Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia sp. Extracts
Abstract
:1. Introduction
2. Results
2.1. Spectrophotometrical Assays for the Quantification of Total Phenolic Compounds
2.2. The Antioxidant Activity
2.3. The HPLC–MS/MS Analysis
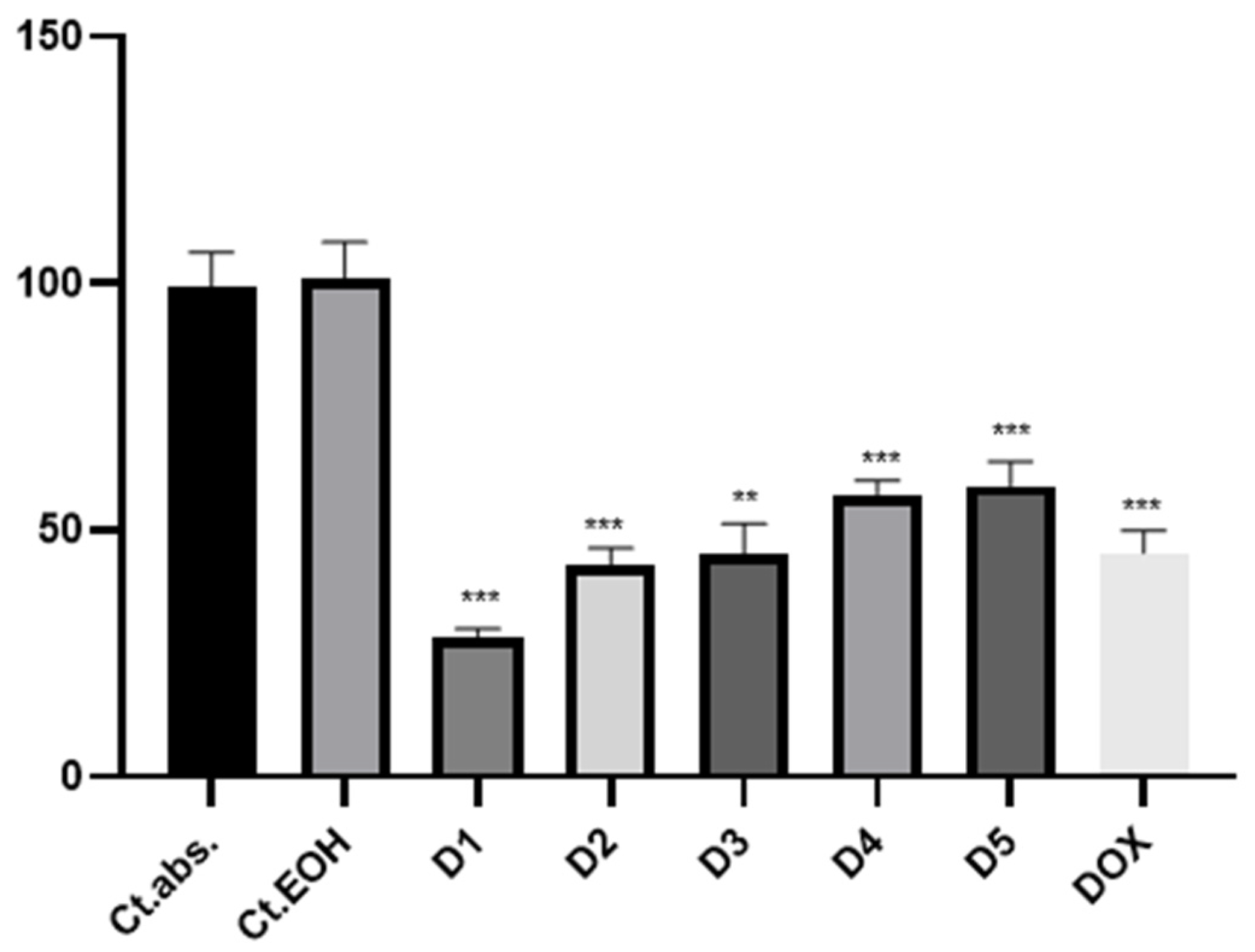
2.4. The Cytototoxic Activity
2.5. The Antimicrobial Activity
2.5.1. The Agar-Well Diffusion Method
2.5.2. The Broth Microdilution Method
2.5.3. The Anti-Biofilm Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemical Agents
4.3. Extraction Method
4.4. Spectrophotometrical Assays
4.5. LC–MS/MS Analysis of Phenolic Compounds in Euphrasia Extracts
4.6. Antioxidant Activity
4.6.1. DPPH Assay
4.6.2. FRAP Assay
4.6.3. Xanthine Oxidase Assay
4.7. Cytotoxicity Assays
4.8. Antimicrobial Activity Assays
4.8.1. Agar-Well Diffusion Method
4.8.2. Broth Microdilution Method
4.8.3. Anti-Biofilm Assay
4.9. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sârbu, I.; Ştefan, N.; Oprea, A. Plante Vasculare Din România; Editura Victor B Victor: Bucureşti, Romania, 2013; pp. 528–530. [Google Scholar]
- Teixeira, R.; Silva, L.R. Bioactive Compounds and in Vitro Biological Activity of Euphrasia rostkoviana Hayne Extracts. Ind. Crops Prod. 2013, 50, 680–689. [Google Scholar] [CrossRef]
- Gawenda-Kempczyńska, D.; Olech, M.; Balcerek, M.; Nowak, R.; Załuski, T.; Załuski, D. Phenolic Acids as Chemotaxonomic Markers Able to Differentiate the Euphrasia Species. Phytochemistry 2022, 203, 113342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hwang, E.; Ngo, H.T.T.; Perumalsamy, H.; Kim, Y.J.; Li, L.; Yi, T.H. Protective Effects of Euphrasia officinalis Extract against Ultraviolet B-Induced Photoaging in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2018, 19, 3327. [Google Scholar] [CrossRef] [PubMed]
- Gruľová, D.; De Feo, V. Euphrasia rostkoviana Hayne-Active Components and Biological Activity for the Treatment of Eye Disorders. Sci. Bull. Uzhgorod Univ. 2017, 1, 5–13. [Google Scholar]
- Blazics, B.; Ludanyi, K.; Szarka, S.; Kery, A. Investigation of Euphrasia rostkoviana Hayne Using GC-MS and LC-MS. Chromatographia 2008, 68 (Suppl. S1), 119–124. [Google Scholar] [CrossRef]
- Blazics, B.; Alberti, Á.; Béni, S.; Kursinszki, L.; Tölgyesi, L.; Kéry, Á. Identification and LC-MS-MS Determination of Acteoside, the Main Antioxidant Compound of Euphrasia rostkoviana, Using the Isolated Target Analyte as External Standard. J. Chromatogr. Sci. 2011, 49, 203–208. [Google Scholar] [CrossRef]
- Lorenz, P.; Knittel, D.N.; Conrad, J.; Lotter, E.M.; Heilmann, J.; Stintzing, F.C.; Kammerer, D.R. 1-Acetyl-3-[(3R)-Hydroxyfatty Acyl]Glycerols: Lipid Compounds from Euphrasia rostkoviana Hayne and E. tetraquetra (Bréb.) Arrond. Chem. Biodivers. 2016, 13, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Woźniak, A.; Niedziela, P.; Rejdak, R. Assessment of Eyebright (Euphrasia officinalis L.) Extract Activity in Relation to Human Corneal Cells Using In Vitro Tests. Balkan Med. J. 2014, 31, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Porchezhian, E.; Ansari, S.H.; Shreedharan, N.K.K. Antihyperglycemic Activity of Euphrasia officinale Leaves. Fitoterapia 2000, 71, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Hristova, L.; Damianova, E.; Yordanova, Y.; Petrova, N.; Kapchina-Toteva, V. Antioxidant Activity and Secondary Metabolites in Different Extracts of Euphrasia officinalis Growing in Bulgaria. Sci. Technol. 2014, 4, 465–469. [Google Scholar]
- Jafri, S.A.A.; Zafar, M.K.; Khan, M.R.; Ashraf, S.; Ahmad, N.; Karami, A.M.; Rafique, E.; Ouladsmane, M.; Al Suliman, N.M.S.; Aslam, S. Evaluation of Some Essential Traditional Medicinal Plants for Their Potential Free Scavenging and Antioxidant Properties. J. King Saud Univ. Sci. 2023, 35, 102562. [Google Scholar] [CrossRef]
- Jafri, S.A.A.; Khalid, Z.M.; Khan, M.Z.; Jogezai, N.U. Evaluation of Phytochemical and Antioxidant Potential of Various Extracts from Traditionally Used Medicinal Plants of Pakistan. Open Chem. 2022, 20, 1337–1356. [Google Scholar] [CrossRef]
- Haratym, W.; Weryszko-Chmielewska, E. Structural Features of Flower Trichomes in Drug Eyebright (Euphrasia stricta D. Wolff EX J. F. Lehm.). Acta Agrobot. 2013, 66, 35–44. [Google Scholar] [CrossRef]
- Petrichenko, V.M.; Sukhinina, T.V.; Babiyan, L.K.; Shramm, N.I. Chemical Composition and Antioxidant Properties of Biologically Active Compounds from Euphrasia brevipila. Pharm. Chem. J. 2006, 40, 312–316. [Google Scholar] [CrossRef]
- Shestakova, T.S.; Petrichenko, V.M.; Sukhinina, T.V. Elemental Composition of Euphrasia brevipila Herbs and Extracts. Pharm. Chem. J. 2008, 42, 460–462. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Pharmacological Activities of an Eye Drop Containing Matricaria Chamomilla and Euphrasia officinalis Extracts in UVB-Induced Oxidative Stress and Inflammation of Human Corneal Cells. J. Photochem. Photobiol. B Biol. 2017, 173, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Marcotullio, M.C.; Loizzo, M.R.; Messina, F.; Temperini, A.; Tundis, R.; Menichini, F.; Curini, M. Bioassay-Guided Fractionation of Euphrasia pectinata Ten. and Isolation of Iridoids with Antiproliferative Activity. Phytochem. Lett. 2015, 12, 252–256. [Google Scholar] [CrossRef]
- Mari, A.; Ciocarlan, A.; Aiello, N.; Scartezzini, F.; Pizza, C.; D’Ambrosio, M. Research Survey on Iridoid and Phenylethanoid Glycosides among Seven Populations of Euphrasia rostkoviana Hayne from the Alps. Phytochemistry 2017, 137, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Novy, P.; Davidova, H.; Serrano-Rojero, C.S.; Rondevaldova, J.; Pulkrabek, J.; Kokoska, L. Composition and Antimicrobial Activity of Euphrasia rostkoviana Hayne Essential Oil. Evid.-Based Complement. Altern. Med. 2015, 2015, 734101. [Google Scholar] [CrossRef] [PubMed]
- Varzaru, I.; Untea, A.E.; Van, I. Determination of Bioactive Compounds with Benefic Potential on Health in Several Medicinal Plants. Rom. Biotechnol. Lett. 2015, 20, 10773–10783. [Google Scholar]
- Sticher, O.; Salama, O.; Chaudhuri, R.K.; Winkler, T. Structural Analysis of Eukovoside, A New Phenylpropanoid Glycoside from Euphrasia rostkoviana HAYNE. Helv. Chim. Acta 1982, 65, 1538–1542. [Google Scholar] [CrossRef]
- Miladinovic, D.L.; Ilic, B.S.; Nikolic, D.M.; Markovic, M.S.; Nikolic, N.D.; Miladinovic, L.C.; Miladinovic, M.D. Volatile Constituents of Euphrasia stricta. Chem. Nat. Compd. 2014, 49, 1146–1147. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, S.; Kim, Y.J.; Perumalsamy, H.; Lee, S.; Hwang, E.; Yi, T.H. Green Synthesis of Gold Nanoparticles Using Euphrasia officinalis Leaf Extract to Inhibit Lipopolysaccharide-Induced Inflammation through NF-ΚB and JAK/STAT Pathways in RAW 264.7 Macrophages. Int. J. Nanomed. 2019, 14, 2945–2959. [Google Scholar] [CrossRef] [PubMed]
- Meier-Girard, D.; Gerstenberg, G.; Stoffel, L.; Kohler, T.; Klein, S.D.; Eschenmoser, M.; Mitter, V.R.; Nelle, M.; Wolf, U. Euphrasia Eye Drops in Preterm Neonates with Ocular Discharge: A Randomized Double-Blind Placebo-Controlled Trial. Front. Pediatr. 2020, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Ghedira, K.; Goetz, P. Euphrasia officinalis L. (Scrophulariaceae): Euphraise. Phytotherapie 2011, 9, 369–372. [Google Scholar] [CrossRef]
- Stoss, M.; Michels, C.; Peter, E.; Beutke, R.; Gorter, R.W. Prospective Cohort Trial of Euphrasia Single-Dose Eye Drops in Conjunctivitis. J. Altern. Complement. Med. 2000, 6, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Mokkapatti, R. An Experimental Double-Blind Study to Evaluate the Use of Euphrasia in Preventing Conjunctivitis. Br. Homeopath. J. 1992, 81, 22–24. [Google Scholar] [CrossRef]
- de Macedo, L.M.; Mendes dos Santos, É.; Militao, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia Rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Dolghi, A.; Buzatu, R.; Marcovici, I.; Pinzaru, I.; Dehelean, C.A.; Dobrescu, A.; Olaru, F.; Popescu, G.A.; Navolan, D.; Cretu, O.M.; et al. Phytochemical Analysis and in Vitro Cytotoxic Activity against Colorectal Adenocarcinoma Cells of Hippophae rhamnodies L., Cymbopogon citratus (D.C.) Stapf and Ocimum basilicum L. Essential Oils. Plants 2021, 10, 2752. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Xu, S.; Fang, J.; Jiang, H. The Protective Effect of Polyphenols for Colorectal Cancer. Front. Immunol. 2020, 11, 555805. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Benedec, D.; Olah, N.K.; Ranga, F.; Mirel, S.; Tiperciuc, B.; Oniga, I. Research on Enzyme Inhibition Potential and Phenolic Compounds from Origanum Vulgare Ssp. Vulgare. Farmacia 2020, 68, 1075–1080. [Google Scholar] [CrossRef]
- Olawuwo, O.S.; Famuyide, I.M.; McGaw, L.J. Antibacterial and Antibiofilm Activity of Selected Medicinal Plant Leaf Extracts Against Pathogens Implicated in Poultry Diseases. Front. Vet. Sci. 2022, 9, 820304. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products. Assessment Report on Euphrasia officinalis L. and Euphrasia rostkoviana Hayne Herba. Eur. Med. Agency 2010, 44, 13. [Google Scholar]
- Suchinina, T.V.; Shestakova, T.S.; Petrichenko, V.M.; Novikova, V.V. Solvent Polarity Effect on the Composition of Biologically Active Substances, UV Spectral Characteristics, and Antibacterial Activity of Euphrasia brevipila Herb Extracts. Pharm. Chem. J. 2011, 44, 683–686. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly Synthesis of Silver and Gold Nanoparticles by Euphrasia officinalis Leaf Extract and Its Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Meccatti, V.M.; Santos, L.F.; de Carvalho, L.S.; Souza, C.B.; Carvalho, C.A.T.; Marcucci, M.C.; Hasna, A.A.; de Oliveira, L.D. Antifungal Action of Herbal Plants’ Glycolic Extracts against Candida Species. Molecules 2023, 28, 2857. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Oniga, I.; Muresan, B.; Mot, A.C.; Damian, G.; Nistor, A.; Silaghi-Dumitrescu, R.; Hanganu, D.; Duma, M.; Vlase, L. Contrast between Water- and Ethanol-Based Antioxidant Assays: Aspen (Populus tremula) and Black Poplar (Populus nigra) Extracts as a Case Study. J. Food Qual. 2014, 37, 259–267. [Google Scholar] [CrossRef]
- Toiu, A.; Vlase, L.; Oniga, I.; Benedec, D.; Tǎmaş, M. HPLC Analysis of Salicylic Derivatives from Natural Products. Farmacia 2011, 59, 106–112. [Google Scholar]
- Sevastre-Berghian, A.C.; Ielciu, I.; Mitre, A.O.; Filip, G.A.; Oniga, I.; Vlase, L.; Benedec, D.; Gheldiu, A.-M.; Toma, V.A.; Mihart, B.; et al. Targeting Oxidative Stress Reduction and Inhibition of HDAC1, MECP2, and NF-KB Pathways in Rats with Experimentally Induced Hyperglycemia by Administration of Thymus Marshallianus Willd. Extracts. Front. Pharmacol. 2020, 11, 581470. [Google Scholar] [CrossRef] [PubMed]
- Ielciu, I.; Vlase, L.; Frédérich, M.; Hanganu, D.; Păltinean, R.; Cieckiewicz, E.; Olah, N.-K.; Gheldiu, A.-M.; Crişan, G. Polyphenolic Profile and Biological Activities of the Leaves and Aerial Parts of Echinocystis Lobata (Michx.) Torr. et A. Gray (Cucurbitaceae). Farmacia 2017, 65, 179–183. [Google Scholar]
- Ielciu, I.; Hanganu, D.; Păltinean, R.; Vlase, L.; Frédérich, M.; Gheldiu, A.-M.; Benedec, D.; Crişan, G. Antioxidant Capacity and Polyphenolic Content of the Echinocystis Lobata (Michx.) Torr. et A.Gray Flowers. Pak. J. Pharm. Sci. 2018, 31 (Suppl. S2), 677–683. [Google Scholar] [PubMed]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The Effect of Extraction Methods on Phytochemicals and Biological Activities of Green Coffee Beans Extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Moldovan, C.; Popa, D.S.; Tomuta, I.; Vlase, L. Process Optimization for Improved Phenolic Compounds Recovery from Walnut (Juglans regia L.) Septum: Phytochemical Profile and Biological Activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Hanganu, D.; Oniga, I.; Tiperciuc, B.; Olah, N.-K.; Raita, O.; Bischin, C.; Silaghi-Dumitrescu, R.; Vlase, L. Assessment of Rosmarinic Acid Content in Six Lamiaceae Species Extracts and Their Antioxidant and Antimicrobial Potential. Pak. J. Pharm. Sci. 2015, 28 (Suppl. S6), 2297–2303. [Google Scholar] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Olah, N.-K.; Osser, G.; Câmpean, R.F.; Furtuna, F.R.; Benedec, D.; Filip, L.; Raita, O.; Hanganu, D. The Study of Polyphenolic Compounds Profile of Some Rosmarinus officinalis L. Extracts. Pak. J. Pharm. Sci. 2016, 29 (Suppl. S6), 2355–2361. [Google Scholar] [PubMed]
- Ielciu, I.; Niculae, M.; Pall, E.; Barbălată, C.; Tomuţă, I.; Olah, N.K.; Burtescu, R.F.; Benedec, D.; Oniga, I.; Hanganu, D. Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes. Molecules 2022, 27, 3988. [Google Scholar] [CrossRef] [PubMed]
- Ielciu, I.; Sevastre, B.; Olah, N.-K.; Turdean, A.; Chişe, E.; Marica, R.; Oniga, I.; Uifălean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of Hepatoprotective Activity and Oxidative Stress Reduction of Rosmarinus officinalis L. Shoots Tincture in Rats with Experimentally Induced Hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef] [PubMed]
- Lupșe, I.; Pall, E.; Tudoran, L.B.; Bulboacă, A.E.; Ciurea, A.; Micu, I.C.; Roman, A.; Delean, A.G.; Muntean, A.; Soancă, A. Cytotoxic Effects on Gingival Mesenchymal Stromal Cells and Root Surface Modifications Induced by Some Local Antimicrobial Products Used in Periodontitis Treatment. Materials 2021, 14, 5049. [Google Scholar] [CrossRef] [PubMed]
- Pall, E.; Roman, A.; Olah, D.; Beteg, F.I.; Cenariu, M.; Spînu, M. Enhanced Bioactive Potential of Functionalized Injectable Platelet-Rich Plasma. Molecules 2023, 28, 1943. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O.; et al. Biological Activities of Some Isoquinoline Alkaloids from Fumaria Schleicheri Soy. Will. Plants 2022, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Niculae, M.; Spinu, M. Antimicrobial Potential of Some Lamiaceae Essential Oils against Animal Multiresistant Bacteria Microbial Pathogens View Project Immune Modulating Effects of Vegetal Extracts View Project. Lucr. Scintifice Med. Vet. Timoara 2009, XLII, 170–175. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method; EUCAST: Copenhagen, Denmark, 2020. [Google Scholar]
- Simea, S.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Burtescu, R.F.; Olah, N.; Cenariu, M.; Oniga, I.; Benedec, D.; et al. Evaluation of the Cytotoxic, Antioxidative and Antimicrobial Effects of Dracocephalum Moldavica L. Cultivars. Molecules 2023, 28, 1604. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.M.; Semreen, M.H.; El-Keblawy, A.A.; Abdullah, A.; Uppuluri, P.; Ibrahim, A.S. Assessment of Herbal Drugs for Promising Anti-Candida Activity. BMC Complement. Altern. Med. 2017, 17, 257. [Google Scholar] [CrossRef] [PubMed]

| Sample | TP (mg GAE/g) | TF (mg RE/g) | CAD (mg CAE/g) | DPPH (IC50 µg/mL) | FRAP (µM TE/mL) | XO | |
|---|---|---|---|---|---|---|---|
| I% | I (mg AE/mL) | ||||||
| EO | 92.10 ± 2.90 | 24.72 ± 0.29 | 45.08 ± 1.92 c | 50.93 ± 2.19 d | 520.21 ± 13.79 | 16.73 ±0.35 f | 21.75 ± 0.51 g |
| ES | 74.91 ± 1.28 a | 10.81 ± 0.19 b | 55.02 ± 1.87 | 71.57 ± 3.42 d | 255.33 ± 9.67 e | 71.90 ± 1.38 f | 93.46± 0.42 |
| Trolox | - | - | 11.88 ± 0.02 | - | - | ||
| Allopurinol | - | - | - | - | - | 90.04 ± 2.35 | - |
| No. | Phenolic Compound | m/z Value | tR ± SD (min) | Concentration (μg/mL) EO | Concentration (μg/mL) ES |
|---|---|---|---|---|---|
| 1. | Protocatechuic acid | 153 | 2.80 ± 0.01 | 1.85 ± 0.05 | 0.87 ± 0.03 |
| 2. | Gentisic acid | 179 | 3.52 ± 0.04 | 0.46 ± 0.02 | <0.02 |
| 3. | Caftaric acid | 311 | 3.54 ± 0.05 | 0.31 ± 0.02 | <0.02 |
| 4. | Chlorogenic acid | 353 | 5.62 ± 0.05 | 23.72 ± 0.28 | 353.86 ± 9.83 |
| 5. | Vanillic acid | 167 | 6.70 ± 0.01 | 1.14 ± 0.01 | - |
| 6. | p-Coumaric acid | 163 | 9.48 ± 0.08 | 9.40 ± 0.20 | 16.21 ± 0.25 |
| 7. | Ferulic acid | 193 | 12.8 ± 0.10 | 2.32 ± 0.08 | 4.80 ± 0.01 |
| 8. | Catechin | 289 | 6.00 ± 0.07 | 0.26 ± 0.01 | - |
| 9. | Rosmarinic acid | 359 | 2.20 ± 0.02 | 5.83 ± 0.29 | 0.82 ± 0.03 |
| 10. | Hyperoside | 463 | 19.00 ± 0.2 | 6.72 ± 0.17 | 1.336 ± 0.01 |
| 11. | Isoquercitrin | 463 | 19.90 ± 0.10 | 5.59 ± 0.11 | 20.38 ± 0.16 |
| 12. | Rutin | 609 | 20.20 ± 0.15 | 61.57 ± 1.55 | 57.41 ± 1.58 |
| 13. | Quercitrin | 447 | 23.64 ± 0.13 | <0.02 | 6.90 ± 0.15 |
| 14. | Luteolin | 285 | 29.10 ± 0.19 | 0.46 ± 0.02 | 0.53 ± 0.02 |
| 15. | Apigenin | 269 | 33.10 ± 0.15 | 0.67 ± 0.03 | 1.06 ± 0.02 |
| Tested Products | Diameters of Inhibition Zone (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| MSSA | MRSA | Bacillus cereus | Enterococcus faecalis | Listeria monocytogenes | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | |
| EO | 14.50 ± 0.50 a | 12.00 ± 0.71 a | 13.75 ± 0.43 a | 12.5 ± 0.50 | 12.75 ± 0.43 a | 9.75 ± 0.43 a | 0 | 10 ± 0.00 c |
| ES | 17.00 ± 0.71 a | 16.25 ± 0.43 b | 15 ± 0.00 a | 14 ± 0.00 | 14.75 ± 0.43 a | 9.75 ± 0.43 a | 0 | 10 ± 0.00 c |
| Gentamicin | 19 ± 0.00 | 17 ± 0.25 | 20 ± 0.00 | 10 ± 0.00 a | 22 ± 0.50 | 19 ± 0.00 | 18 ± 0.00 | - |
| Fluconazole | - | - | - | - | - | - | - | 21 ± 0.00 |
| Samples | Microorganisms | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSSA | MRSA | Bacillus cereus | Enterococcus faecalis | Listeria monocytogenes | Escherichia coli | Candida albicans | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| EO | 27.08 | 27.08 | 27.08 | 27.08 | 27.08 | 27.08 | 54.17 | 54.17 | 54.17 | 54.17 | 54.17 | >54.17 | 54.17 | >54.17 |
| ES | 5.50 | 22.02 | 11.01 | 11.01 | 11.01 | 11.01 | 44.05 | 44.05 | 44.05 | 44.05 | 44.05 | >44.05 | 44.05 | >44.05 |
| Gentamicin MIC (mg/L) | 3 | 4 | 3 | 3 | 3 | 4 | - | |||||||
| Fluconazole MIC (mg/L) | - | - | - | - | - | - | 8 | |||||||
| % Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples | Staphylococcus aureus | Listeria monocytogenes | Escherichia coli | Candida albicans | ||||
| T0 | T24 | T0 | T24 | T0 | T24 | T0 | T24 | |
| EO | + | + | + | - | - | - | - | + |
| ES | ++ | ++ | + | + | - | - | - | + |
| Gentamicin | + | ++ | + | ++ | - | ++ | - | - |
| Fluconazole | - | - | - | - | - | - | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedec, D.; Oniga, I.; Hanganu, D.; Vlase, A.-M.; Ielciu, I.; Crișan, G.; Fiţ, N.; Niculae, M.; Bab, T.; Pall, E.; et al. Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia sp. Extracts. Plants 2024, 13, 1790. https://doi.org/10.3390/plants13131790
Benedec D, Oniga I, Hanganu D, Vlase A-M, Ielciu I, Crișan G, Fiţ N, Niculae M, Bab T, Pall E, et al. Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia sp. Extracts. Plants. 2024; 13(13):1790. https://doi.org/10.3390/plants13131790
Chicago/Turabian StyleBenedec, Daniela, Ilioara Oniga, Daniela Hanganu, Ana-Maria Vlase, Irina Ielciu, Gianina Crișan, Nicodim Fiţ, Mihaela Niculae, Timea Bab, Emoke Pall, and et al. 2024. "Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia sp. Extracts" Plants 13, no. 13: 1790. https://doi.org/10.3390/plants13131790
APA StyleBenedec, D., Oniga, I., Hanganu, D., Vlase, A.-M., Ielciu, I., Crișan, G., Fiţ, N., Niculae, M., Bab, T., Pall, E., & Vlase, L. (2024). Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia sp. Extracts. Plants, 13(13), 1790. https://doi.org/10.3390/plants13131790













