A Highly Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation Method of Idesia polycarpa and the Generation of Transgenic Plants
Abstract
1. Introduction
2. Results
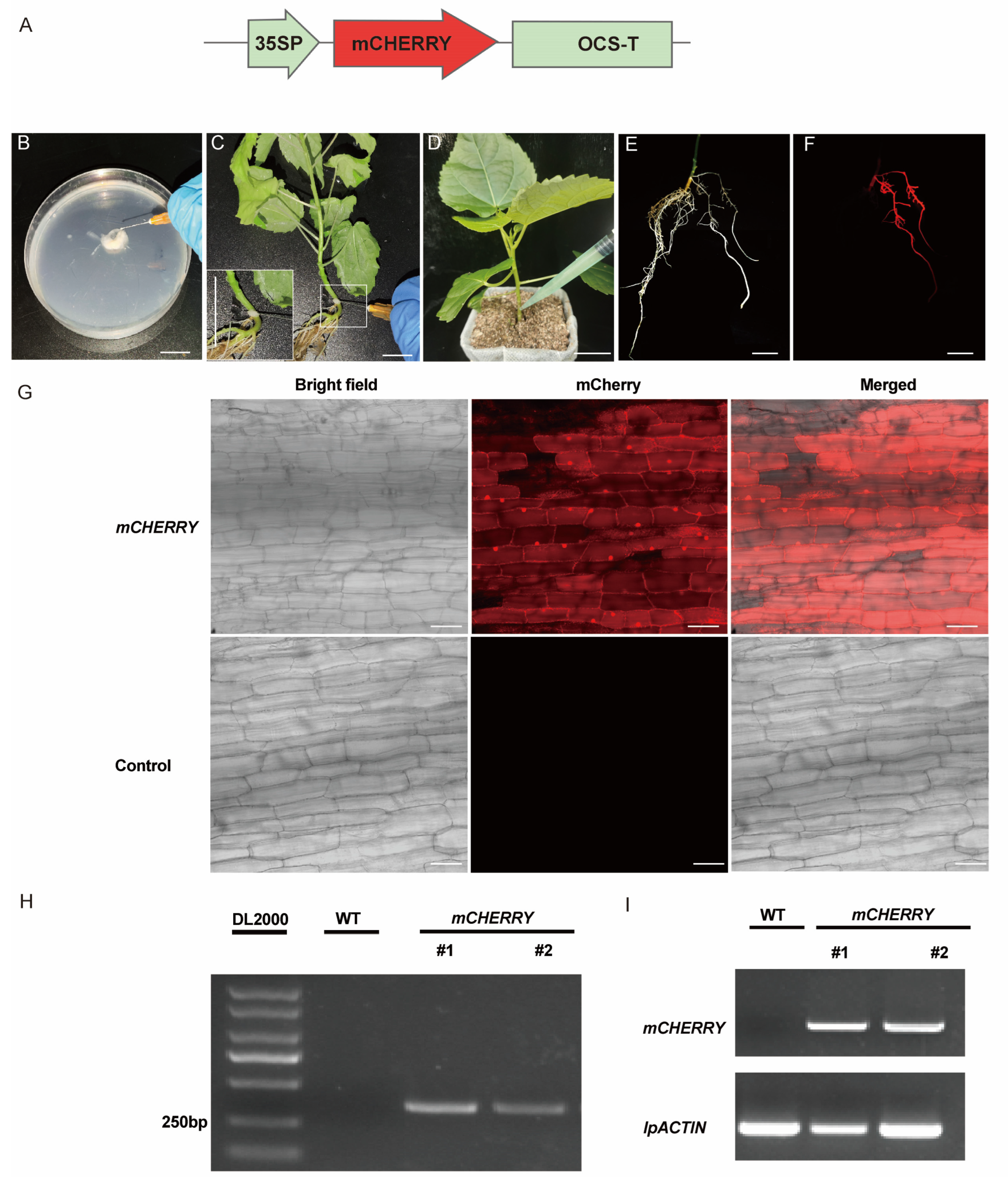
2.1. Establishment of Transformation System Using Whole Seedlings as Explants
2.2. Establishment of Transformation System Using Rootless Seedlings as Explants
2.3. Establishment of Transformation System Using Leaf Petioles as Explants
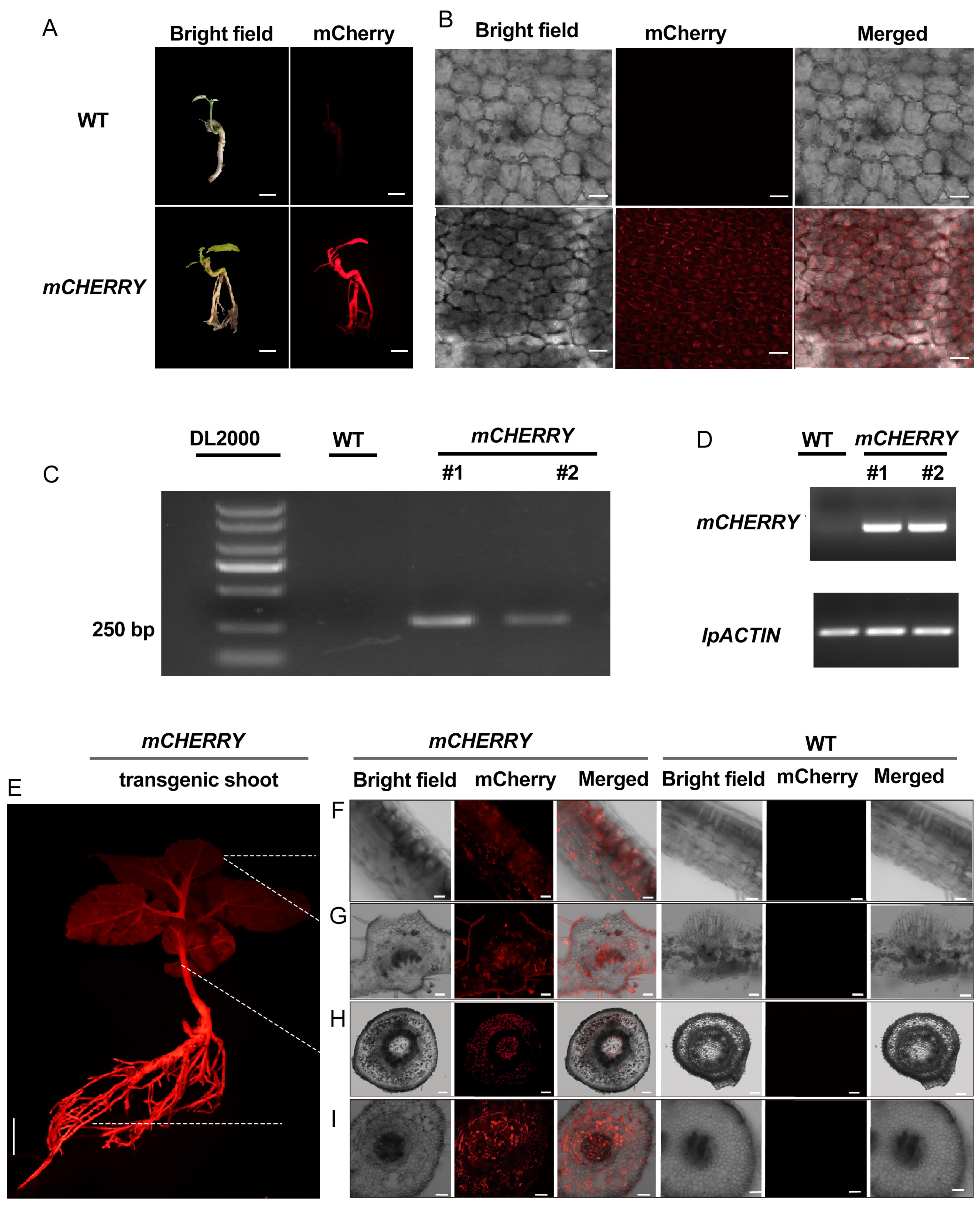
2.4. Regeneration of Transgenic Shoots from Transgenic Roots
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Growth Conditions
5.2. Preparation of Agrobacterium rhizogenes
5.3. Agrobacterium rhizogenes Infection
5.4. Regeneration of Transgenic Shoots
5.5. Fluorescence Observation
5.6. PCR Analysis
5.7. RT-PCR Analysis
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Yang, F.-X.; Su, Y.-Q.; Li, X.-H.; Zhang, Q.; Sun, R.-C. Preparation of biodiesel from Idesia polycarpa var. vestita fruit oil. Ind. Crops Prod. 2009, 29, 622–628. [Google Scholar] [CrossRef]
- Li, R.J.; Gao, X.; Li, L.M.; Liu, X.L.; Wang, Z.Y.; Lü, S.Y. De novo Assembly and Characterization of the Fruit Transcriptome of Idesia polycarpa Reveals Candidate Genes for Lipid Biosynthesis. Front. Plant Sci. 2016, 7, 801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, C.; Karrar, E.; Du, M.; Jin, Q.; Wang, X. Analysis of Chemical Composition and Antioxidant Activity of Idesia polycarpa Pulp Oil from Five Regions in China. Foods 2023, 12, 1251. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wen, L.; Wang, Z.; Yang, G.; Mao, J.; An, X.; Kan, J. A comprehensive study on physicochemical properties, bioactive compounds, and emulsified lipid digestion characteristics of Idesia polycarpa var. vestita Diels fruits oil. Food Chem. 2023, 404 Pt A, 134634. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Jemim, R.S.; Li, Z.; Geng, X.; Wang, Y.; Cai, Q.; Liu, Z. Study of the pattern of reproductive allocation and fruit development in young dioecious trees of Idesia polycarpa Maxim. S. Afr. J. Bot. 2022, 146, 472–480. [Google Scholar] [CrossRef]
- Ji, X.; Yang, B.; Wang, D. Achieving Plant Genome Editing While Bypassing Tissue Culture. Trends Plant Sci. 2020, 25, 427–429. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Niazian, M.; Belzile, F.; Torkamaneh, D. CRISPR/Cas9 in Planta Hairy Root Transformation: A Powerful Platform for Functional Analysis of Root Traits in Soybean. Plants 2022, 11, 1044. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Wang, P.; Wang, C.; Wang, J.; Wang, X.; Cheng, H. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene editing analysis in cotton. Front. Plant Sci. 2022, 13, 1059404. [Google Scholar] [CrossRef]
- Meng, D.; Yang, Q.; Dong, B.; Song, Z.; Niu, L.; Wang, L.; Cao, H.; Li, H.; Fu, Y. Development of an efficient root transgenic system for pigeon pea and its application to other important economically plants. Plant Biotechnol. J. 2019, 17, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Gajurel, G.; Nopo-Olazabal, L.; Hendrix, E.; Medina-Bolivar, F. Production and Secretion of Isowighteone in Hairy Root Cultures of Pigeon Pea (Cajanus cajan) Co-Treated with Multiple Elicitors. Plants 2022, 11, 834. [Google Scholar] [CrossRef]
- Ajdanian, L.; Niazian, M.; Torkamaneh, D. Optimizing ex vitro one-step RUBY-equipped hairy root transformation in drug- and hemp-type Cannabis. Plant Biotechnol. J. 2024, 22, 1957. [Google Scholar] [CrossRef] [PubMed]
- Barrera, K.; González-Cortazar, M.; Reyes-Pérez, R.; Pérez-García, D.; Herrera-Ruiz, M.; Arellano-García, J.; Cruz-Sosa, F.; Nicasio-Torres, P. Production of Two Isomers of Sphaeralcic Acid in Hairy Roots from Sphaeralcea angustifolia. Plants 2023, 12, 1090. [Google Scholar] [CrossRef]
- Ma, H.; Liu, N.; Sun, X.; Zhu, M.; Mao, T.; Huang, S.; Meng, X.; Li, H.; Wang, M.; Liang, H. Establishment of an efficient transformation system and its application in regulatory mechanism analysis of biological macromolecules in tea plants. Int. J. Biol. Macromol. 2023, 244, 125372. [Google Scholar] [CrossRef]
- Wang, M.; Qin, Y.Y.; Wei, N.N.; Xue, H.Y.; Dai, W.S. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation in citrus seeds and its application in gene functional analysis. Front. Plant Sci. 2023, 14, 1293374. [Google Scholar] [CrossRef]
- Jia, H.; Chen, J.; Zhang, L.; Zhang, L. The First Report on Transgenic Hairy Root Induction from the Stem of Tung Tree (Vernicia fordii). Plants 2022, 11, 1315. [Google Scholar] [CrossRef]
- Cao, X.; Xie, H.; Song, M.; Zhao, L.; Liu, H.; Li, G.; Zhu, J.-K. Simple method for transformation and gene editing in medicinal plants. J. Integr. Plant Biol. 2024, 66, 17–19. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Deng, S.; Wang, M.; Wu, Y.; Li, M.; Dong, J.; Lu, S.; Su, C.; Li, G.; et al. A method of genetic transformation and gene editing of succulents without tissue culture. Plant Biotechnol. J. 2024, 22, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xie, H.; Song, M.; Lu, J.; Ma, P.; Huang, B.; Wang, M.; Tian, Y.; Chen, F.; Peng, J.; et al. Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation 2023, 4, 100345. [Google Scholar] [CrossRef]
- Ying, W.; Wen, G.; Xu, W.; Liu, H.; Ding, W.; Zheng, L.; He, Y.; Yuan, H.; Yan, D.; Cui, F.; et al. Agrobacterium rhizogenes: Paving the road to research and breeding for woody plants. Front. Plant Sci. 2023, 14, 1196561. [Google Scholar] [CrossRef] [PubMed]
- Bosselut, N.; Van Ghelder, C.; Claverie, M.; Voisin, R.; Onesto, J.-P.; Rosso, M.-N.; Esmenjaud, D. Agrobacterium rhizogenes-mediated transformation of Prunus as an alternative for gene functional analysis in hairy-roots and composite plants. Plant Cell Rep. 2011, 30, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lai, E.; Zhao, L.; Cai, Y.; Ogutu, C.; Cherono, S.; Han, Y.; Zheng, B. Development of a fast and efficient root transgenic system for functional genomics and genetic engineering in peach. Sci. Rep. 2020, 10, 2836. [Google Scholar] [CrossRef]
- Ma, H.; Meng, X.; Xu, K.; Li, M.; Gmitter, F.G., Jr.; Liu, N.; Gai, Y.; Huang, S.; Wang, M.; Wang, M.; et al. Highly efficient hairy root genetic transformation and applications in citrus. Front. Plant Sci. 2022, 13, 1039094. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, D.; Fu, J.; Zhang, Z.; Qin, Y.; Hu, G.; Zhao, J. Agrobacterium rhizogenes-mediated hairy root transformation as an efficient system for gene function analysis in Litchi chinensis. Plant Methods 2021, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhao, F.; Zhang, G.; Pan, Y.; Sun, L.; Bao, N.; Qin, P.; Chen, L.; Yu, J.; Zhang, Y.; et al. Control of de novo root regeneration efficiency by developmental status of Arabidopsis leaf explants. J. Genet. Genom. 2019, 46, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, L.; Sun, L.; Zhu, Z. ETHYLENE INSENSITIVE 3 suppresses plant de novo root regeneration from leaf explants and mediates age-regulated regeneration decline. Development 2020, 147, dev179457. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, Z. The Molecular Basis of Age-Modulated Plant De Novo Root Regeneration Decline in Arabidopsis thaliana. Plant Cell Physiol. 2021, 62, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.; Janocha, D.; Dong, Y.; Medzihradszky, A.; Schöne, S.; Daum, G.; Suzaki, T.; Forner, J.; Langenecker, T.; Rempel, E.; et al. Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 2016, 5, e17023. [Google Scholar] [CrossRef]
- van Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor. Plant Cell 2018, 30, 101–116. [Google Scholar] [CrossRef]


| Seedling Age | Rooting Rates (%) | Transgenic Rates (%) |
|---|---|---|
| 20 days old | 96.45 ± 1.86 a | 49.94 ± 4.52 b |
| 30 days old | 100 ± 0 a | 58.09 ± 4.10 ab |
| 40 days old | 98.25 ± 1.67 a | 71.91 ± 5.97 a |
| 100 days old | 10.88 ± 1.00 b | 0.00 ± 0 c |
| Seedling Age | Rooting Rates (%) | Transgenic Rates (%) |
|---|---|---|
| 20 days old | 97.92 ± 2.00 a | 9.72 ± 5.00 c |
| 30 days old | 98.33 ± 1.67 a | 30.00 ± 10.00 b |
| 40 days old | 100 ± 0 a | 58.22 ± 2.50 a |
| 100 days old | 13.6 ± 4.48 b | 0.05 ± 0.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cheng, K.; Li, T.; Lan, X.; Shen, L.; Zhao, H.; Lü, S. A Highly Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation Method of Idesia polycarpa and the Generation of Transgenic Plants. Plants 2024, 13, 1791. https://doi.org/10.3390/plants13131791
Wang H, Cheng K, Li T, Lan X, Shen L, Zhao H, Lü S. A Highly Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation Method of Idesia polycarpa and the Generation of Transgenic Plants. Plants. 2024; 13(13):1791. https://doi.org/10.3390/plants13131791
Chicago/Turabian StyleWang, Hui, Kaimao Cheng, Tongjie Li, Xiaoyu Lan, Li Shen, Huayan Zhao, and Shiyou Lü. 2024. "A Highly Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation Method of Idesia polycarpa and the Generation of Transgenic Plants" Plants 13, no. 13: 1791. https://doi.org/10.3390/plants13131791
APA StyleWang, H., Cheng, K., Li, T., Lan, X., Shen, L., Zhao, H., & Lü, S. (2024). A Highly Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation Method of Idesia polycarpa and the Generation of Transgenic Plants. Plants, 13(13), 1791. https://doi.org/10.3390/plants13131791







