New Intrinsic Ecological Mechanisms of Leaf Nutrient Resorption in Temperate Deciduous Trees
Abstract
1. Introduction
2. Results
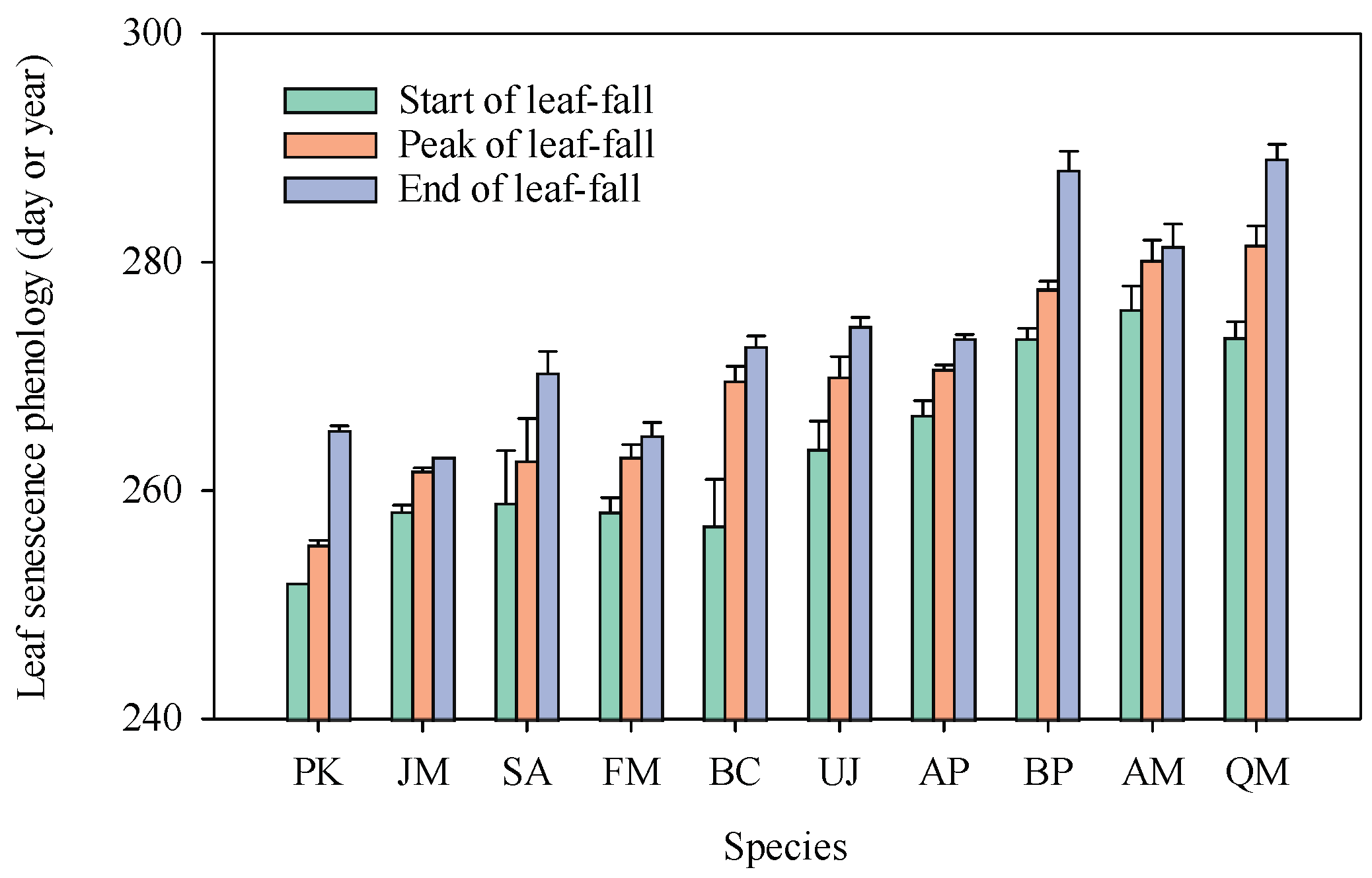
2.1. Leaf Senescence Phenology
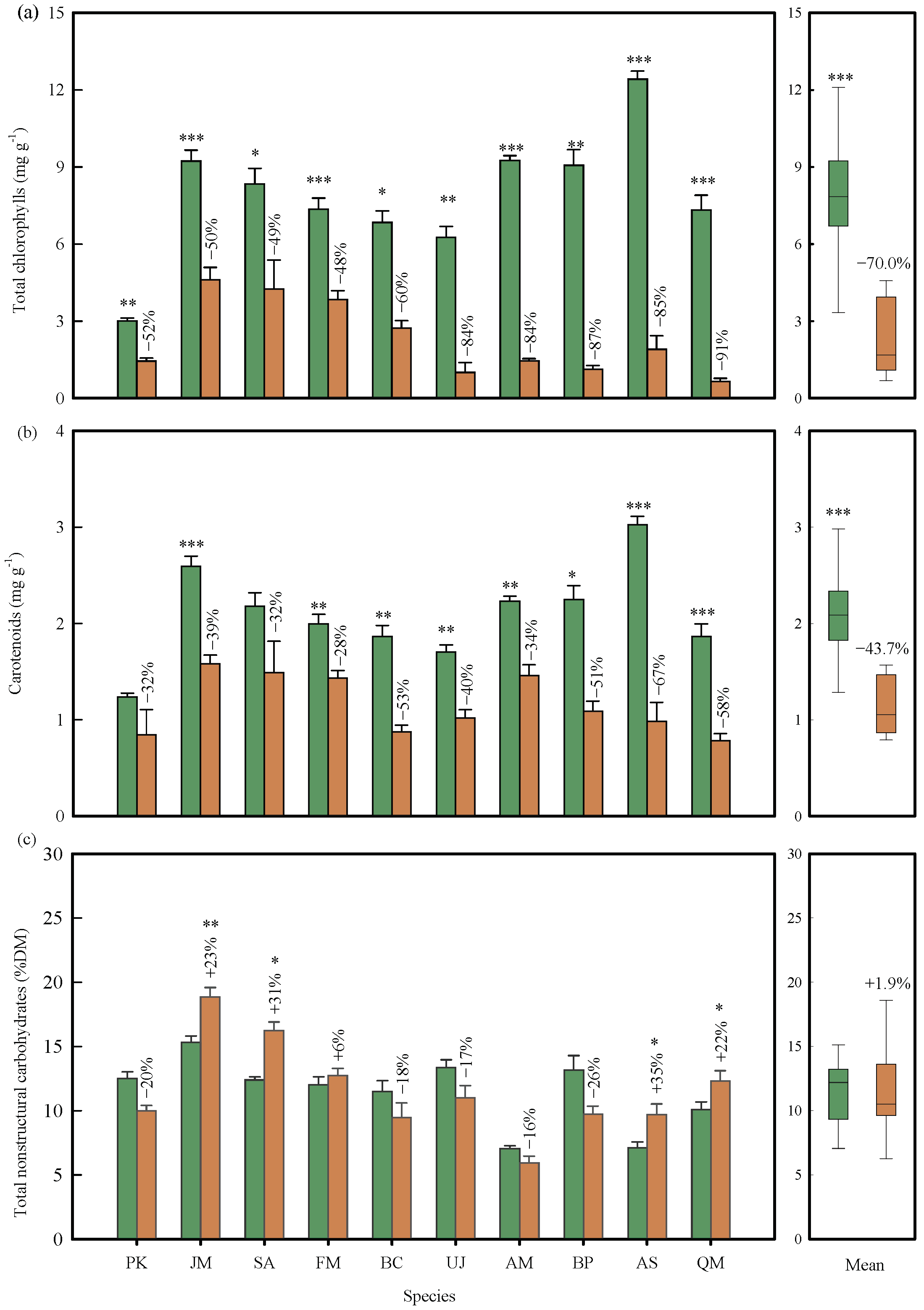
2.2. Changes in Leaf Pigments and Nonstructural Carbohydrates
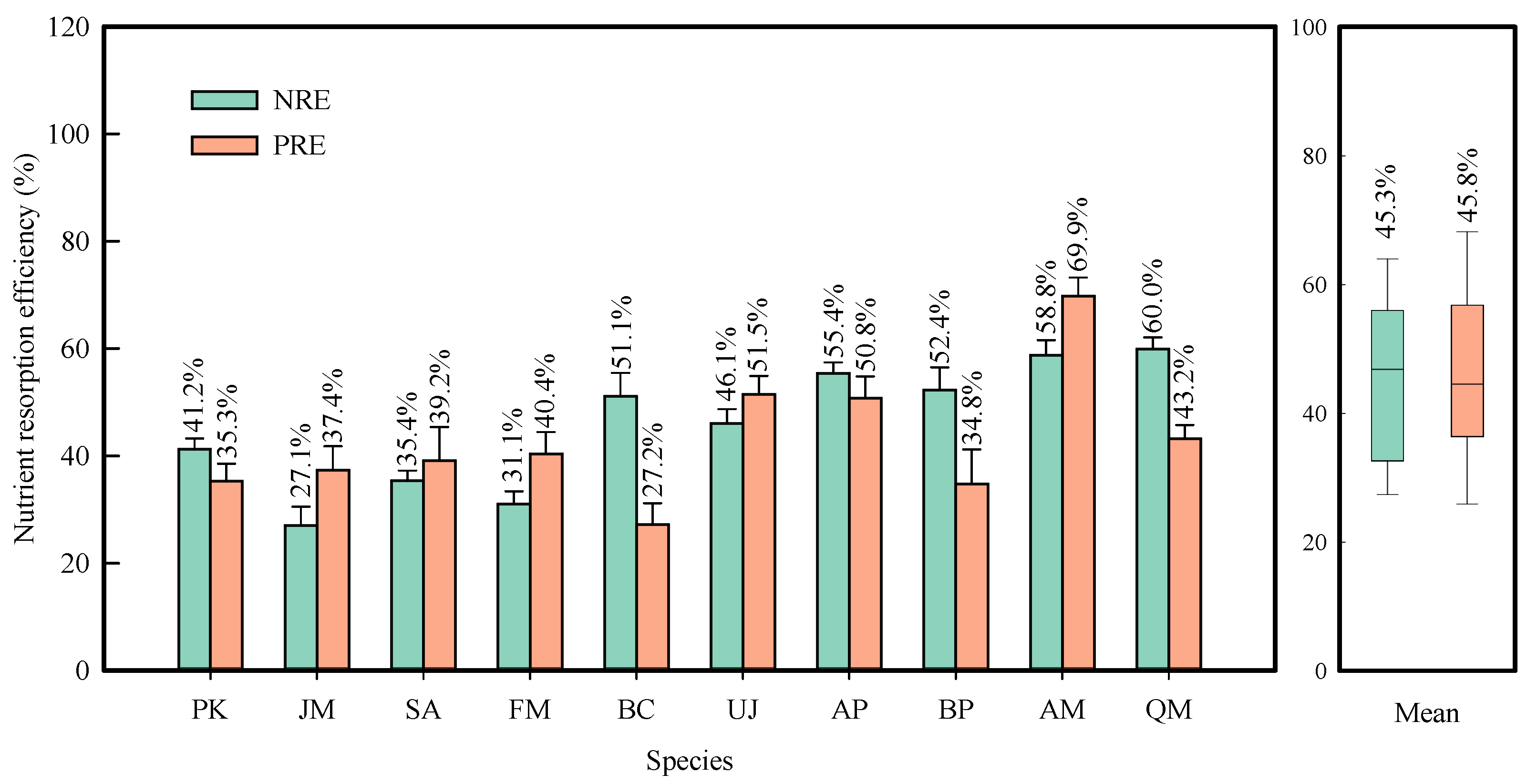
2.3. Correlations of Resorption Efficiency with Leaf Phenology, Pigments, and Nonstructural Carbohydrates
2.4. Intrinsic Factor Apportionment of Resorption Efficiency
3. Discussion
3.1. Leaf Senescence Phenology Hypothesis: Delayed Leaf Senescence Facilitates Nutrient Resorption
3.2. Leaf Pigments Hypothesis: Degradation of Chlorophylls and Carotenoids and Synthesis of Anthocyanins Facilitate Nutrient Resorption
3.3. Energy Residual Hypothesis: NSC Accumulation Suppresses Nutrient Resorption
3.4. Synthesis
4. Materials and Methods
4.1. Site Description
4.2. Field and Laboratorial Measurements
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wieder, W.R.; Cleveland, C.C.; Smith, W.K.; Todd-Brown, K. Future productivity and carbon storage limited by terrestrial nutrient availability. Nat. Geosci. 2015, 8, 441–444. [Google Scholar] [CrossRef]
- Estiarte, M.; Campioli, M.; Mayol, M.; Penuelas, J. Variability and limits of nitrogen and phosphorus resorption during foliar senescence. Plant Commun. 2023, 4, 100503. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, X.; Meng, D.; Liu, Z.; Gao, D.; Chang, Q.; Bai, E. Ectomycorrhizal trees rely on nitrogen resorption less than arbuscular mycorrhizal trees globally. Ecol. Lett. 2024, 27, e14346. [Google Scholar] [CrossRef] [PubMed]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Guo, L.; Deng, M.; Li, X.; Schmid, B.; Huang, J.; Wu, Y.; Peng, Z.; Yang, L.; Liu, L. Evolutionary and ecological forces shape nutrient strategies of mycorrhizal woody plants. Ecol. Lett. 2024, 27, e1433. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, G.; Tang, X.; Wang, W.; Wang, G.; Ma, K.; Han, S.; Du, S.; Li, S.; Yan, J.; et al. Different spatial patterns of nitrogen and phosphorus resorption efficiencies in China’s forests. Sci. Rep. 2017, 7, 10584. [Google Scholar] [CrossRef] [PubMed]
- Brant, A.N.; Chen, H.Y.H. Patterns and mechanisms of nutrient resorption in plants. Crit. Rev. Plant Sci. 2015, 34, 471–486. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Global-scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Glob. Ecol. Biogeogr. 2009, 18, 11–18. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Liu, F.; Zhao, R.; Quan, X.; Wang, C. Nutrient resorption estimation compromised by leaf mass loss and area shrinkage: Variations and solutions. For. Ecol. Manag. 2020, 472, 118232. [Google Scholar] [CrossRef]
- Du, B.; Ji, H.; Liu, S.; Kang, H.; Yin, S.; Liu, C. Nutrient resorption strategies of three oak tree species in response to interannual climate variability. For. Ecosyst. 2021, 8, 70. [Google Scholar] [CrossRef]
- Sun, X.; Li, D.; Lü, X.; Fang, Y.; Ma, Z.; Wang, Z.; Chu, C.; Li, M.; Chen, H. Widespread controls of leaf nutrient resorption by nutrient limitation and stoichiometry. Funct. Ecol. 2023, 37, 1653–1662. [Google Scholar] [CrossRef]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, Y.; Guo, L.; Yang, L.; Wang, B.; Wang, X.; Liu, W.; Su, Y.; Wu, J.; Liu, L. Foliar nutrient resorption stoichiometry and microbial phosphatase catalytic efficiency together alleviate the relative phosphorus limitation in forest ecosystems. New Phytol. 2023, 238, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.; Turner, B.L.; Lambers, H.; Laliberté, E. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J. Ecol. 2014, 102, 396–410. [Google Scholar] [CrossRef]
- Reed, S.C.; Townsend, A.R.; Davidson, E.A.; Cleveland, C.C. Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytol. 2012, 196, 173–180. [Google Scholar] [CrossRef]
- Chen, H.; Reed, S.C.; Lü, X.; Xiao, K.; Wang, K.; Li, D. Coexistence of multiple leaf nutrient resorption strategies in a single ecosystem. Sci. Total Environ. 2021, 772, 144951. [Google Scholar] [CrossRef]
- Shi, Z.; Meng, Q.; Luo, Y.; Zhang, M.; Han, W. Broadleaf trees switch from phosphorus to nitrogen limitation at lower latitudes than conifers. Sci. Total Environ. 2024, 914, 169924. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Piao, S.L.; Liu, Q.; Chen, A.P.; Janssens, I.A.; Fu, Y.S.; Dai, J.H.; Liu, L.L.; Lian, X.; Shen, M.G.; Zhu, X.L. Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 2019, 25, 1922–1924. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Furze, M.E.; Seyednasrollah, B.; Krassovski, M.B.; Latimer, J.M.; Nettles, W.R.; Heiderman, R.R.; et al. Ecosystem warming extends vegetation activity but heightens vulnerability to cold temperatures. Nature 2018, 560, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Zohner, C.M.; Mirzagholi, L.; Renner, S.S.; Mo, L.; Rebindaine, D.; Bucher, R.; Palouš, D.; Vitasse, Y.; Fu, Y.H.; Stocker, B.D.; et al. Effect of climate warming on the timing of autumn leaf senescence reverses after the summer solstice. Science 2023, 381, eadf5098. [Google Scholar] [CrossRef]
- Estiarte, M.; Penuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Change Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef]
- Wang, X.C.; Wang, Q.; Chen, Y.; Zhao, R.; Zhang, J.H.; Quan, X.K.; Liu, F.; Wang, C.K. Coloration and phenology manifest nutrient variability in senesced leaves of 46 temperate deciduous woody species. J. Plant Ecol. 2022, 15, 700–710. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Schaberg, P.G.; Van den Berg, A.K.; Murakami, P.F.; Shane, J.B.; Donnelly, J.R. Factors influencing red expression in autumn foliage of sugar maple trees. Tree Physiol. 2003, 23, 325–333. [Google Scholar] [CrossRef]
- Hughes, N.M.; Lev-Yadun, S. Review: Why do some plants have leaves with red or purple undersides? Environ. Exp. Bot. 2023, 205, 105126. [Google Scholar] [CrossRef]
- Marien, B.; Balzarolo, M.; Dox, I.; Leys, S.; Lorene, M.J.; Geron, C.; Portillo-Estrada, M.; AbdElgawad, H.; Asard, H.; Campioli, M. Detecting the onset of autumn leaf senescence in deciduous forest trees of the temperate zone. New Phytol. 2019, 224, 166–176. [Google Scholar] [CrossRef]
- Joly, S. Red and yellow pigments in autumn leaves are associated with higher nitrogen resorption A comment on Pena-Novas and Marchetti 2021. J. Evol. Biol. 2022, 35, 180–182. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef]
- Cazzonelli, C.I. Carotenoids in nature: Insights from plants and beyond. Funct. Plant Biol. 2011, 38, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B. Carotenoid catabolism during leaf senescence and its control by light. J. Photochem. Photobiol. B Biol. 1995, 30, 3–13. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.M.; George, C.O.; Gumpman, C.B.; Neufeld, H.S. Coevolution and photoprotection as complementary hypotheses for autumn leaf reddening: A nutrient-centered perspective. New Phytol. 2022, 233, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Zohner, C.M. Trees growing in Eastern North America experience higher autumn solar irradiation than their European relatives, but is nitrogen limitation another factor explaining anthocyanin-red autumn leaves? A comment on Pena-Novas and Marchetti 2021. J. Evol. Biol. 2022, 35, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Landi, M.; van Klink, J.W.; Schwinn, K.E.; Brummell, D.A.; Albert, N.W.; Chagne, D.; Jibran, R.; Kulshrestha, S.; Zhou, Y.; et al. Evolution and function of red pigmentation in land plants. Ann. Bot. 2022, 130, 613–636. [Google Scholar] [CrossRef]
- Lev-Yadun, S. The phenomenon of red and yellow autumn leaves: Hypotheses, agreements and disagreements. J. Evol. Biol. 2022, 35, 1245–1282. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; Casper, B.B.; Helliker, B.R. Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. J. Ecol. 2017, 105, 412–424. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.; Bergquist, G.; Gardestrom, P.; Jansson, S. A cellular timetable of autumn senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Fracheboud, Y.; Luquez, V.; Bjorken, L.; Sjodin, A.; Tuominen, H.; Jansson, S. The control of autumn senescence in European aspen. Plant Physiol. 2009, 149, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; del Arco, J.M. Ecological Significance of the Phenology of Leaf Abscission. Oikos 1987, 49, 11–14. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Yang, Q.-Y.; Lee, D.W.; Goldstein, G.; Cao, K.-F. Extended leaf senescence promotes carbon gain and nutrient resorption: Importance of maintaining winter photosynthesis in subtropical forests. Oecologia 2013, 173, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Song, H.M.; Liu, F.; Quan, X.K.; Wang, C.K. Timing of leaf fall and changes in litter nutrient concentration compromise estimates of nutrient fluxes and nutrient resorption efficiency. For. Ecol. Manag. 2022, 513, 120188. [Google Scholar] [CrossRef]
- Niinemets, U.; Tamm, U. Species differences in timing of leaf fall and foliage chemistry modify nutrient resorption efficiency in deciduous temperate forest stands. Tree Physiol. 2005, 25, 1001–1014. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Pietrasiak, N.; Short, T.H. Global temporal patterns in plant nutrient resorption plasticity. Glob. Ecol. Biogeogr. 2019, 28, 728–743. [Google Scholar] [CrossRef]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Change Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Marqués, L.; Hufkens, K.; Bigler, C.; Crowther, T.W.; Zohner, C.M.; Stocker, B.D. Acclimation of phenology relieves leaf longevity constraints in deciduous forests. Nat. Ecol. Evol. 2023, 7, 198–204. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Wang, L.; Aranibar, J.; Bauters, M.; Boeckx, P.; Crowley, B.E.; Dawes, M.A.; Delzon, S.; Fajardo, A.; et al. Isotopic evidence for oligotrophication of terrestrial ecosystems. Nat. Ecol. Evol. 2018, 2, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, H.; Ge, Q. Interpretable machine learning algorithms to predict leaf senescence date of deciduous trees. Agric. For. Meteorol. 2023, 340, 109623. [Google Scholar] [CrossRef]
- Gill, A.L.; Gallinat, A.S.; Sanders-DeMott, R.; Rigden, A.J.; Gianotti, D.J.S.; Mantooth, J.A.; Templer, P.H. Changes in autumn senescence in northern hemisphere deciduous trees: A meta-analysis of autumn phenology studies. Ann. Bot. 2015, 116, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Zohner, C.M. The occurrence of red and yellow autumn leaves explained by regional differences in insolation and temperature. New Phytol. 2019, 224, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Koide, D.; Ide, R.; Oguma, H. Detection of autumn leaf phenology and color brightness from repeat photography: Accurate, robust, and sensitive indexes and modeling under unstable field observations. Ecol. Indic. 2019, 106, 105482. [Google Scholar] [CrossRef]
- Mayta, M.L.; Hajirezaei, M.-R.; Carrillo, N.; Lodeyro, A.F. Leaf snescence: The chloroplast connection comes of age. Plants 2019, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Pena-Novas, I.; Archetti, M. A test of the photoprotection hypothesis for the evolution of autumn colours: Chlorophyll resorption, not anthocyanin production, is correlated with nitrogen translocation. J. Evol. Biol. 2021, 34, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Junker, L.V.; Ensminger, I. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Hoch, W.A.; Zeldin, E.L.; McCown, B.H. Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol. 2001, 21, 1–8. [Google Scholar] [CrossRef]
- Hoch, W.A.; Singsaas, E.L.; McCown, B.H. Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiol. 2003, 133, 1296–1305. [Google Scholar] [CrossRef]
- Pei, Z.; Huang, Y.; Ni, J.; Liu, Y.; Yang, Q. For a colorful life: Recent advances in anthocyanin biosynthesis during leaf senescence. Biology 2024, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T.; Pallardy, S.G.; Kramer, P.J. Physiology of Woody Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Lee, D.W.; O’Keefe, J.; Holbrook, N.M.; Feild, T.S. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecol. Res. 2003, 18, 677–694. [Google Scholar] [CrossRef]
- Bu, W.-S.; Chen, F.-S.; Wang, F.-C.; Fang, X.-M.; Mao, R.; Wang, H.-M. The species-specific responses of nutrient resorption and carbohydrate accumulation in leaves and roots to nitrogen addition in a subtropical mixed plantation. Can. J. For. Res. 2019, 49, 826–835. [Google Scholar] [CrossRef]
- Kim, J. Sugar metabolism as input signals and fuel for leaf senescence. Genes Genom. 2019, 41, 737–746. [Google Scholar] [CrossRef]
- Zani, D.; Crowther, T.W.; Mo, L.; Renner, S.S.; Zohner, C.M. Response to Comment on “Increased growing-season productivity drives earlier autumn leaf senescence in temperate trees”. Science 2021, 371, eabg2679. [Google Scholar] [CrossRef] [PubMed]
- Zani, D.; Crowther, T.W.; Mo, L.; Renner, S.S.; Zohner, C.M. Increased growing-season productivity drives earlier autumn leaf senescence in temperate trees. Science 2020, 370, 1066–1071. [Google Scholar] [CrossRef]
- Li, N.; He, N.; Yu, G.; Wang, Q.; Sun, J. Leaf non-structural carbohydrates regulated by plant functional groups and climate: Evidences from a tropical to cold-temperate forest transect. Ecol. Indic. 2016, 62, 22–31. [Google Scholar] [CrossRef]
- Sun, T.; Hobbie, S.E.; Berg, B.; Zhang, H.; Wang, Q.; Wang, Z.; Hättenschwiler, S. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl. Acad. Sci. USA 2018, 115, 10392–10397. [Google Scholar] [CrossRef]
- Prieto, I.; Querejeta, J.I. Simulated climate change decreases nutrient resorption from senescing leaves. Glob. Change Biol. 2020, 26, 1795–1807. [Google Scholar] [CrossRef]
- Asad, M.A.U.; Yan, Z.; Zhou, L.; Guan, X.; Cheng, F. How abiotic stresses trigger sugar signaling to modulate leaf senescence? Plant Physiol. Biochem. 2024, 210, 108650. [Google Scholar] [CrossRef]
- Sun, X.; Liu, F.; Zhang, Q.; Li, Y.; Zhang, L.; Wang, J.; Zhang, H.; Wanga, C.; Wang, X. Biotic and climatic controls on interannual variation in canopy litterfall of a species-rich deciduous broad-leaved forest. Agric. For. Meteorol. 2021, 307, 108483. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, F.; Zhu, Y.; Li, J.; Ruan, Y.; Quan, X.; Wang, X.; Wang, C. Degree of shade tolerance shapes seasonality of chlorophyll, nitrogen and phosphorus levels of trees and herbs in a temperate deciduous forest. J. For. Res. 2024, 35, 72. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Wang, X.C.; Liu, F.; Wang, C.K. Towards a standardized protocol for measuring leaf area index in deciduous forests with litterfall collection. For. Ecol. Manag. 2019, 447, 87–94. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. Aoac Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, C.K.; Wang, X.C. Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees-Struct. Funct. 2014, 28, 77–89. [Google Scholar] [CrossRef]


| Variable Group | Abbreviation | Definition |
|---|---|---|
| Phenology variables for leaf senescence phenology mechanism | StartDOY | Start of leaf fall |
| PeakDOY | Peak of leaf fall | |
| EndDOY | End of leaf fall | |
| Pigment variables for leaf pigments mechanism | Chl_sen | Chlorophyll in senesced leaves |
| Car_sen | Carotenoids in senesced leaves | |
| TAC_sen | Anthocyanins in senesced leaves | |
| DeltaChl | Degradation of chlorophyll | |
| DeltaChl_per | Degradation percentage of chlorophyll | |
| DeltaCar | Degradation of carotenoids | |
| DeltaCar_per | Degradation percentage of carotenoids | |
| Energy variables for energy residual mechanism | TNCgreen | Total nonstructural carbohydrates in green leaves |
| TNC_sen | Total nonstructural carbohydrates in senesced leaves | |
| DeltaTNC | Change in total nonstructural carbohydrates (TNCs) | |
| DeltaTNC_per | Change percentage of TNCs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, Y.; Wang, Q.; Pan, J.; Quan, X.; Gu, J.; Wang, C. New Intrinsic Ecological Mechanisms of Leaf Nutrient Resorption in Temperate Deciduous Trees. Plants 2024, 13, 1659. https://doi.org/10.3390/plants13121659
Wang X, Guo Y, Wang Q, Pan J, Quan X, Gu J, Wang C. New Intrinsic Ecological Mechanisms of Leaf Nutrient Resorption in Temperate Deciduous Trees. Plants. 2024; 13(12):1659. https://doi.org/10.3390/plants13121659
Chicago/Turabian StyleWang, Xingchang, Yanmin Guo, Qi Wang, Jun Pan, Xiankui Quan, Jiacun Gu, and Chuankuan Wang. 2024. "New Intrinsic Ecological Mechanisms of Leaf Nutrient Resorption in Temperate Deciduous Trees" Plants 13, no. 12: 1659. https://doi.org/10.3390/plants13121659
APA StyleWang, X., Guo, Y., Wang, Q., Pan, J., Quan, X., Gu, J., & Wang, C. (2024). New Intrinsic Ecological Mechanisms of Leaf Nutrient Resorption in Temperate Deciduous Trees. Plants, 13(12), 1659. https://doi.org/10.3390/plants13121659








