Soil Acidification Can Be Improved under Different Long-Term Fertilization Regimes in a Sweetpotato–Wheat Rotation System
Abstract
:1. Introduction
2. Results
2.1. Soil pH Changes under Different Fertilization Treatments
2.2. Changes in Soil CEC under Different Fertilization Treatments
2.3. Changes in Exchangeable Base Cation Ion Content in Soil under Different Fertilization Treatments
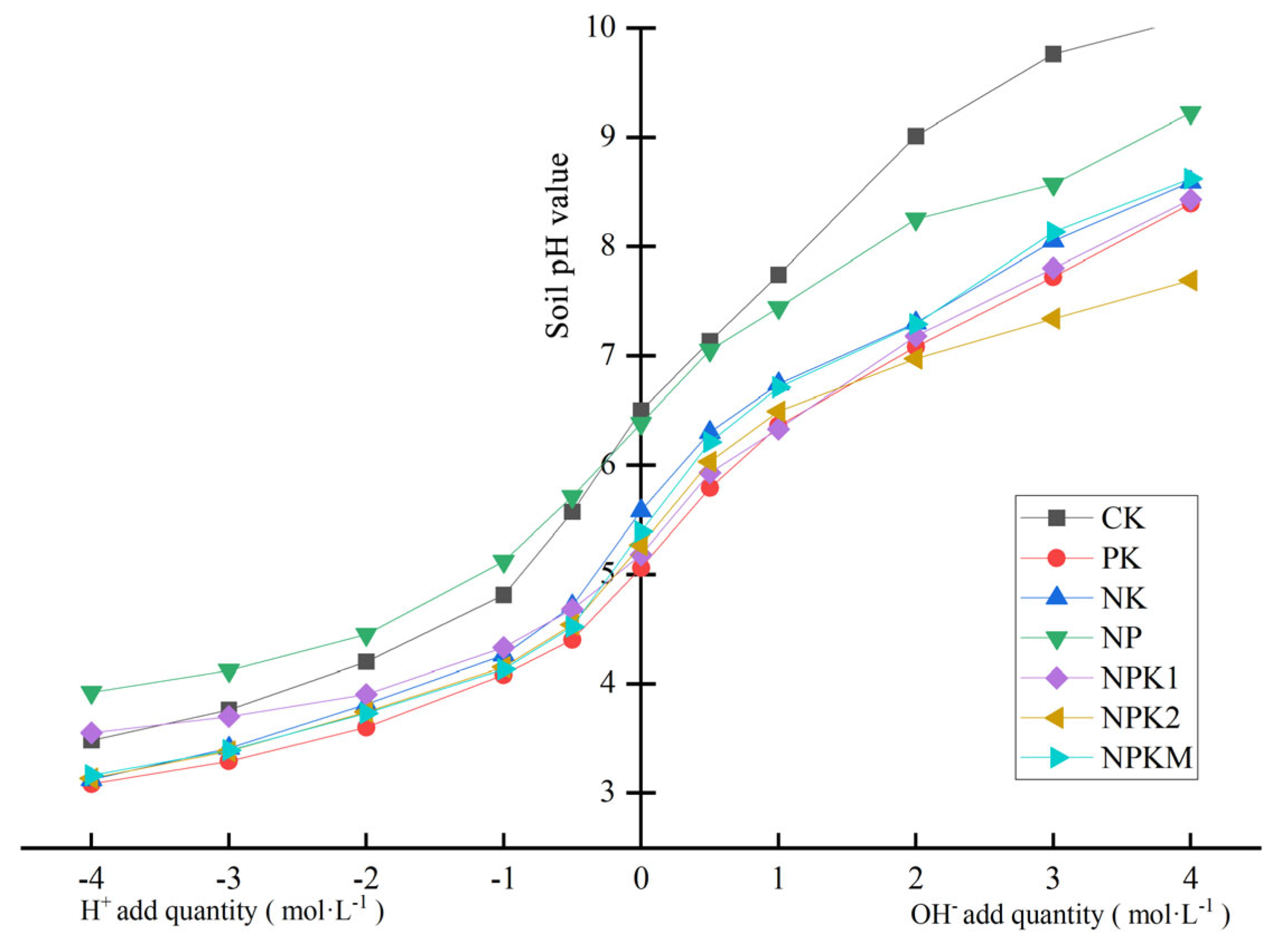
2.4. Changes in Soil-Buffering Capacity under Different Fertilization Treatments
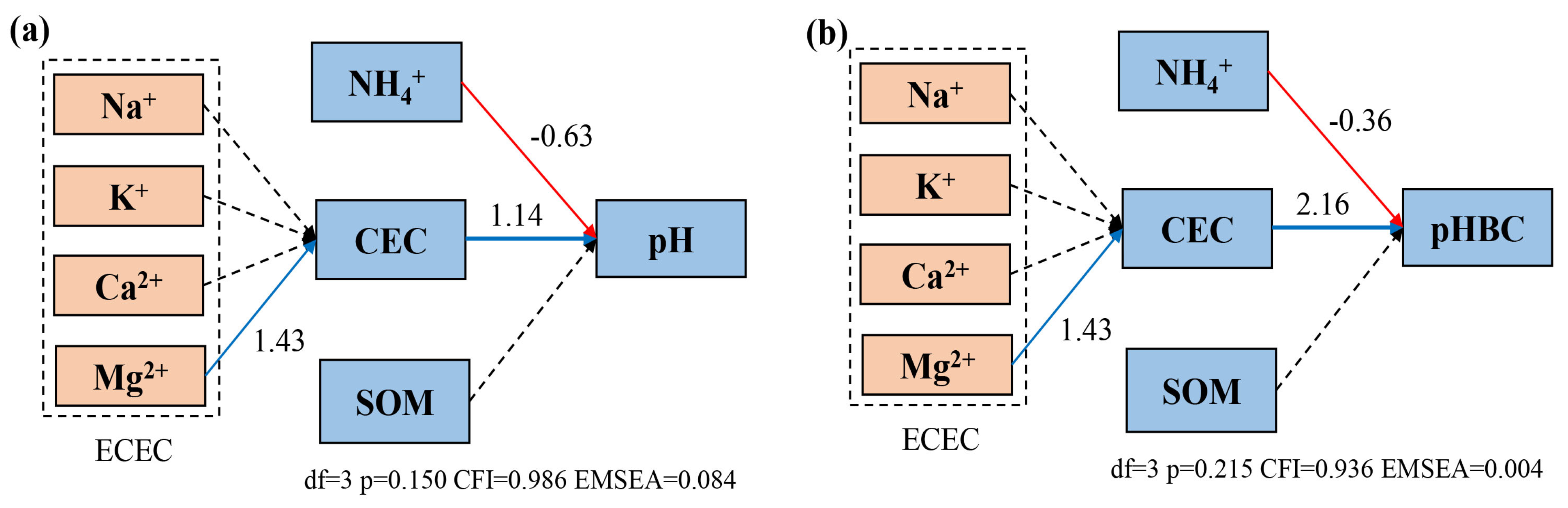
2.5. Correlation Analysis of Soil Physicochemical Properties
3. Discussion
3.1. Relationship between Crop Rotation Systems and Soil Acidification Characteristics
3.2. Relationship between Different Fertilization Treatments and Soil Acidification Characteristics
4. Materials and Methods
4.1. Experiment Site Description
4.2. Experimental Design
4.3. Soil Sample Collection and Physicochemical Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goulding, K. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil. Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Chang. Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.; Han, W.; Zhang, W.; Christie, P.; Goulding, K.; Vitousek, P.; Zhang, F. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Song, X.; Wang, Y.-P.; Canadell, J.G.; Luo, Y.; Ciais, P.; Chen, A.; Hong, S.; Wang, Y.; Tao, F. Size, distribution, and vulnerability of the global soil inorganic carbon. Science 2024, 384, 233–239. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sed. 2015, 15, 260–270. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Yan, X.; Jia, L.; Chen, N.; Liu, J.; Zhao, P.; Zhou, L.; Cao, Q. Effect of nitrogen, phosphorus and potassium fertilization management on soil properties and leaf traits and yield of Sapindus mukorossi. Front. Plant Sci. 2024, 15, 1300683. [Google Scholar] [CrossRef]
- Yao, R.; Li, H.; Yang, J.; Zhu, W.; Yin, C.; Wang, X.; Xie, W.; Zhang, X. Combined application of biochar and N fertilizer shifted nitrification rate and amoA gene abundance of ammonia-oxidizing microorganisms in salt-affected anthropogenic-alluvial soil. Appl. Soil. Ecol. 2022, 171, 104348. [Google Scholar] [CrossRef]
- Tkaczyk, P.; Mocek-Płóciniak, A.; Skowrońska, M.; Bednarek, W.; Kuśmierz, S.; Zawierucha, E. The mineral fertilizer-dependent chemical parameters of soil acidification under field conditions. Sustainability 2020, 12, 7165. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.; Xu, Y.; Mandal, S.; Gleeson, D.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C. Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv. Agron. 2016, 139, 1–71. [Google Scholar]
- Kurabachew, H. The Role of Orange Fleshed Sweet Potato (Ipomea batatas) for Combating Vitamin A Deficiency in Ethiopia. Int. J. Food Sci. Nutr. Eng. 2015, 5, 141–146. [Google Scholar]
- Nanbol, K.K.; Namo, O. The contribution of root and tuber crops to food security: A review. J. Agric. Sci. Technol. B 2019, 9, 221–233. [Google Scholar]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. S. Afr. J. Sci. 2015, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yi, Z.; Fang, Y.; He, K.; Huang, Y.; Zhu, H.; Du, A.; Tan, L.; Zhao, H.; Jin, Y. Improving the quality of barren rocky soil by culturing sweetpotato, with special reference to plant-microbes-soil interactions. Microbiol. Res. 2023, 268, 127294. [Google Scholar] [CrossRef] [PubMed]
- Urban, F.; Nordensvärd, J. Low carbon development: Origins, concepts and key issues. In Low Carbon Development; Routledge: New York, NY, USA, 2013; pp. 25–44. [Google Scholar]
- Zhao, Y.; Wang, L.; Lei, X.; Wang, B.; Cui, J.; Xu, Y.; Chen, Y.; Sui, P. Reducing carbon footprint without compromising grain security through relaxing cropping rotation system in the North China Plain. J. Clean Prod. 2021, 318, 128465. [Google Scholar] [CrossRef]
- Duan, J.; Liu, Y.-J.; Yang, J.; Tang, C.-J.; Shi, Z.-H. Role of groundcover management in controlling soil erosion under extreme rainfall in citrus orchards of southern China. J. Hydrol. 2020, 582, 124290. [Google Scholar] [CrossRef]
- Bashir, S.; Javed, A.; Bibi, I.; Ahmad, N. Soil and Water Conservation; University of Agriculture: Faisalabad, Pakistan, 2017; pp. 263–286. [Google Scholar]
- Gebremedhin, B.; Schwab, G. The Economic Importance of Crop Rotation Systems: Evidence from the Literature; Michigan State University, Department of Agricultural, Food, and Resource Economics: East Lansing, MI, USA, 1998; Volume 11690, p. 30. [Google Scholar]
- Zhao, J.; Yang, Y.; Zhang, K.; Jeong, J.; Zeng, Z.; Zang, H. Does crop rotation yield more in China? A meta-analysis. Field Crops Res. 2020, 245, 107659. [Google Scholar] [CrossRef]
- Li, Q.; Li, S.; Xiao, Y.; Zhao, B.; Wang, C.; Li, B.; Gao, X.; Li, Y.; Bai, G.; Wang, Y. Soil acidification and its influencing factors in the purple hilly area of southwest China from 1981 to 2012. Catena 2019, 175, 278–285. [Google Scholar] [CrossRef]
- Li, Z.; Xia, S.; Zhang, R.; Zhang, R.; Chen, F.; Liu, Y. N2O emissions and product ratios of nitrification and denitrification are altered by K fertilizer in acidic agricultural soils. Environ. Pollut. 2020, 265, 115065. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Xu, C.; Yuan, J.; Xu, X.; Wang, J.; Zhang, Y. Long-term nitrogen fertilization and sweetpotato cultivation in the wheat-sweetpotato rotation system decrease alkaline phosphomonoesterase activity by regulating soil phoD-harboring bacteria communities. Sci. Total Environ. 2023, 900, 165916. [Google Scholar] [CrossRef]
- Shao, G.; Ai, J.; Sun, Q.; Hou, L.; Dong, Y. Soil quality assessment under different forest types in the Mount Tai, central Eastern China. Ecol. Indic. 2020, 115, 106439. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Dakora, F.D.; Phillips, D. A Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Tang, C.; Rengel, Z.; Diatloff, E.; Gazey, C. Responses of wheat and barley to liming on a sandy soil with subsoil acidity. Aust. J. Exp. Agric. 1999, 39, 395–401. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Reich, P.B.; Oleksyn, J.; Ogdahl, M.; Zytkowiak, R.; Hale, C.; Karolewski, P. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 2006, 87, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, H.; Moir, J. Total and Organic Carbon. Soil Sampling and Methods of Analysis. Martin R. Carter (Ed.). Can. J. Soil Sci. 1993, 187–201. [Google Scholar]
- Zhu, X.; Ros, G.H.; Xu, M.; Xu, D.; Cai, Z.; Sun, N.; Duan, Y.; de Vries, W. The contribution of natural and anthropogenic causes to soil acidification rates under different fertilization practices and site conditions in southern China. Sci. Total Environ. 2024, 934, 172986. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Houba, V.; Temminghoff, E.; Gaikhorst, G.; Van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Shi, R.-y.; Li, J.-y.; Xu, R.-k. Combined application of Pseudomonas fluorescens and urea can mitigate rapid acidification of cropland Ultisol. Sci. Total Environ. 2024, 906, 167652. [Google Scholar] [CrossRef]
- Santos, R.A.; Reis, B.R.; Azevedo, A.C.; Sermarini, R.A. Potential Errors in Cation Exchange Capacity Measurement in Soils Amended with Rock Dust: Two Case Studies. Commun. Soil Sci. Plant Anal. 2024, 55, 329–342. [Google Scholar] [CrossRef]
- RColorBrewer, S.; Liaw, M.A. Package ‘Randomforest’; University of California: Berkeley, CA, USA, 2018. [Google Scholar]
- Archer, E.; Archer, M.E. Package ‘rfPermute’; R Project: Indianapolis, IN, USA, 2016. [Google Scholar]
- Raut, N.; Dörsch, P.; Sitaula, B.K.; Bakken, L.R. Soil acidification by intensified crop production in South Asia results in higher N2O/(N2+ N2O) product ratios of denitrification. Soil Biol. Biochem. 2012, 55, 104–112. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Haynes, R.J.; Mokolobate, M.S. Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: A critical review of the phenomenon and the mechanisms involved. Nutr. Cycl. Agroecosyst. 2001, 59, 47–63. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Wagoner, P.; Sarrantonio, M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998, 396, 262–265. [Google Scholar] [CrossRef]
- De Datta, S.K. Principles and Practices of Rice Production; International Rice Research Institute: Los Baños, Philippines, 1981. [Google Scholar]
- Poss, R.; Smith, C.; Dunin, F.; Angus, J. Rate of soil acidification under wheat in a semi-arid environment. Plant Soil 1995, 177, 85–100. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Liu, D.; An, S.-S. Effects of slope aspect on soil nitrogen and microbial properties in the Chinese Loess region. Catena 2015, 125, 135–145. [Google Scholar] [CrossRef]
- Ulrich, B. Natural and anthropogenic components of soil acidification. Z. Pflanzenernährung. Bodenkd. 1986, 149, 702–717. [Google Scholar] [CrossRef]
- Lesturgez, G.; Poss, R.; Noble, A.; Grünberger, O.; Chintachao, W.; Tessier, D. Soil acidification without pH drop under intensive cropping systems in Northeast Thailand. Agric. Ecosyst. Environ. 2006, 114, 239–248. [Google Scholar] [CrossRef]
- Aitken, R.; Moody, P. The effect of valence and ionic-strength on the measurement of pH buffer capacity. Soil Res. 1994, 32, 975–984. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, X.; Yi, X.; Chen, Z.; Dong, X.; Chen, R.; Shen, R. Excessive application of nitrogen and phosphorus fertilizers induces soil acidification and phosphorus enrichment during vegetable production in Yangtze River Delta, China. Soil Use Manag. 2013, 29, 161–168. [Google Scholar] [CrossRef]
- Zarif, N.; Khan, A.; Wang, Q. Linking soil acidity to P fractions and exchangeable base cations under increased N and P fertilization of mono and mixed plantations in Northeast China. Forests 2020, 11, 1274. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Haro, R.; Benito, B. The role of soil fungi in K+ plant nutrition. Int. J. Mol. Sci. 2019, 20, 3169. [Google Scholar] [CrossRef]
- Madar, R.; Singh, Y.; Meena, M.C.; Gaind, S.; Das, T.K.; Verma, R.K.; Halli, H. Crop residue and potassium management on crop and soil properties of maize and wheat in no-tillage systems. Commun. Soil Sci. Plant Anal. 2021, 52, 769–791. [Google Scholar] [CrossRef]



| Treatment | Exchangeable Sodium (Na+) | Exchangeable Potassium (K+) | Exchangeable Calcium (Ca2+) | Exchangeable Magnesium (Mg2+) | ECEC |
|---|---|---|---|---|---|
| CK | 0.125 a | 0.21 e | 4.23 a | 2.14 a | 13.1 a |
| PK | 0.128 a | 0.61 a | 4.65 a | 1.31 d | 12.7 a |
| NK | 0.116 bc | 0.64 a | 1.94 c | 1.06 f | 6.7 c |
| NP | 0.117 bc | 0.17 e | 4.43 a | 1.51 c | 12.2 a |
| NPK1 | 0.123 ab | 0.31 d | 4.68 a | 1.52 c | 12.8 a |
| NPK2 | 0.124 a | 0.46 b | 2.87 b | 1.19 e | 8.7 b |
| NPKM | 0.111 c | 0.40 c | 4.25 a | 1.66 b | 12.3 a |
| Treatment | Slope | Intercept | R2 | pHBC (mmol·kg−1·pH−1) |
|---|---|---|---|---|
| CK | 1.118 | 6.570 | 0.9725 | 18.87 b |
| PK | 1.310 | 5.518 | 0.9876 | 24.42 a |
| NK | 1.370 | 5.392 | 0.9859 | 21.64 ab |
| NP | 1.196 | 6.340 | 0.9931 | 21.28 ab |
| NPK1 | 1.190 | 5.138 | 0.9866 | 21.52 ab |
| NPK2 | 1.050 | 5.290 | 0.9854 | 22.86 ab |
| NPKM | 1.234 | 5.296 | 0.9888 | 24.57 a |
| pH | pHBC | CEC | ECEC | Na+ | K+ | Ca2+ | Mg2+ | AN | NH4+ | NO3− | SOM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 0.964 ** | 0.840 ** | NS | NS | 0.633 * | NS | NS | −0.858 ** | NS | NS | −0.785 |
| pHBC | 1 | 0.810 ** | NS | NS | NS | 0.643 ** | NS | NS | NS | NS | 0.800 * | |
| CEC | 1 | NS | NS | NS | 0.518 * | NS | NS | NS | NS | 0.503 * | ||
| ECEC | 1 | NS | 0.658 ** | 0.980 ** | 0.726 ** | −0.449 * | −0.548 * | NS | NS | |||
| Na+ | 1 | NS | NS | NS | −0.541 ** | NS | −0.620 * | NS | ||||
| K+ | 1 | −0.607 ** | −0.449 * | −0.811 ** | NS | 0.439 * | 0.487 * | |||||
| Ca2+ | 1 | 0.579 ** | NS | −0.533 | NS | NS | ||||||
| Mg2+ | 1 | 0.442 * | NS | NS | −0.616 * | |||||||
| AN | 1 | NS | 0.515 * | 0.859 ** | ||||||||
| NH4+ | 1 | NS | NS | |||||||||
| NO3− | 1 | NS | ||||||||||
| SOM | 1 |
| Treatment | Wheat (kg·ha−1) | Sweetpotato (kg·ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | P | K | Organic Fertilizer | N | P | K | Organic Fertilizer | |
| CK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NP | 210 | 39 | 0 | 0 | 120 | 26 | 0 | 0 |
| NK | 210 | 0 | 75 | 0 | 120 | 0 | 149 | 0 |
| PK | 0 | 39 | 75 | 0 | 0 | 26 | 149 | 0 |
| NPK1 | 210 | 39 | 75 | 0 | 120 | 26 | 149 | 0 |
| NPK2 | 210 | 39 | 75 | 0 | 120 | 26 | 149 | 0 |
| NPKM | 210 | 39 | 75 | 3060 | 120 | 26 | 149 | 2040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, L.; Fu, W.; Xu, C.; Zhang, H.; Xu, X.; Ma, H.; Wang, J.; Zhang, Y. Soil Acidification Can Be Improved under Different Long-Term Fertilization Regimes in a Sweetpotato–Wheat Rotation System. Plants 2024, 13, 1740. https://doi.org/10.3390/plants13131740
Zhang H, Wang L, Fu W, Xu C, Zhang H, Xu X, Ma H, Wang J, Zhang Y. Soil Acidification Can Be Improved under Different Long-Term Fertilization Regimes in a Sweetpotato–Wheat Rotation System. Plants. 2024; 13(13):1740. https://doi.org/10.3390/plants13131740
Chicago/Turabian StyleZhang, Huan, Lei Wang, Weiguo Fu, Cong Xu, Hui Zhang, Xianju Xu, Hongbo Ma, Jidong Wang, and Yongchun Zhang. 2024. "Soil Acidification Can Be Improved under Different Long-Term Fertilization Regimes in a Sweetpotato–Wheat Rotation System" Plants 13, no. 13: 1740. https://doi.org/10.3390/plants13131740
APA StyleZhang, H., Wang, L., Fu, W., Xu, C., Zhang, H., Xu, X., Ma, H., Wang, J., & Zhang, Y. (2024). Soil Acidification Can Be Improved under Different Long-Term Fertilization Regimes in a Sweetpotato–Wheat Rotation System. Plants, 13(13), 1740. https://doi.org/10.3390/plants13131740






