Investigating the Mechanism of Cadmium-Tolerant Bacterium Cellulosimicrobium and Ryegrass Combined Remediation of Cadmium-Contaminated Soil
Abstract
:1. Introduction
2. Results
2.1. Changes in Growth, Physiology, and Biochemistry of Ryegrass
2.1.1. Changes in Biomass and Chlorophyll Content of Ryegrass
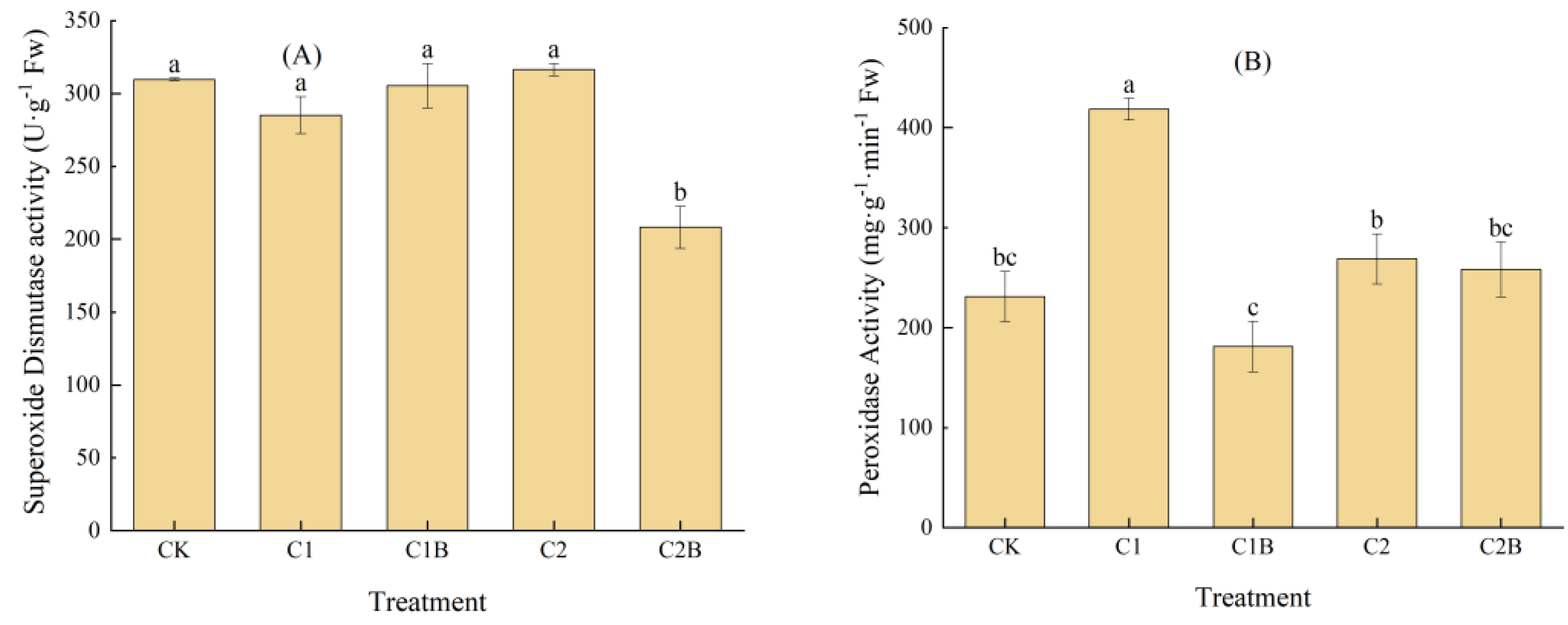
2.1.2. Changes in SOD and POD Activities of Ryegrass
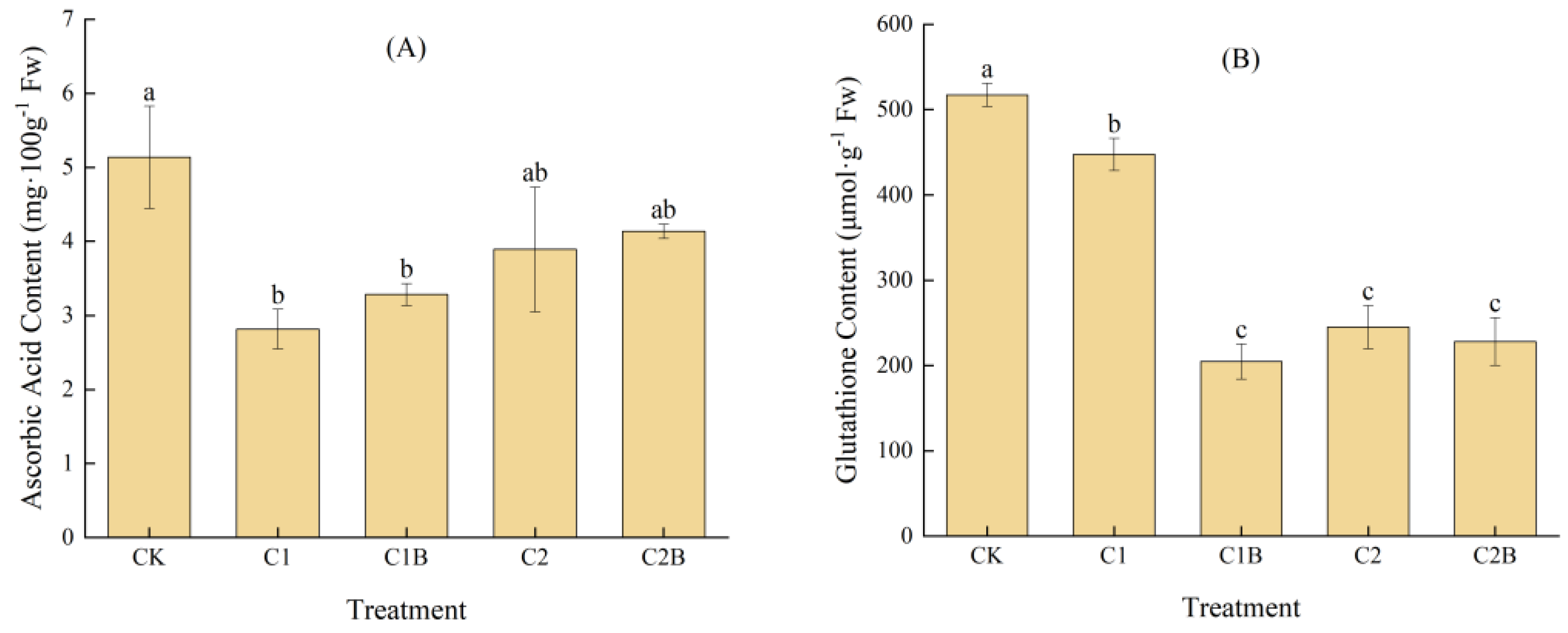
2.1.3. Changes in ASA and GSH Contents of Ryegrass
2.2. Changes in Soil Basal Respiration and Enzyme Activity
2.3. Effects of Cd-Tolerant Bacteria on Soil Cd Forms and Cd Accumulation in Ryegrass
2.3.1. The Content and Changes in Soil Cd Forms
2.3.2. Changes in the Cd Content of Ryegrass
2.4. Correlation of Cd Accumulation in Ryegrass with Microorganisms, Physiology of Ryegrass
3. Discussion
3.1. Effects of Cd-Tolerant Bacteria Inoculation on the Physiology and Biochemistry of Ryegrass
3.2. Effects of Cd-Tolerant Bacteria on Microbial Activity
3.3. Effects of Cd-Tolerant Bacteria on Soil Cd Forms and Cd Accumulation of Ryegrass
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Methods
4.3.1. Determination of Physiological and Biochemical Characteristics of Ryegrass
4.3.2. Determination of Soil Microbial Activity
4.3.3. Determination of Cd Content
4.3.4. Identification of Tolerant Bacteria
4.4. Instruments
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2014, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment of the People’s Pepublic of China. Bulletin on Ecological Environment in China; Ministry of Ecology and Environment of the People’s Pepublic of China: Beijing, China, 2021. (In Chinese)
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Peng, R.; Zhu, B.; Tian, Y.; Xu, J.; Wang, B.; Fu, X.; Han, H.; Wang, L.; Zhang, F.; et al. Enhanced phytoremediation of TNT and cobalt co-contaminated soil by AfSSB transformed plant. Ecotoxicol. Environ. Saf. 2021, 220, 112407. [Google Scholar] [CrossRef] [PubMed]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Yang, X.; Wang, P.; Mcgrath, S.P.; Zhao, F. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 2018, 207, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Lu, H.; Xu, D.; Kong, T.; Wang, D. Characteristics of enzyme activities during phytoremediation of Cd-contaminated soil. Sustainability 2022, 14, 9350. [Google Scholar] [CrossRef]
- Lou, Y.; Lou, H.; Hu, T.; Li, H.; Fu, J. Toxic effects, uptake, and translocation of Cd and Pb in perennial ryegrass. Ecotoxicology 2013, 22, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, K.; Song, L.; Li, W.; Jiang, H.; Huang, G. Exogenous indoleacetic acid induces cadmium accumulation and growth in Cinnamomum camphora. Sci. Hortic. 2024, 323, 112518. [Google Scholar] [CrossRef]
- He, S.; Wu, Q.; He, Z. Synergetic effects of DA-6/GA3 with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 2014, 117, 132–138. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, H.; He, Z.; Wang, L. Effects of a new-type cleaning agent and a plant growth regulator on phytoextraction of cadmium from a contaminated soil. Pedosphere 2019, 29, 161–169. [Google Scholar] [CrossRef]
- Fine, P.; Rathod, P.H.; Beriozkin, A.; Mingelgrin, U. Uptake of cadmium by hydroponically grown, mature Eucalyptus camaldulensis saplings and the effect of organic ligands. Int. J. Phytoremed. 2013, 15, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, A.; Perez-Sanz, A.; Semane, B.; Carleer, R.; Vangronsveld, J. Cadmium accumulation and tolerance of two Salix genotypes hydroponically grown in presence of Cadmium. J. Plant. Nutr. 2005, 28, 2159–2177. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Yang, L.; Chu, Y. Research on progress in combined remediation technologies of heavy metal polluted sediment. Int. J. Environ. Res. Public Health 2019, 16, 5098. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Luan, Y.; Ning, Y.; Wang, L. Effects and mechanisms of microbial remediation of heavy metals in soil: A critical review. Appl. Sci. 2018, 8, 1336. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Hu, F.; Li, H.; Ma, L.; Xu, L. Exogenous IAA treatment enhances phytoremediation of soil contaminated with phenanthrene by promoting soil enzyme activity and increasing microbial biomass. Environ. Sci. Pollut. Res. 2016, 23, 10656–10664. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Trasar-Cepada, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F. Limitations of soil enzymes as indicators of soil pollution. Soil Biol. Biochem. 2000, 32, 867–875. [Google Scholar] [CrossRef]
- Luo, J.; Qi, S.; Gu, X.W.S.; Hou, T.; Lin, L. Ecological risk assessment of EDTA-assisted phytoremediation of Cd under different cultivation systems. Bull. Environ. Contam. Toxicol. 2016, 96, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Z.; Rasheed, F.; Khan, W.R.; Mohsin, M.; Rashid, M.Z.; Magiman, M.M.; Raza, Z.; Rosli, Z.; Afzal, S.; Bakar, F.A. The change in growth, osmolyte production and antioxidant ezymes activity explains the cadmium tolerance in four tree species at the saplings stage. Forests 2022, 13, 1343. [Google Scholar] [CrossRef]
- Zhang, M.; Hua, M.; Guo, D.; Xue, Y.; Chen, X.; Rui, L.; Zhou, N. Effects of plant growth-promoting rhizobacteria on growth indicators and physiological characteristics of Peucedanum praeruptorum Dunn leaves. Plant Signal. Behav. 2023, 18, 2203571. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, S.; Wu, F.; Leung, H.M.; Lin, X.; Wong, M.H. Arbuscular mycorrhizal fungi enhance both absorption and stabilization of Cd by Alfred stonecrop (Sedum alfredii Hance) and perennial ryegrass (Lolium perenne L.) in a Cd-contaminated acidic soil. Chemosphere 2013, 93, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, X.; Amombo, E.; Fu, J.; Xie, Y. Cadmium tolerance of perennial ryegrass induced by Aspergillus aculeatus. Front Microbiol. 2018, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Qian, P.; Jin, R.; Ali, S.; Khan, M.; Aziz, R.; Tian, T.; Zhou, W. Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol. Plant. 2014, 58, 131–138. [Google Scholar] [CrossRef]
- Andresen, E.; Küpper, H. Cadmium toxicity in plants. In Cadmium: From Toxicity to Essentiality; Springer: Dordrecht, The Netherlands, 2013; pp. 395–413. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Desoky, E.S.M.; El-Tarabily, K.A.; AbuQamar, S.F.; Saad, A.M. Exploiting the role of plant growth promoting rhizobacteria in reducing heavy metal toxicity of pepper (Capsicum annuum L.). Environ. Sci. Pollut. Res. 2024, 31, 27465–27484. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, G.S.; El-Esawi, M.A. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef] [PubMed]
- Iori, V.; Pietrini, F.; Zacchini, M. Assessment of ibuprofen tolerance and removal capability in Populus nigra L. by in vitro culture. J. Hazard. Mater. 2012, 229, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Kaipiainen, E.; Salam, M.M.A.; Evstishenkov, N.; Nawrot, N.; Villa, A.; Wojciechowska, E.; Kuittinen, S.; Pappinen, A. Biomass production and removal of nitrogen and phosphorus from processed municipal wastewater by salix schwerinii: A field trial. Water 2021, 13, 2298. [Google Scholar] [CrossRef]
- Hussain, Z.; Rasheed, F.; Tanvir, M.A.; Zafar, Z.; Rafay, M.; Mohsin, M.; Pulkkinen, P.; Ruffner, C. Increased antioxidative enzyme activity mediates the phytoaccumulation potential of Pb in four agroforestry tree species: A case study under municipal and industrial wastewater irrigation. Int. J. Phytorem. 2021, 23, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xi, M.; Jiang, G.; Liu, X.; Bai, Z.; Huang, Y. Effects of IDSA, EDDS and EDTA on heavy metals accumulation in hydroponically grown maize (Zea mays L.). J. Hazard. Mater. 2010, 181, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, H.; Wang, Y.; He, G.; Wang, J.; Guo, D.; Li, T.; Sun, G.; Zhang, H. The role of antioxidant mechanism in photosynthesis under heavy metals Cd or Zn exposure in tobacco leaves. J. Plant Interact. 2021, 16, 354–366. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, G.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Wang, K.R.; Gong, H.; Wang, Y.; van der Zee, S.E.A.T.M. Toxic effects of cadmium on Morus alba L. and Bombyx moril L. Plant Soil 2004, 261, 171–180. [Google Scholar] [CrossRef]
- Hou, W.; Chen, X.; Song, G.; Wang, Q.; Chang, C.C. Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol. Biochem. 2007, 45, 62–69. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Guo, J.; Liu, Y. Antioxidative response in leaves and allelochemical changes in root exudates of Ricinus communis under Cu, Zn, and Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 32747–32755. [Google Scholar] [CrossRef]
- Zhu, T.; Li, L.; Duan, Q.; Liu, X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef] [PubMed]
- Maslennikova, D.; Nasyrova, K.; Chubukova, O.; Akimova, E.; Baymiev, A.; Blagova, D.; Ibragimov, A.; Lastochkina, O. Effects of Rhizobium leguminosarum Thy2 on the growth and tolerance to cadmium stress of wheat plants. Life 2022, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, X.; Zheng, Y.; Hong, W.; Li, X.; Ai, Y.; Wang, Y.; Zhang, Z.; Chen, H.; Huang, Y.; et al. Dynamic mechanisms of cadmium accumulation and detoxification by Lolium perenne grown in soil inoculated with the cadmium-tolerant bacterium strain Cdq4-2. Sci. Total Environ. 2023, 873, 162314. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, C.P.; Venkatesh, J.; Gururani, M.A.; Asnin, L.; Sharma, K.; Ajappala, H.; Park, S.W. Transgenic potato overproducing Lascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol. Lett. 2011, 33, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ba, Q.; Chen, S.; Liu, F.; Li, G. Exogenous application of glycine betaine alleviates cadmium toxicity in super black waxy maize by improving photosynthesis, the antioxidant system and glutathione-ascorbic acid cycle metabolites. Cereal Res. Commun. 2020, 48, 449–458. [Google Scholar] [CrossRef]
- Horikawa, S.; Yoneya, R.; Nagashima, Y.; Hagiwara, K.; Ozasa, H. Prior induction of heme oxygenase-1 with glutathione depletor ameliorates the renal ischemia and reperfusion injury in the rat. FEBS Lett. 2002, 510, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Beiyuan, J.; Hu, W.; Zhang, Z.; Duan, C.; Cui, Q.; Zhu, X.; He, H.; Huang, X.; Fang, L. Phytoremediation of potentially toxic elements (PTEs) contaminated soils using alfalfa (Medicago sativa L.): A comprehensive review. Chemosphere 2022, 293, 133577. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, J.C.; Lolata, G.B.; de Carvalho, G.M.; Da Silva, E.M.; Sousa, J.P.; Nogueira, M.A. Microbial indicators of soil health as tools for ecological risk assessment of a metal contaminated site in Brazil. Appl. Soil. Ecol. 2012, 59, 96–105. [Google Scholar] [CrossRef]
- Kızılkaya, R.; Aşkın, T.; Bayraklı, B.; Sağlam, M. Microbiological characteristics of soils contaminated with heavy metals. Eur. J. Soil Biol. 2004, 40, 95–102. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Czapla, J.; Płociniczak, T.; Piotrowska-Seget, Z. The effect of bioaugmentation of petroleum-contaminated soil with Rhodococcus erythropolis strains on removal of petroleum from soil. Ecotoxicol. Environ. Saf. 2019, 169, 615–622. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Chen, H.; Chen, G.; White, J.C.; Xing, B. Copper stress in flooded soil: Impact on enzyme activities, microbial community composition and diversity in the rhizosphere of Salix integra. Sci. Total Environ. 2020, 704, 135350. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F.; Turley, E.T.; Dawood, M.H. Monitoring soil enzymes activity before and after animal manure application. Agriculture 2020, 10, 166. [Google Scholar] [CrossRef]
- Xie, J.; Xu, X.; Zhang, S.; Yang, Z.; Wang, G.; Li, T.; Pu, Y.; Zhou, W.; Xu, C.; Lv, G.; et al. Activation and tolerance of Siegesbeckia orientalis L. rhizosphere to Cd stress. Front. Plant Sci. 2023, 14, 1145012. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, Y.; Hu, H.; Shaheen, S.M.; Zhong, H.; Tack, F.M.G.; Wu, M.; Li, Y.-F.; Gao, Y.; Rinklebe, J.; et al. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ. Int. 2021, 156, 106749. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Y.; Guo, H.; Lu, L.; Yang, C. Combined effect of ryegrass and Hyphomicrobium sp. GHH on the remediation of EE2-Cd co-contaminated soil. J. Soils Sediments 2019, 20, 425–434. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Li, J.; Wang, Z.; Jiang, Y. Adsorption of soil invertase to goethite, gibbsite and their organic complexes and the effects on enzyme catalytic performance. Colloids Surf. B 2023, 222, 113073. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Toxicology rethinks its central belief. Nature 2003, 421, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Mi, R.; Li, Q.; Lang, J.; Lan, Y.; Huang, N.; Yang, G. Bacillus Thuringiensis Enhances the Ability of Ryegrass to Remediate Cadmium-Contaminated Soil. Sustainability 2023, 15, 5177. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Wang, P.; Zhang, H.; Ali, E.F.; Li, R.; Shaheen, S.M.; Zhang, Z. Lactic acid bacteria promoted soil quality and enhanced phytoextraction of Cd and Zn by mustard: A trial for bioengineering of toxic metal contaminated mining soils. Environ. Res. 2023, 216, 114646. [Google Scholar] [CrossRef]
- Zhang, C.; Tao, Y.; Li, S.; Ke, T.; Wang, P.; Wei, S.; Chen, L. Bioremediation of cadmium-trichlorfon co-contaminated soil by Indian mustard (Brassica juncea) associated with the trichlorfon-degrading microbe Aspergillus sydowii: Related physiological responses and soil enzyme activities. Ecotoxicol. Environ. Saf. 2020, 188, 109756. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Yan, K.; Wang, Z.; Lu, G.; He, Y.; He, W. Differences in the response of soil dehydrogenase activity to Cd contamination are determined by the different substrates used for its determination. Chemosphere 2017, 169, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.; Parkinson, D. Soil biological criteria as indicators of soil quality: Soil microorganisms. Am. J. Altern. Agric. 1992, 7, 33–37. [Google Scholar] [CrossRef]
- Liao, M.; Luo, Y.; Zhao, X.; Huang, C. Toxicity of cadmium to soil microbial biomass and its activity: Effect of incubation time on Cd ecological dose in a paddy soil. J. Zhejiang. Univ. Sci. B 2005, 6, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Chen, T.; Yang, J. Remediation of vanadium contaminated soil by alfalfa (Medicago sativa L.) combined with vanadium-resistant bacterial strain. Environ. Technol. Innov. 2020, 20, 101090. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jia, Q.; Li, Y.; Dong, K.; Xu, S.; Ren, Y.; Zhang, T.; Chen, J.; Shi, N.; Fu, S. Effect of arbuscular mycorrhiza fungus Diversispora eburnea inoculation on Lolium perenne and Amorpha fruticosa growth, cadmium uptake, and soil cadmium speciation in cadmium-contaminated soil. Int. J. Environ. Res. Public Health 2023, 20, 795. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, W.; Zheng, X.; Chen, X.; Fu, W.; Wang, G.; Ji, J.; Jin, C.; Guan, C. Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 2022, 302, 134900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, N.; Geng, Y.; Zhou, J.; Lei, J. Effects of the combined pollution of cadmium, lead and zinc on the phytoextraction efficiency of ryegrass (Lolium perenne L.). RSC Adv. 2019, 9, 20603–20611. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, Y.; Fang, Z.; Shi, G.; Lou, L.; Ren, K.; Cai, Q. Italian ryegrass–rice rotation system for biomass production and cadmium removal from contaminated paddy fields. J. Soils Sediments 2020, 20, 874–882. [Google Scholar] [CrossRef]
- Guo, J.K.; Zhao, J.; Ren, X.H.; Jia, H.L.; Muhammad, H.; Lv, X.; Wei, T.; Hua, L. Effects of Burkholderia sp. D54 on growth and cadmium uptake of tomato, ryegrass and soybean plants. Int. J. Environ. Sci. Technol. 2020, 17, 1149–1158. [Google Scholar] [CrossRef]
- Yung, L.; Blaudez, D.; Maurice, N.; Azou-Barré, A.; Sirguey, C. Dark Septate Endophytes Isolated from Non-hyperaccumulator plants can increase phytoextraction of Cd and Zn by the hyperaccumulator Noccaea caerulescens. Environ. Sci. Pollut. Res. 2021, 28, 16544–16557. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vila, M.; Arenas-Lago, D.; Rodríguez-Seijo, A.; Andrade, M.L.; Vega, F.A. Ability of Cytisus scoparius for phytoremediation of soils from a Pb/Zn mine: Assessment of metal bioavailability and bioaccumulation. J. Environ. Manag. 2019, 235, 152–160. [Google Scholar] [CrossRef]
- Alfaro, M.A.; Alfaro, M.A.; Jarvis, S.C.; Gregory, P.J. Factors affecting potassium leaching in different soils. Soil. Use Manag. 2004, 20, 182–189. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of soil physicochemical properties and enzyme activities to long-term reclamation of coastal saline soil, Eastern China. Sci. Total Environ. 2017; 607–608, 1419–1427. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Pepublic of China. Soil environmental quality Risk control standard for soil contamination of agricultural land; Ministry of Ecology and Environment of the People’s Pepublic of China: Beijing, China, 2018. (In Chinese)
- Yang, Q.; Zhao, Z.; Bai, Z.; Hou, H.; Yuan, Y.; Guo, A.; Li, Y. Effects of mycorrhizae and water conditions on perennial ryegrass growth in rare earth tailings. RSC Adv. 2019, 9, 10881–10888. [Google Scholar] [CrossRef]
- Cvitković, D.; Lisica, P.; Zorić, Z.; Repajić, M.; Pedisić, S.; Dragović-Uzelac, V.; Balbino, S. Composition and antioxidant properties of pigments of Mediterranean herbs and spices as affected by different extraction methods. Foods 2021, 10, 2477. [Google Scholar] [CrossRef]
- Fukami, J.; de la Osa, C.; Ollero, F.J.; Megías, M.; Hungria, M. Co-inoculation of maize with Azospirillum brasilense and Rhizobium tropici as a strategy to mitigate salinity stress. Funct. Plant Biol. 2018, 45, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Samrana, S.; Zhang, Y.; Malik, Z.; Khan, M.D.; Zhu, S. Reduced glutathione protects subcellular compartments from Pb-induced ROS injury in leaves and roots of upland cotton (Gossypium hirsutum L.). Front. Plant Sci. 2020, 11, 412. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Xu, B.; Li, J.; Huang, B. Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Front. Plant Sci. 2016, 7, 1500. [Google Scholar] [CrossRef]
- Xu, Y.; Seshadri, B.; Bolan, N.; Sarkar, B.; Ok, Y.S.; Zhang, W.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; et al. Microbial functional diversity and carbon use feedback in soils as affected by heavy metals. Environ. Int. 2019, 125, 478–488. [Google Scholar] [CrossRef]
- Yang, D.; Tang, L.; Cui, Y.; Chen, J.; Liu, L.; Guo, C. Saline-alkali stress reduces soil bacterial community diversity and soil enzyme activities. Ecotoxicology 2022, 31, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Filipović, L.; Romić, M.; Sikora, S.; Huić Babić, K.; Filipović, V.; Gerke, H.H.; Romić, D. Response of soil dehydrogenase activity to salinity and cadmium species. J. Soil Sci. Plant Nutr. 2020, 20, 530–536. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Shi, G.; Yan, Y.; Yu, Z.; Zhang, L.; Cheng, Y.; Shi, W. Modification-bioremediation of copper, lead, and cadmium-contaminated soil by combined ryegrass (Lolium multiflorum Lam.) and Pseudomonas aeruginosa treatment. Environ. Sci. Pollut. Res. 2020, 27, 37668–37676. [Google Scholar] [CrossRef] [PubMed]
- Guillemaut, P.; Maréchal-Drouard, L. Isolation of plant DNA: Afast, inexpensive, and reliable method. Plant Mol. Biol. Rep. 1992, 10, 60–65. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]




| Treatment | Exchangeable | Potential Bioavailability | Residual |
|---|---|---|---|
| C1 | 2.501 b | 0.295 b | 0.186 ab |
| C1B | 2.637 b | 0.340 b | 0.026 b |
| C2 | 13.522 a | 2.868 a | 0.571 a |
| C2B | 12.038 a | 3.627 a | 0.319 ab |
| Treatment | Cd Pollution Concentration (mg·kg−1) | Cd Tolerant Bacteria |
|---|---|---|
| CK | 0 | Without inoculation |
| C1 | 4 | Without inoculation |
| C1B | 4 | Inoculation |
| C2 | 20 | Without inoculation |
| C2B | 20 | Inoculation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, X.; Song, L.; Na, M.; Xu, S.; Zhang, J.; Huang, Y.; Li, X.; Zheng, X.; Zhou, J. Investigating the Mechanism of Cadmium-Tolerant Bacterium Cellulosimicrobium and Ryegrass Combined Remediation of Cadmium-Contaminated Soil. Plants 2024, 13, 1657. https://doi.org/10.3390/plants13121657
Li J, Xu X, Song L, Na M, Xu S, Zhang J, Huang Y, Li X, Zheng X, Zhou J. Investigating the Mechanism of Cadmium-Tolerant Bacterium Cellulosimicrobium and Ryegrass Combined Remediation of Cadmium-Contaminated Soil. Plants. 2024; 13(12):1657. https://doi.org/10.3390/plants13121657
Chicago/Turabian StyleLi, Jiaqi, Xiaoyang Xu, Lanping Song, Meng Na, Shangqi Xu, Jie Zhang, Yongjie Huang, Xiaoping Li, Xianqing Zheng, and Jihai Zhou. 2024. "Investigating the Mechanism of Cadmium-Tolerant Bacterium Cellulosimicrobium and Ryegrass Combined Remediation of Cadmium-Contaminated Soil" Plants 13, no. 12: 1657. https://doi.org/10.3390/plants13121657







