gong, X.; Jia, X.; Li, X.; Wang, Y.; Wang, P.; Huo, L.; Sun, X.; Che, R.; Li, T. Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple. Int. J. Mol. Sci. 2021, 22, 5517. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyeva, L.I.; Lazareva, E.M.; Shelepova, O.V.; Baranova, E.N.; Kononenko, N.V. Salt-Induced Autophagy and Programmed Cell Death in Wheat. Agronomy 2022, 12, 1909. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ma, C. Atg3 promotes Atg8 lipidation via altering lipid diffusion and rearrangement. Protein Sci. 2020, 29, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H. Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy. Essays Biochem. 2013, 55, 39–50. [Google Scholar] [PubMed]
- Han, S.; Wang, Y.; Zheng, X.; Jia, Q.; Zhao, J.; Bai, F.; Hong, Y.; Liu, Y. Cytoplastic Glyceraldehyde-3-Phosphate Dehydrogenases Interact with ATG3 to Negatively Regulate Autophagy and Immunity in Nicotiana benthamiana. Plant Cell 2015, 27, 1316–1331. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Ma, L.; Tang, Y.; Yang, W.; Yang, Y.; Xi, J.; Wang, X.; Zhu, W.; Xue, J.; Zhang, X.; et al. Maize Autophagy-Related Protein ZmATG3 Confers Tolerance to Multiple Abiotic Stresses. Plants 2024, 13, 1637. https://doi.org/10.3390/plants13121637
Liu M, Ma L, Tang Y, Yang W, Yang Y, Xi J, Wang X, Zhu W, Xue J, Zhang X, et al. Maize Autophagy-Related Protein ZmATG3 Confers Tolerance to Multiple Abiotic Stresses. Plants. 2024; 13(12):1637. https://doi.org/10.3390/plants13121637
Chicago/Turabian StyleLiu, Mengli, Li Ma, Yao Tang, Wangjing Yang, Yuying Yang, Jing Xi, Xuan Wang, Wanchao Zhu, Jiquan Xue, Xinghua Zhang, and et al. 2024. "Maize Autophagy-Related Protein ZmATG3 Confers Tolerance to Multiple Abiotic Stresses" Plants 13, no. 12: 1637. https://doi.org/10.3390/plants13121637
APA StyleLiu, M., Ma, L., Tang, Y., Yang, W., Yang, Y., Xi, J., Wang, X., Zhu, W., Xue, J., Zhang, X., & Xu, S. (2024). Maize Autophagy-Related Protein ZmATG3 Confers Tolerance to Multiple Abiotic Stresses. Plants, 13(12), 1637. https://doi.org/10.3390/plants13121637





