The Response and Evaluation of Morphology, Physiology, and Biochemistry Traits in Triploid Passiflora edulis Sims ‘Mantianxing’ to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Drought Stress Experiment
2.3. Determination of Leaf Morphology Indicators, Growth Yield, and Biomass
2.4. Physiological Index Measurements
2.5. Determination of Antioxidant Enzymes
2.6. Statistical Analysis
3. Results
3.1. The Effect of Drought Stress on the Morphology of Diploid and Triploid P. edulis ‘Mantianxing’
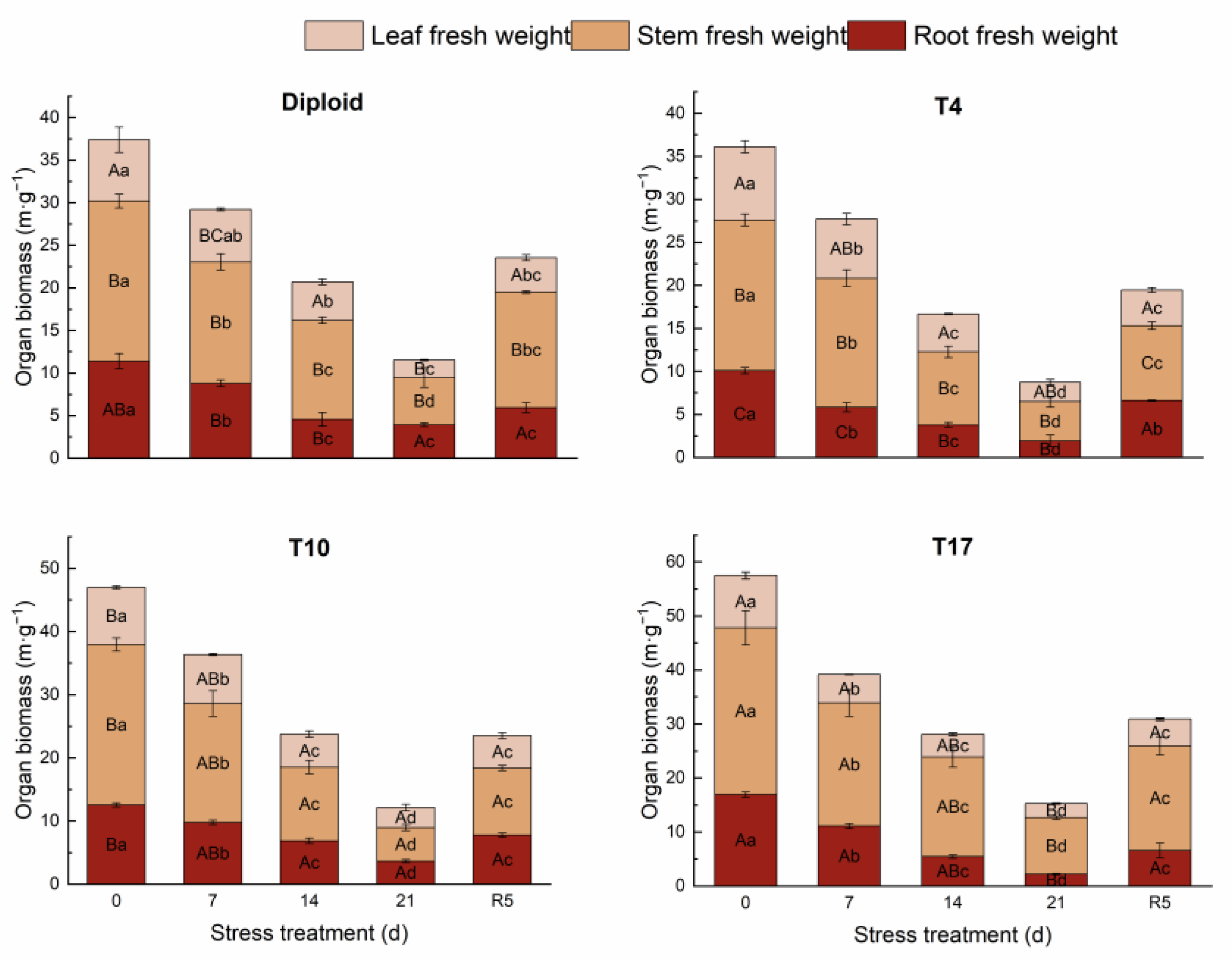
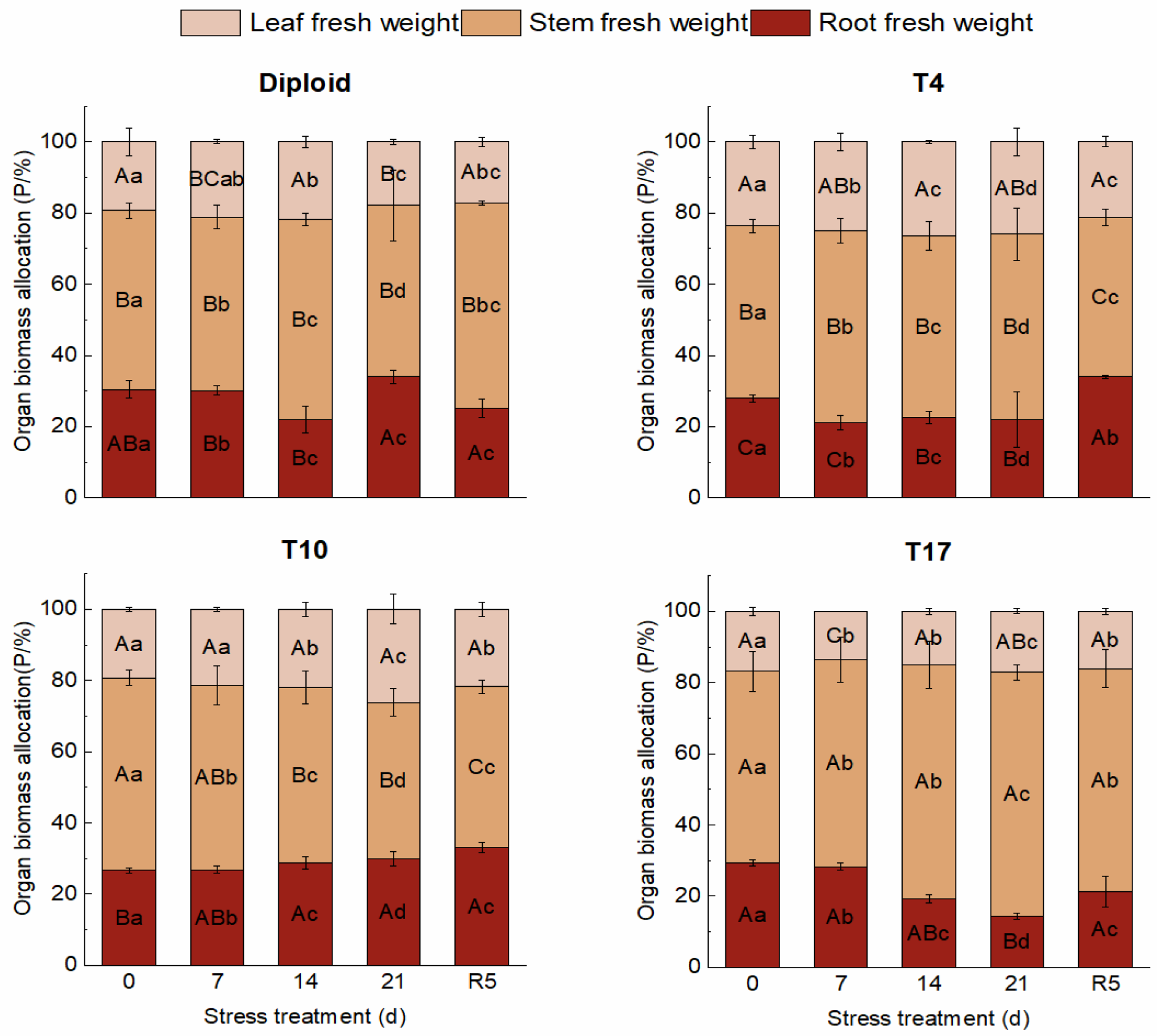
3.2. The Response of Organ Biomass in ‘Mantianxing’ to Drought Stress
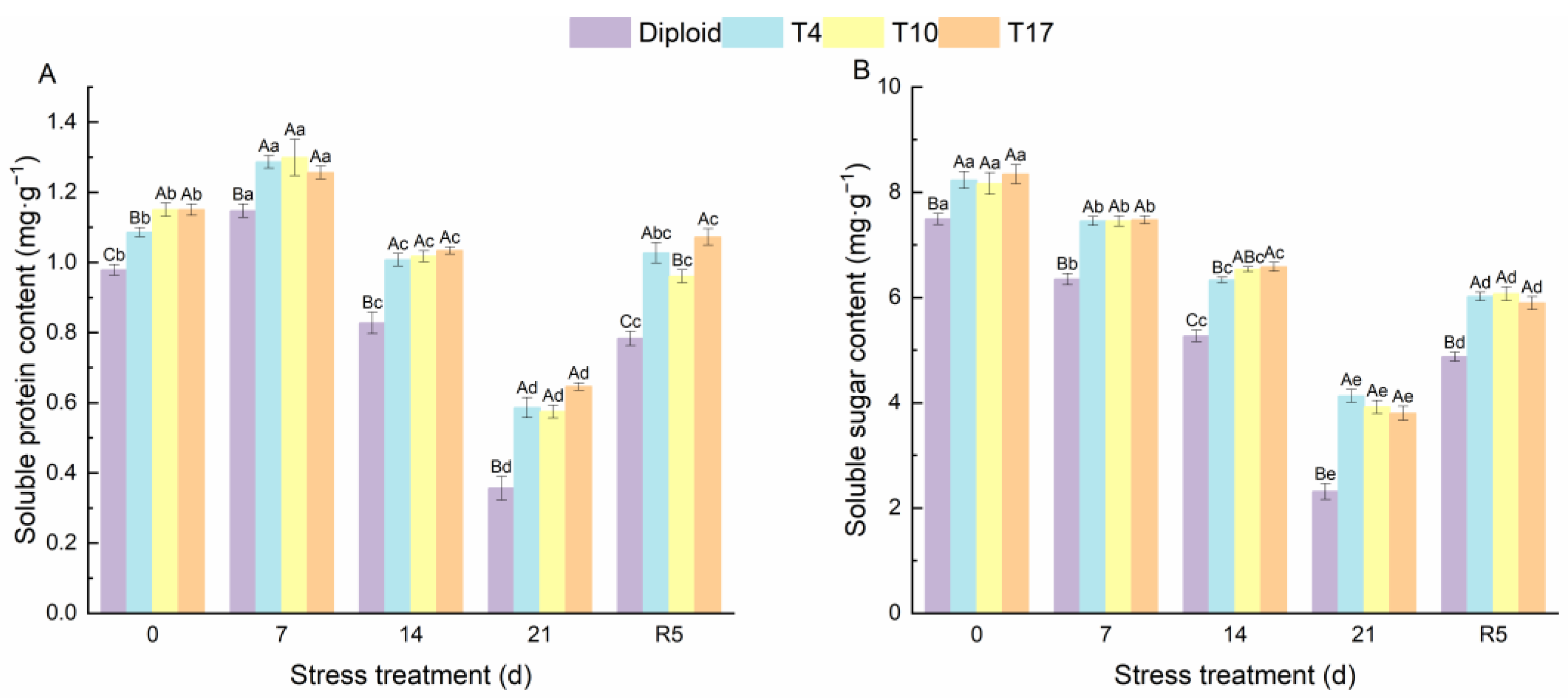
3.3. Chlorophyll Content, Soluble Protein, and Soluble Sugar
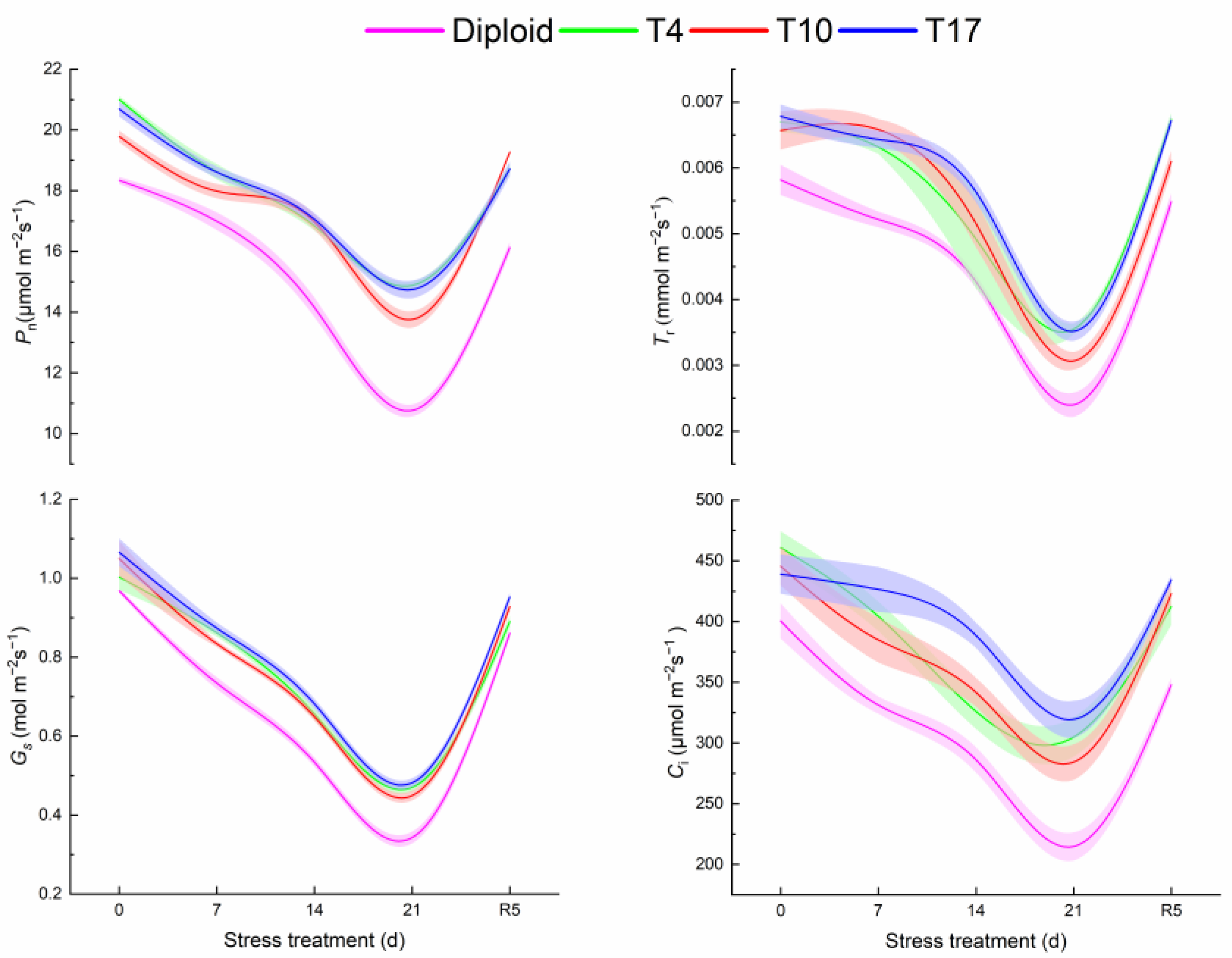
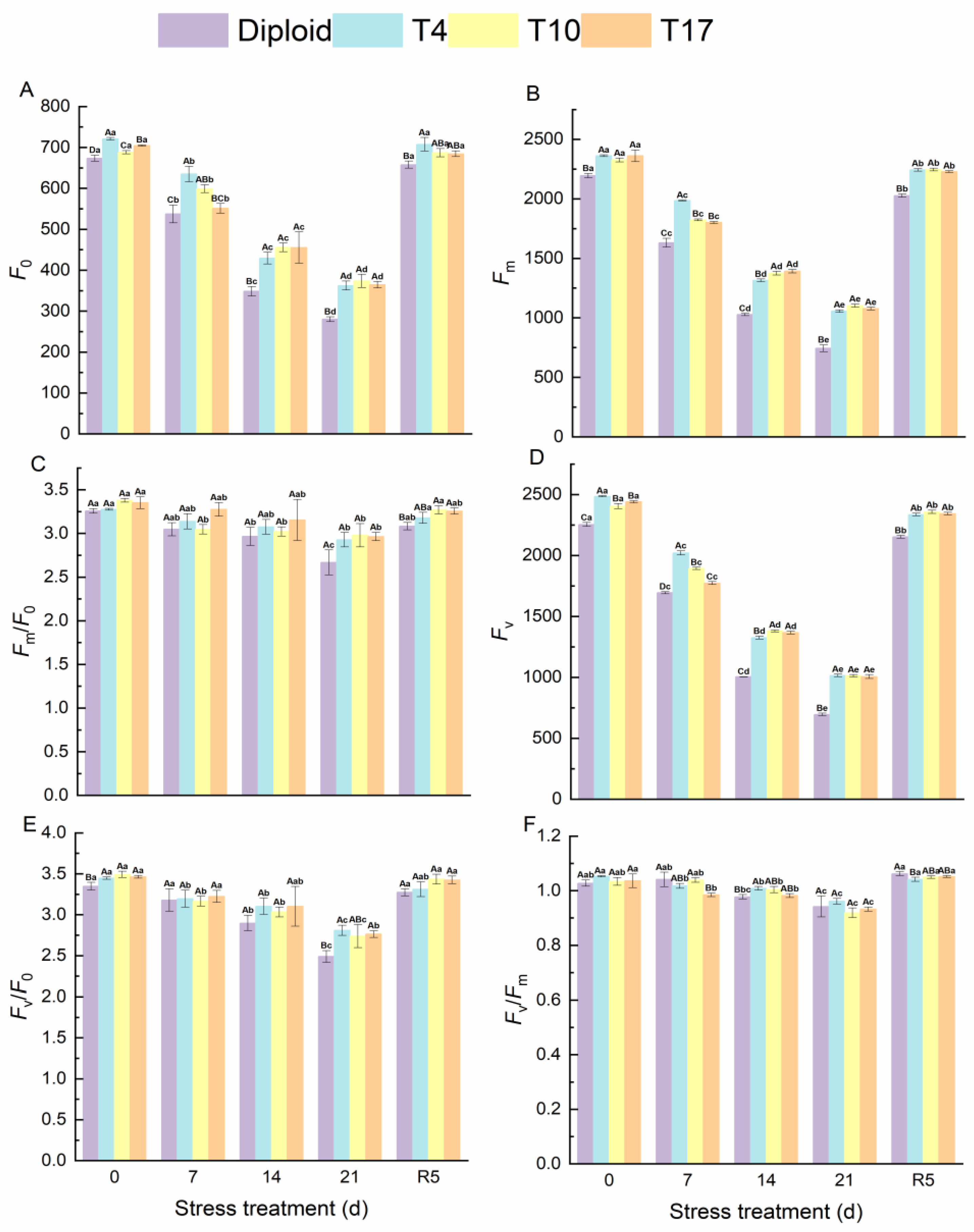
3.4. Physiological Traits in Diploid and Triploid ‘Mantianxing’ under Drought Stress
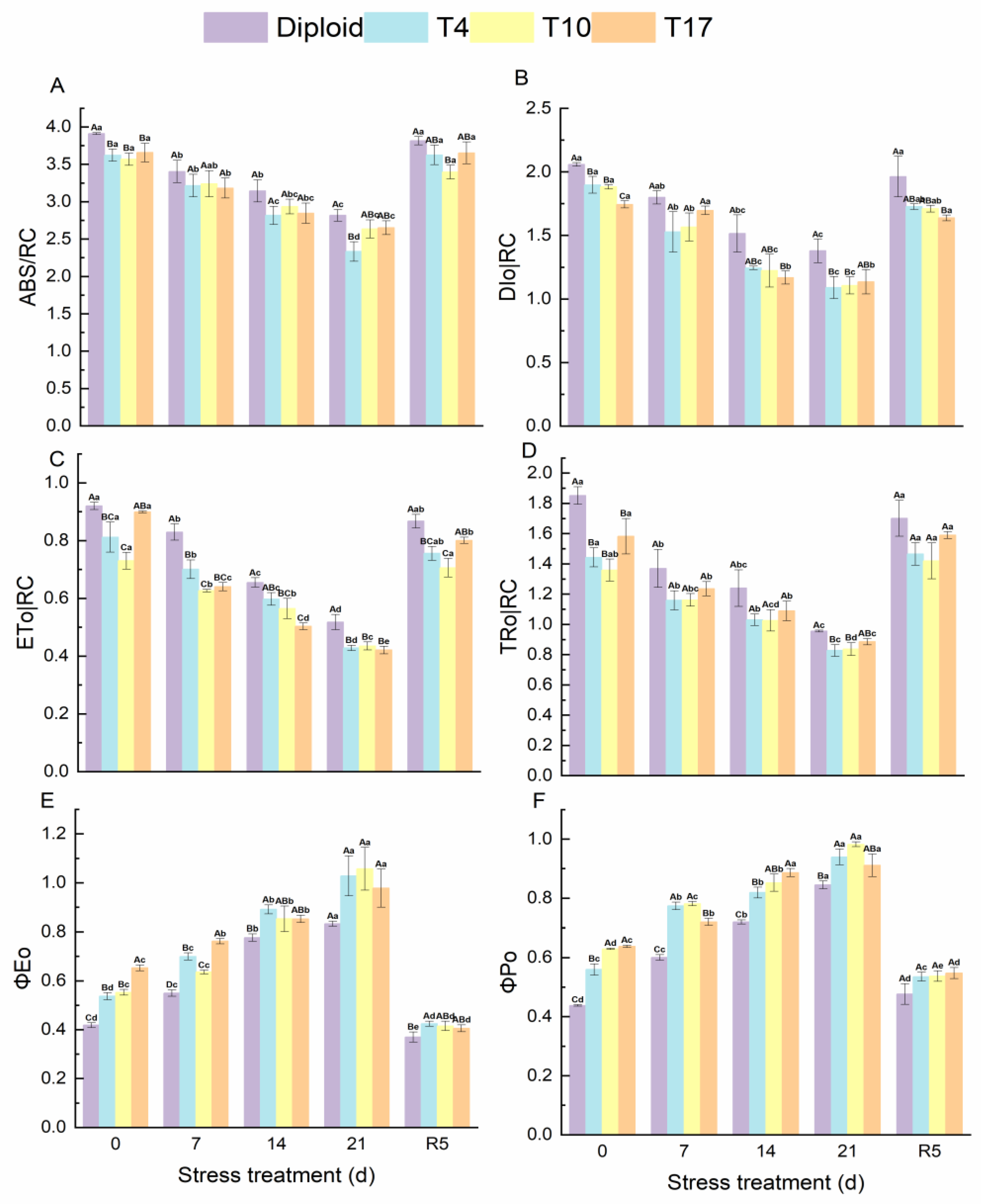
3.5. Energy Flow and Distribution in PS Ⅱ Reaction Center of Diploid and Triploid
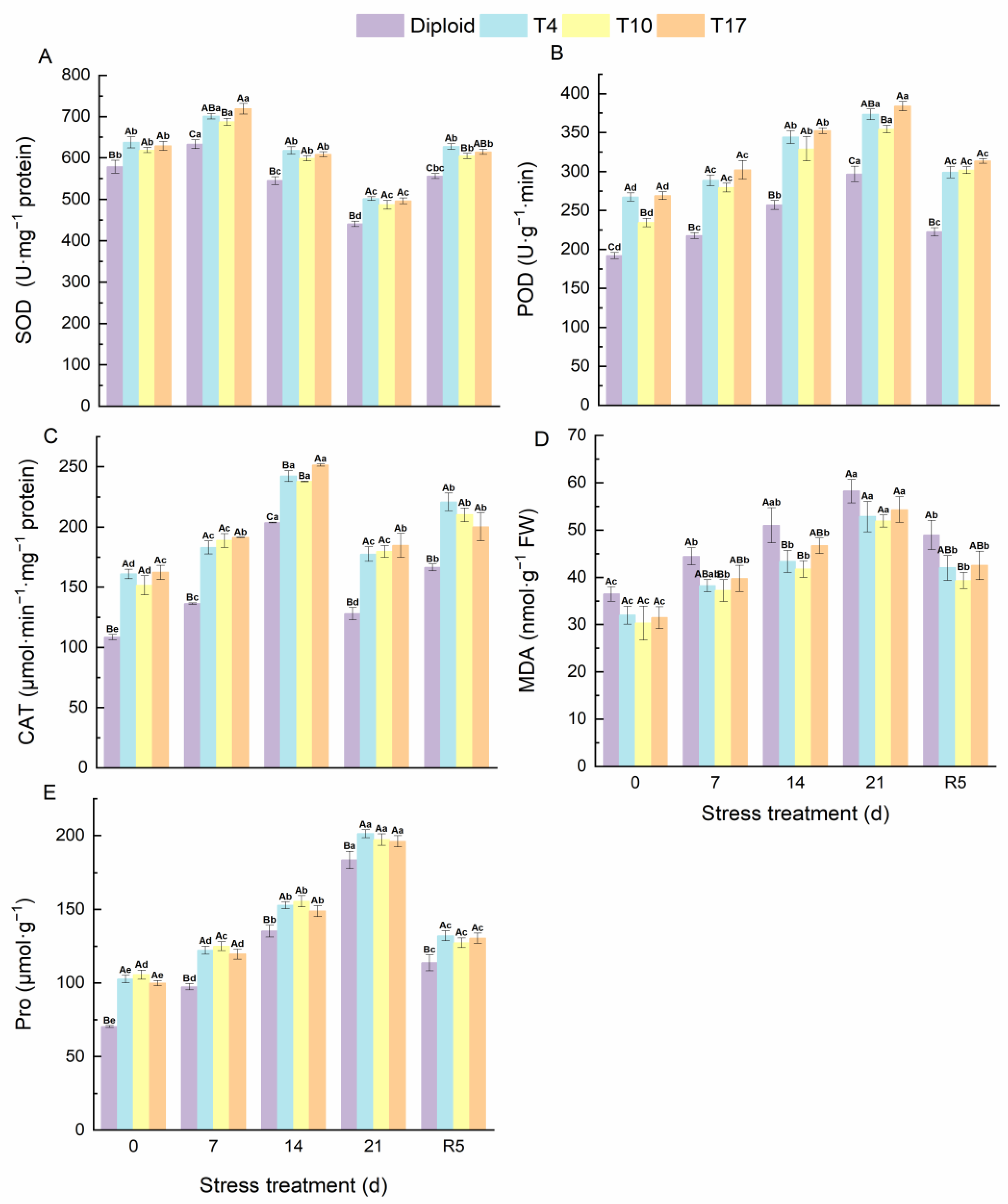
3.6. The Antioxidant Enzyme and MDA, Pro in Diploid and Triploid ‘Mantianxing’ under Drought Stress
3.7. The Correlation Analysis of Indicators in Diploid and Triploid ‘Mantianxing’
4. Discussion
4.1. Effect of Drought Stress on Morphology and Biomass Allocation of Triploid P. edulis ‘Mantianxing’
4.2. Variations in Chlorophyll Content and Photosynthesis in Triploid ‘Mantianxing’ under Drought
4.3. Chlorophyll Fluorescence and Photosynthesis System under Drought
4.4. Osmotic Adjustment Substances of Triploid ‘Mantianxing’ under Drought Stress
4.5. Antioxidant System of Triploid ‘Mantianxing’ under Drought
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, P.O.; Batista, D.S.; Cavalcanti, J.H.F.; Koehler, A.D.; Vieira, L.M.; Fernandes, A.M.; Barrera-Rojas, C.H.; Ribeiro, D.M.; Nogueira, F.T.S.; Otoni, W.C. Leaf Heteroblasty in Passiflora edulis as Revealed by Metabolic Profiling and Expression Analyses of the Micrornas Mir156 and Mir172. Ann. Bot. 2019, 123, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An Insight into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Araújo Galdino, O.; de Souza Gomes, I.; Ferreira de Almeida Júnior, R.; Conceição Ferreira de Carvalho, M.I.; Abreu, B.J.; Abbott Galvão Ururahy, M.; Cabral, B.; Zucolotto Langassner, S.M.; Costa de Souza, K.S.; Augusto de Rezende, A. The Nephroprotective Action of Passiflora edulis in Streptozotocin-Induced Diabetes. Sci. Rep. 2022, 12, 17546. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, H.-S.; Lee, H.-H.; Kim, T.-H. Role Identification of Passiflora incarnata Linnaeus: A Mini Review. J. Menopausal Med. 2017, 23, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Villada Ramos, J.A.; Aguillón Osma, J.; Restrepo Cortes, B.; Loango Chamarro, N.; Maldonado Celis, M.E. Identification of Potential Bioactive Compounds of Passiflora edulis Leaf Extract against Colon Adenocarcinoma Cells. Biochem. Biophys. Rep. 2023, 34, 101453. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, S.; Xiao, J.; He, W.; Zhou, Y.; Qin, Q.; Zhang, C.; Liu, Y. Polyploid Organisms. Sci. China Life Sci. 2012, 55, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Rothfels, C.J. Polyploid Phylogenetics. New Phytol. 2021, 230, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Sheng, Y.; Hao, Z.; Long, X.; Fu, F.; Liu, Y.; Tang, Z.; Ali, A.; Peng, Y.; Liu, Y.; et al. Transcriptome and Proteome Analysis Suggest Enhanced Photosynthesis in Tetraploid Liriodendron sino-americanum. Tree Physiol. 2021, 41, 1953–1971. [Google Scholar] [CrossRef] [PubMed]
- Dudits, D.; Török, K.; Cseri, A.; Paul, K.; Nagy, A.V.; Nagy, B.; Sass, L.; Ferenc, G.; Vankova, R.; Dobrev, P.; et al. Response of Organ Structure and Physiology to Autotetraploidization in Early Development of Energy Willow Salix viminalis. Plant Physiol. 2022, 170, 1504–1523. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, B.; Zou, J.; Luo, Z.; Yang, H.; Zhou, P.; Chen, X.; Zhou, W. Induction of Tetraploids in Paper Mulberry (Broussonetia papyrifera (L.) L’Hér. Ex Vent.) by Colchicine. BMC Plant Biol. 2023, 23, 574. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Song, L.J.; Qi, Q.; Du, K.; Han, Q.; Kang, X.Y. Study of Variation in the Growth, Photosynthesis, and Content of Secondary Metabolites in Eucommia triploids. Trees Struct. Funct. 2019, 33, 817–826. [Google Scholar] [CrossRef]
- Yao, X.Z.; Qi, Y.; Chen, H.F.; Zhang, B.H.; Chen, Z.W.; Lu, L. Study of Camellia sinensis Diploid and Triploid Leaf Development Mechanism Based on Transcriptome and Leaf Characteristics. PLoS ONE 2023, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Moller, C.A.; Mitchell, N.G.; Martin, D.G.; Sacks, E.J.; Saikia, S.; Labonte, N.R.; Baldwin, B.S.; Morrison, J.I.; Ferguson, J.N.; et al. The Leaf Economics Spectrum of Triploid and Tetraploid C4 Grass Miscanthus x giganteus. Plant Cell Environ. 2022, 45, 3462–3475. [Google Scholar] [CrossRef]
- Castro, H.; Dias, M.C.; Castro, M.; Loureiro, J.; Castro, S. Impact of Genome Duplications in Drought Tolerance and Distribution of the Diploid-Tetraploid Jasione maritima. Front. Plant Sci. 2023, 14, 1144678. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J. Polyploidy and Ecological Adaptation in Wild Yarrow. Proc. Natl. Acad. Sci. USA 2011, 108, 7096–7101. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-Analysis of Drought and Heat Stress Combination Impact on Crop Yield and Yield Components. Physiol. Plant. 2020, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of Drought Stress on Photosynthetic Response, Leaf Water Potential, and Stem Sap Flow in Two Cultivars of Bi-Leader Apple Trees (Malus x domestica Borkh.). Sci. Hortic amsterdam 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to Drought Stress in Prunus sargentii and Larix kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- DiTommaso, A.; Averill, K.M.; Qin, Z.; Ho, M.; Westbrook, A.S.; Mohler, C.L. Biomass Allocation of Vincetoxicum rossicum and V. nigrum in Contrasting Competitive Environments. Am. J. Bot. 2021, 108, 1646–1661. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, D.; Song, W.; Wang, X.; Duan, W.; Wang, C.; Wang, P. Tobacco Roots Increasing Diameter and Secondary Lateral Density in Response to Drought Stress. Plant Physiol. Biochem. 2023, 204, 108122. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Su, Y.; He, Q.; Wang, J.; Qiu, Q.; Ma, J. A Morphophysiological Analysis of the Effects of Drought and Shade on Catalpa bungei Plantlets. Acta Physiol. Plant. 2017, 39, 80. [Google Scholar] [CrossRef]
- Sinha, R.; Fritschi, F.B.; Zandalinas, S.I.; Mittler, R. The Impact of Stress Combination on Reproductive Processes in Crops. Plant Sci. 2021, 311, 111007. [Google Scholar] [CrossRef] [PubMed]
- Hatzig, S.; Zaharia, L.I.; Abrams, S.; Hohmann, M.; Legoahec, L.; Bouchereau, A.; Nesi, N.; Snowdon, R.J. Early Osmotic Adjustment Responses in Drought-Resistant and Drought-Sensitive Oilseed Rape. J. Integr. Plant Biol. 2014, 56, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative Role of Sugar Signaling and Transport during Drought Stress Responses in Plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and Its Actions during the Drought Stress in Plants. Physiol. Plant. 2020, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chai, H.H.; Ho, W.K.; Mayes, S.; Massawe, F. Deciphering the Molecular Basis for Photosynthetic Parameters in Bambara Groundnut (Vigna subterranea L. Verdc) under Drought Stress. BMC Plant Biol. 2023, 23, 287. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Kordrostami, M.; Mohamadhasani, F.; Chaeikar, S.S. Antioxidant Gene Expression Analysis and Evaluation of Total Phenol Content and Oxygen-Scavenging System in Tea Accessions under Normal and Drought Stress Conditions. BMC Plant Biol. 2021, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Shang, X.H.; Cao, S.; Xie, X.Y.; Zeng, W.D.; Lu, L.Y.; Chen, S.B.; Yan, H.B. Comparative Physiology and Transcriptome Analysis Allows for Identification of Lncrnas Imparting Tolerance to Drought Stress in Autotetraploid Cassava. BMC Genom. 2019, 20, 514. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Hou, L.; Yang, W.; Liu, S.; Pang, X.; Li, Y. Multiple Responses Contribute to the Enhanced Drought Tolerance of the Autotetraploid Ziziphus jujuba Mill. var. spinosa. Cell Biosci. 2021, 11, 119. [Google Scholar] [CrossRef]
- Fonollá, A.; Hormaza, J.I.; Losada, J.M. Foliar Pectins and Physiology of Diploid and Autotetraploid Mango Genotypes under Water Stress. Plants 2023, 12, 3738. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Chen, S.; Yang, Z.; Luo, X.; Xu, Y.; Wang, D.; Cai, N. Comparison of Morphology, Chlorophyll and Chlorophyll Fluorescence Parameters of Passiflora edulis with Different Ploidy. J. ShandongAgricultural Univ. Nat. Sci. Ed. 2022, 53, 755–762. [Google Scholar]
- Yang, Z.; Zhu, Y.; Lu, Z.; Luo, X.; Su, X.; Cheng, S.; Cai, N.; Xu, Y.; Wang, D. Photosynthetic Characteristics of the Different Ploidy of Passiflora edulis Mantianxing. J. Plant Genet. Resour. 2023, 24, 1659–1668. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Wang, X.; Wang, B.; Xiao, F.; He, K. Physiological Response and Drought Resistance Evaluation of Gleditsia sinensis Seedlings under Drought-Rehydration State. Sci. Rep. 2023, 13, 19963. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Buysse, J.; Merckx, R. An Improved Colorimetric Method to Quantify Sugar Content of Plant-Tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.Y.; Su, Y.K.; Song, Z.Q.; Wang, J.H. The Posttranscriptional Mechanism in Salvia miltiorrhiza Bunge Leaves in Response to Drought Stress Using Phosphoproteomics. Agronomy 2022, 12, 781. [Google Scholar] [CrossRef]
- Shi, H.; He, X.; Xu, L.; Pan, C.; Gong, C.; Wang, Y.; Liu, X.; Yu, Y. Drought Resistance of Camellia oleifera under Drought Stress: Changes in Physiology and Growth Characteristics. PLoS ONE 2020, 15, e0235795. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, H.L.; Yan, L.P.; Gao, L.; Zhai, S.S.; Xu, Y. Physiological, Photosynthetic and Stomatal Ultrastructural Responses of Quercus acutissima Seedlings to Drought Stress and Rewatering. Forests 2024, 15, 71. [Google Scholar] [CrossRef]
- Hajibarat, Z.; Saidi, A. Senescence-Associated Proteins and Nitrogen Remobilization in Grain Filling under Drought Stress Condition. J. Genet. Eng. Biotechnol. 2022, 20, 101–114. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.D.; Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Antioxidant Response Resides in the Shoot in Reciprocal Grafts of Drought-Tolerant and Drought-Sensitive Cultivars in Tomato under Water Stress. Plant Sci. 2012, 188, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Weng, B.; Jing, L.; Bi, W. Effects of Drought Stress on Water Content and Biomass Distribution in Summer Maize (Zea mays L.). Front. Plant sci. 2023, 14, 1118131. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Boot, R.G.A.; Vanderaart, P.J.M. The Relation between Aboveground and Belowground Biomass Allocation Patterns and Competitive Ability. Oecologia 1991, 87, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Ding, G.D.; Gao, G.L.; Liu, Z.H.; Wang, C.Y. Double Effects of Age and Environment on Resource Allocation Trade-offs of Salix psammophila in Different Microtopographic Habitats of a Sand Dune. J. Plant Growth Regul. 2020, 39, 544–552. [Google Scholar] [CrossRef]
- Huang, Y.X.; Zhao, X.Y.; Zhou, D.W.; Zhao, H.L.; Zhang, H.X.; Zuo, X.A.; Mao, W. Allometry of Salsola collina in Response to Soil Nutrients, Water Supply and Population Density. Nord. J. Bot. 2009, 27, 539–547. [Google Scholar] [CrossRef]
- Xu, B.; Wang, J.N.; Shi, F.S. Impacts Of Ontogenetic and Altitudinal Changes on Morphological Traits and Biomass Allocation Patterns of Fritillaria unibracteata. J. Mt. Sci. 2020, 17, 83–94. [Google Scholar] [CrossRef]
- Li, W.D.; Biswas, D.K.; Xu, H.; Xu, C.Q.; Wang, X.Z.; Liu, J.K.; Jiang, G.M. Photosynthetic Responses to Chromosome Doubling in Relation to Leaf Anatomy in Lonicera japonica Subjected to Water Stress. Funct. Plant Biol. 2009, 36, 783–792. [Google Scholar] [CrossRef]
- Godfree, R.C.; Marshall, D.J.; Young, A.G.; Miller, C.H.; Mathews, S. Empirical Evidence of Fixed and Homeostatic Patterns of Polyploid Advantage in A Keystone Grass Exposed to Drought and Heat Stress. R. Soc. Open Sci. 2017, 4, 170934. [Google Scholar] [CrossRef] [PubMed]
- Sabater, B.; Rodriguez, M.T. Control of Chlorophyll Degradation in Detached Leaves of Barley and Oat Through Effect of Kinetin on Chlorophyllase Levels. Physiol. Plant 1978, 43, 274–276. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effect of Water-Stress, Abscisic-Acid and Proline on Cotton Plants. J. Arid. Environ. 1995, 30, 315–325. [Google Scholar] [CrossRef]
- Yu, K.Y.; Wang, J.S.; Sun, C.Y.; Liu, X.Q.; Xu, H.Q.; Yang, Y.M.; Dong, L.D.; Zhang, D. High-Density QTL Mapping of Leaf-Related Traits and Chlorophyll Content in Three Soybean RIL Populations. BMC Plant Biol. 2020, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Casaretto, E.; Signorelli, S.; Gallino, J.P.; Vidal, S.; Borsani, O. Endogenous •NO Accumulation in Soybean Is Associated with Initial Stomatal Response to Water Deficit. Physiol. Plant. 2021, 172, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Z.; Bao, W.K.; Li, F.L.; Wu, N. Effects of Water Stress and Nitrogen Supply on Leaf Gas Exchange and Fluorescence Parameters of Sophora davidii Seedlings. Photosynthetica 2008, 46, 40–48. [Google Scholar] [CrossRef]
- Dong, B.; Wang, H.; Liu, T.; Cheng, P.; Chen, Y.; Chen, S.; Guan, Z.; Fang, W.; Jiang, J.; Chen, F. Whole Genome Duplication Enhances the Photosynthetic Capacity of Chrysanthemum nankingense. Mol. Genet. 2017, 292, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Wang, Y.; Xu, C.P.; Li, Y.; Kang, X.Y. Adaptive Photosynthetic and Physiological Responses to Drought and Rewatering in Triploid Populus Populations. Photosynthetica 2018, 56, 578–590. [Google Scholar] [CrossRef]
- Hui, T.; Bao, L.; Shi, X.; Zhang, H.; Xu, K.; Wei, X.; Liang, J.; Zhang, R.; Qian, W.; Zhang, M.; et al. Grafting Seedling Rootstock Strengthens Tolerance to Drought Stress in Polyploid Mulberry (Morus alba L.). Plant Physiol. Biochem. 2024, 208, 108441. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.Y.; Liu, Z.G.; Huo, H.Y.; Li, Z.L.; Nerry, F.; Wang, Q.S.; Li, X.W. Early Water Stress Detection using Leaf-Level Measurements of Chlorophyll Fluorescence and Temperature Data. Remote Sens. 2015, 7, 3232–3249. [Google Scholar] [CrossRef]
- Zhao, J.; Lang, Y.; Zhang, S.; Zhao, Q.; Zhang, C.; Xia, J. Photosynthetic Characteristics and Chlorophyll A Fluorescence Transient In Lonicera japonica under Drought Stress. Acta Physiol. Plant. 2019, 41, 124. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought Stress Strengthens the Link between Chlorophyll Fluorescence Parameters and Photosynthetic Traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef]
- Yang, X.Q.; Zhang, S.Q.; Liang, Z.S.; Shan, Y. Effects of Water Stress on Chlorophyll Fluorescence Parameters of Different Drought Resistance Winter Wheat Cultivars Seedlings. Xibei Zhiwu Xuebao 2004, 24, 812–816. [Google Scholar]
- Mao, H.T.; Chen, M.Y.; Su, Y.Q.; Wu, N.; Yuan, M.; Yuan, S.; Brestic, M.; Zivcak, M.; Zhang, H.Y.; Chen, Y. Comparison on Photosynthesis and Antioxidant Defense Systems in Wheat with Different Ploidy Levels and Octoploid Triticale. Int. J. Mol. Sci. 2018, 19, 3006. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, M.Q.; Xu, F.L.; Liu, S.; Meng, F.J. The Effects of Elevated CO2 (0.5%) on Chloroplasts in the Tetraploid Black Locust (Robinia pseudoacacia L.). Ecol. Evol. 2017, 7, 10546–10555. [Google Scholar] [CrossRef] [PubMed]
- López-Jurado, J.; Picazo-Aragonés, J.; Alonso, C.; Balao, F.; Mateos-Naranjo, E.; Lunn, J. Physiology, Gene Expression, and Epiphenotype of Two Dianthus broteri Polyploid Cytotypes under Temperature Stress. J. Exp. Bot. 2024, 75, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Sauer, N.; Neuhaus, H.E. Sugar Transport Across the Plant Vacuolar Membrane: Nature and Regulation of Carrier Proteins. Curr. Opin. Plant Biol. 2015, 25, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Conocono, E.A.; Egdane, J.A.; Setter, T.L. Estimation of Canopy Photosynthesis in Rice by Means of Daily Increases in Leaf Carbohydrate Concentrations. Crop. Sci. 1998, 38, 987–995. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect Of Various Drought Stresses and Subsequent Recovery on Proline, Total Soluble Sugar and Starch Metabolisms in Rice (Oryza sativa L.) Varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Deligoz, A.; Gur, M. Morphological, Physiological and Biochemical Responses to Drought Stress of Stone Pine (Pinus pinea L.) Seedlings. Acta Physiol. Plant. 2015, 37, 243. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Cao, P.; Zeng, X.; Xu, B.; Luo, F.; Yang, X.; Wang, X.; Wang, X.; Xiao, X.; et al. Morphological Structure and Physiological and Biochemical Responses to Drought Stress of Iris japonica. Plants 2023, 12, 3729. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, Physiochemical and Antioxidant Responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of Morphological, Physiological, and Biochemical Traits for Assessing Drought Resistance in Eleven Tree Species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Ahmad, P.; Umar, S.; Sharma, S. Mechanism of Free Radical Scavenging and Role of Phytohormones in Plants Under Abiotic Stresses. In Plant Adaptation and Phytoremediation; Springer: Dordrecht, The Netherlands, 2010; pp. 99–118. [Google Scholar] [CrossRef]
- Dvorak, P.; Krasylenko, Y.; Zeiner, A.; Samaj, J.; Takac, T. Signaling toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, G.S.; Salinas-Arcienega, A.J.; Galviz-Fajardo, Y.C.; do Amarante, L.; Pedroso, C.E.D.; Oliveira, J.C.P.; Köpp, M.M.; Martins, A.B.N.; de Tunes, L.V.M. Seed Germination and Antioxidant Enzyme Activity in Seedlings of Diploid and Tetraploid Bahiagrass under Water Restriction. Cienc. Rural. 2020, 50, e20190382. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hu, C.G.; Yao, J.L. Tetraploidization of Diploid Dioscorea Results in Activation of the Antioxidant Defense System and Increased Heat Tolerance. J. Plant Physiol. 2010, 167, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.J.; Pang, H.Y.; Huang, F.L.; Liu, L.; Wang, Y.J. Tetraploid Black Locust (Robinia pseudoacacia L.) Increased Salt Tolerance by Activation of the Antioxidant System. Biotechnol. Biotechnol. Equip. 2012, 26, 3351–3358. [Google Scholar] [CrossRef]
- Zhang, J.X.; Kirkham, M.B. Drought-Stress-Induced Changes in Activities of Superoxide-Dismutase, Catalase, and Peroxidase in Wheat Species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Fakhrzad, F.; Jowkar, A. Water Stress and Increased Ploidy Level Enhance Antioxidant Enzymes, Phytohormones, Phytochemicals and Polyphenol Accumulation of Tetraploid Induced Wallflower. Ind. Crop. Prod. 2023, 206, 117612. [Google Scholar] [CrossRef]




| Indicator | Ploidy | 0 | 7 | 14 | 21 | R5 |
|---|---|---|---|---|---|---|
| Root length (cm) | Diploid | 636.32 ± 42.95 Bb | 1450.41 ± 313.32 Aa | 543.00 ± 50.72 Aab | 507.55 ± 58.77 Ab | 952.11 ± 307.17 Ab |
| T4 | 823.78 ± 139.13 Aa | 897.48 ± 145.42 Aa | 769.77 ± 228.89 Aa | 671.71 ± 195.53 Aa | 822.73 ± 79.76 ABa | |
| T10 | 924.42 ± 98.28 Aa | 1333.88 ± 677.14 Aa | 819.12 ± 72.98 Aa | 619.70 ± 24.68 Ba | 924.42 ± 98.28 Aa | |
| T17 | 975.07 ± 315.70 Aa | 1227.95 ± 273.99 Aa | 886.43 ± 113.72 Aa | 827.97 ± 116.06 Aa | 1026.90 ± 287.79 Aa | |
| Leaf length (cm) | Diploid | 9.35 ± 0.21 Ba | 8.63 ± 0.15 Cb | 8.05 ± 0.19 Bb | 7.36 ± 0.27 Bc | 9.89 ± 0.23 Ba |
| T4 | 9.47 ± 0.15 Bb | 8.76 ± 0.11 BCc | 8.24 ± 0.11 Bd | 7.78 ± 0.11 ABe | 10.07 ± 0.14 Ba | |
| T10 | 9.51 ± 0.23 Bb | 9.18 ± 0.20 Bb | 8.38 ± 0.24 Bc | 7.86 ± 0.25 ABc | 10.39 ± 0.38 Ba | |
| T17 | 10.48 ± 0.21 Ab | 9.82 ± 0.23 Ab | 9.06 ± 0.22 Ac | 8.09 ± 0.20 Ad | 11.58 ± 0.32 Aa | |
| Leaf width (cm) | Diploid | 6.47 ± 0.15 Aa | 6.03 ± 0.12 Ab | 5.20 ± 0.10 Ac | 4.40 ± 0.18 Ad | 6.57 ± 0.13 Aa |
| T4 | 4.46 ± 0.15 Cb | 4.16 ± 0.09 Cb | 3.64 ± 0.15 Cc | 3.42 ± 0.14 Ac | 5.13 ± 0.18 Ba | |
| T10 | 5.30 ± 0.25 Bb | 4.80 ± 0.23 Bbc | 4.40 ± 0.14 Bc | 3.58 ± 0.21 Ad | 6.26 ± 0.26 Aa | |
| T17 | 5.38 ± 0.20 Ba | 4.89 ± 0.21 Ba | 4.20 ± 0.26 Ba | 3.58 ± 0.09 Aa | 6.08 ± 0.29 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Yang, Z.; Zhou, C.; Geng, S.; Chen, S.; Cai, N.; Tang, J.; Chen, L.; Xu, Y. The Response and Evaluation of Morphology, Physiology, and Biochemistry Traits in Triploid Passiflora edulis Sims ‘Mantianxing’ to Drought Stress. Plants 2024, 13, 1685. https://doi.org/10.3390/plants13121685
Su X, Yang Z, Zhou C, Geng S, Chen S, Cai N, Tang J, Chen L, Xu Y. The Response and Evaluation of Morphology, Physiology, and Biochemistry Traits in Triploid Passiflora edulis Sims ‘Mantianxing’ to Drought Stress. Plants. 2024; 13(12):1685. https://doi.org/10.3390/plants13121685
Chicago/Turabian StyleSu, Xin, Zhenxin Yang, Chiyu Zhou, Shili Geng, Shi Chen, Nianhui Cai, Junrong Tang, Lin Chen, and Yulan Xu. 2024. "The Response and Evaluation of Morphology, Physiology, and Biochemistry Traits in Triploid Passiflora edulis Sims ‘Mantianxing’ to Drought Stress" Plants 13, no. 12: 1685. https://doi.org/10.3390/plants13121685





