Overexpression of RpKTI2 from Robinia pseudoacacia Affects the Photosynthetic Physiology and Endogenous Hormones of Tobacco
Abstract
:1. Introduction
2. Results
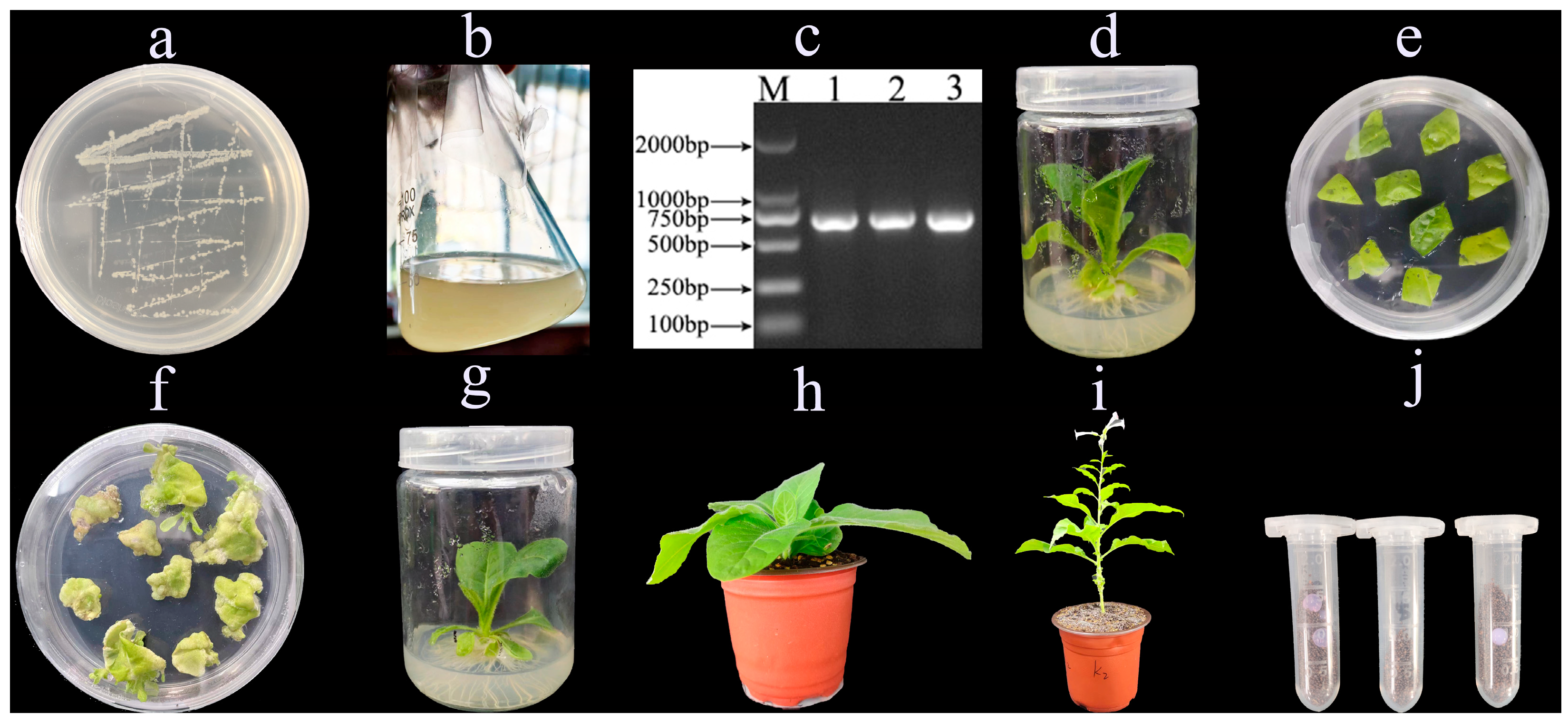
2.1. Cultivation and Identification of RpKTI2-Expressing Transgenic Tobacco Plants
2.2. Changes in RpKTI2 Levels in Transgenic Tobacco
2.3. Changes of Chlorophyll Content in RpKTI2-Overexpressing Tobacco
2.4. Changes of Chlorophyll Fluorescence Parameters in RpKTI2-Overexpressing Tobacco
2.5. Photosynthetic Function of RpKTI2-Overexpressing Tobacco
2.6. Observation of Stomatal Morphology in Leaves of RpKTI2-Overexpressing Tobacco
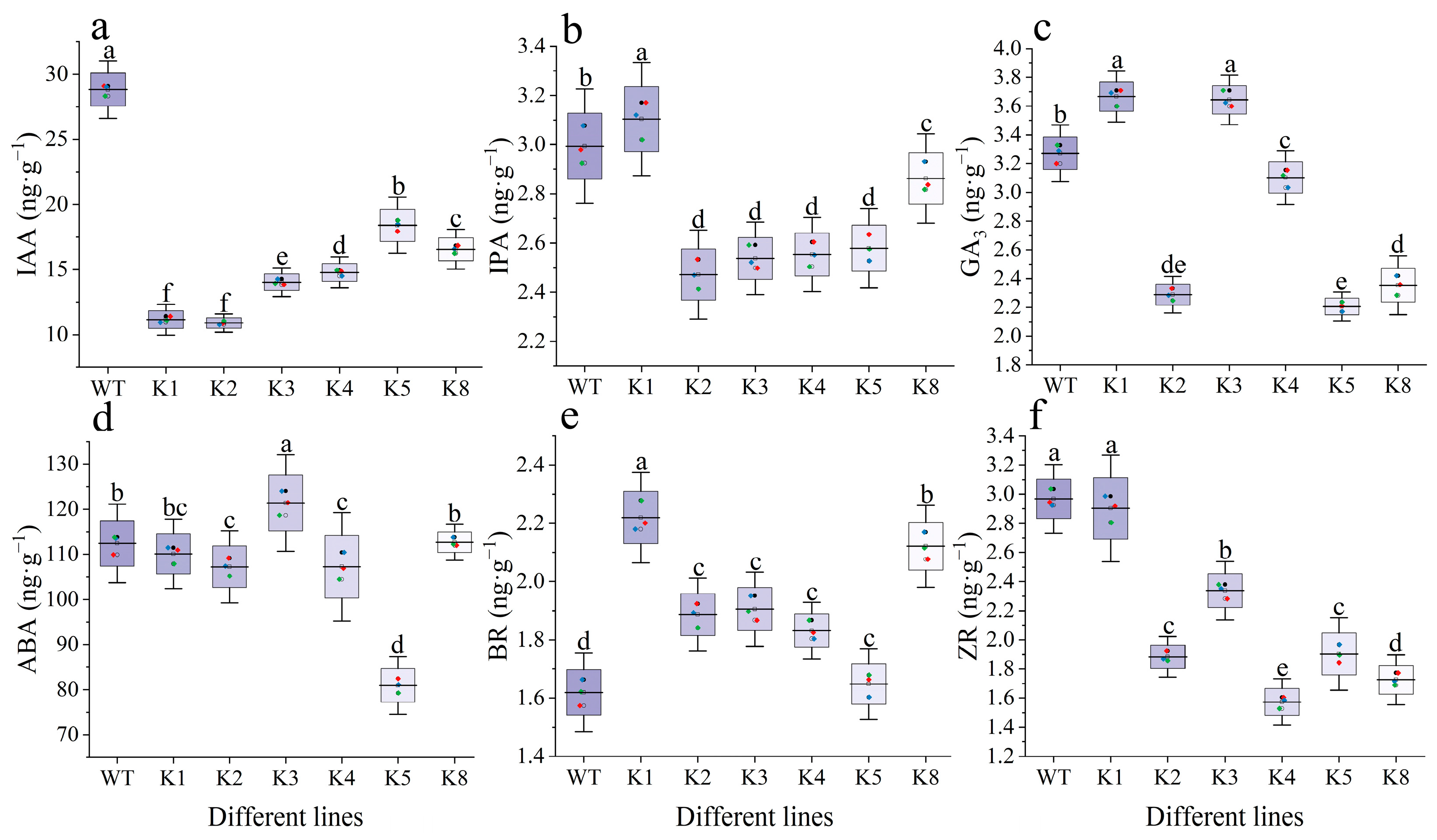
2.7. Endogenous Hormone Content of RpKTI2-Overexpressing Tobacco Leaves
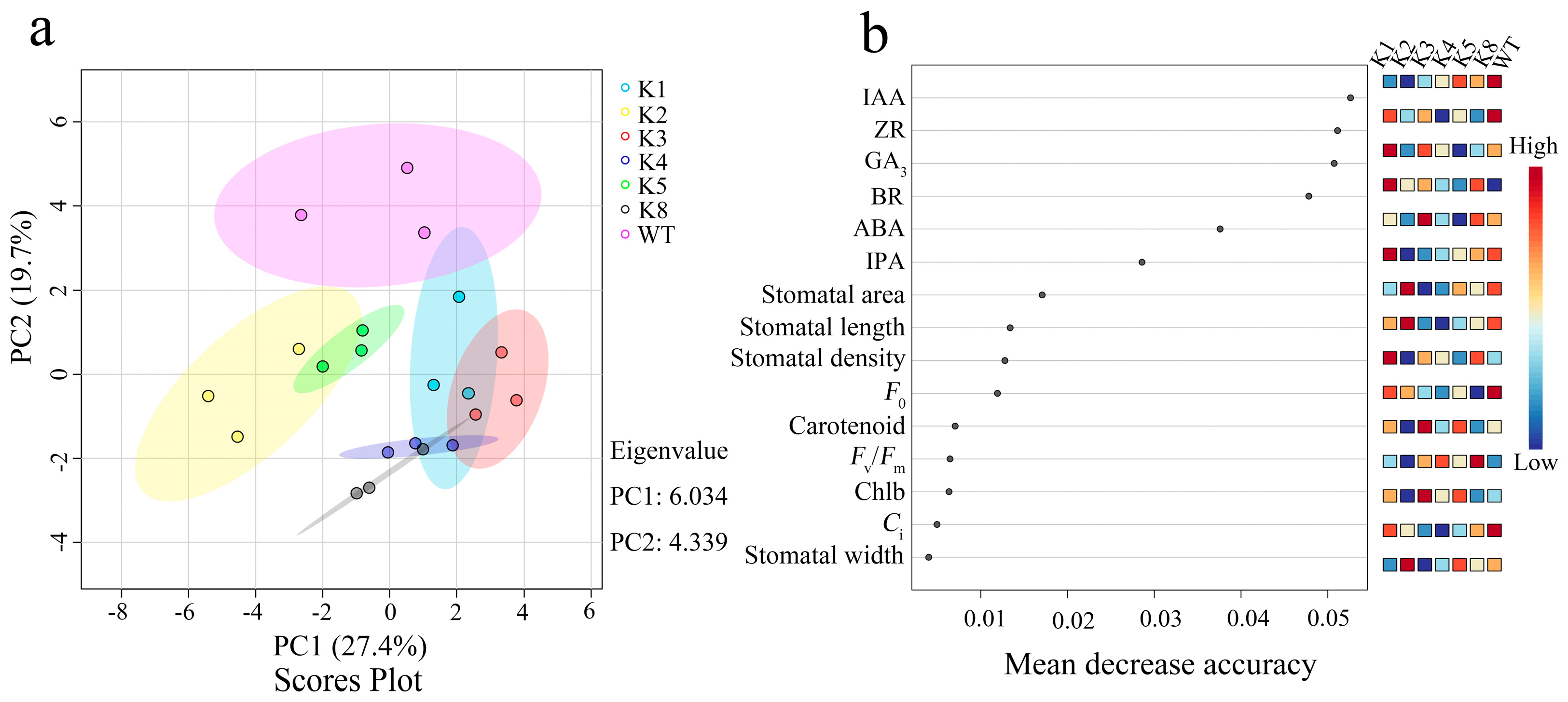
2.8. PCA and Random Forest Regression Analysis of Various Indicators in RpKTI2-Overexpressing Tobacco
3. Discussion
3.1. Influence of RpKTI2 Overexpression on Chlorophyll Content and Chlorophyll Fluorescence Characteristics of Tobacco
3.2. Influence of RpKTI2 Overexpression on the Photosynthetic Function and Stomatal Characteristics of Tobacco
3.3. Influence of RpKTI2 Overexpression on Endogenous Hormones of Tobacco
4. Materials and Methods
4.1. Materials
4.2. Cultivation and Identification of Transgenic Tobacco Plants Expressing RpKTI2
4.3. Extraction and Quantitative Analysis of RpKTI2
4.4. Measurement of Chlorophyll Content
4.5. Chlorophyll Fluorescence Parameter Measurements
4.6. Measurement of Photosynthetic Function
4.7. Observation of Stomatal Morphology
4.8. Measurement of Endogenous Hormone Content
4.9. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y. Trypsin Inhibitor and Its Influence on Immunological Enhancement of Mulberry in Mice. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2016. (In Chinese). [Google Scholar]
- Luo, Y.J.; Li, B.; Shu, H.P.; Jiang, L.P. Research advances in Kunitz trypsin inhibitor. Chin. J. Biochem. Pharm. 2012, 33, 316–319. (In Chinese) [Google Scholar]
- Chang, X. Insect Resistance Mechanisms of KTI Gene Families in Model Plants. Master’s Thesis, Tianjin University, Tianjin, China, 2012. (In Chinese). [Google Scholar]
- Arnaiz, A.; Talavera-Mateo, L.; Gonzalez-Melendi, P.; Martinez, M.; Isabel, D.; Santamaria, M.E. Arabidopsis kunitz trypsin inhibitors in defense against spider mites. Front. Plant Sci. 2018, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.S.; Mazarei, M.; Jurat-Fuentes, J.L.; Hewezi, T.; Millwood, R.J.; Stewart, C.N., Jr. Overexpression of soybean trypsin inhibitor genes decreases defoliation by corn earworm (Helicoverpa zea) in soybean (Glycine max) and Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1129454. [Google Scholar] [CrossRef]
- Domash, V.I.; Sharpio, T.P.; Zabreiko, S.A.; Sosnovskaya, T.F. Proteolytic enzymes and trypsin inhibitors of higher plants under stress conditions. Russ. J. Bioorg. Chem. 2008, 34, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Xu, C.; Zhang, Y.B.; Yan, X.; Jin, X.H.; Yao, X.Q.; Chen, P.; Zheng, B. PtKTI12 genes influence wobble uridine modifications and drought stress tolerance in hybrid poplar. Tree Physiol. 2020, 40, 1778–1791. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.X.; Duan, Y.X.; Chen, L.J.; Wang, X.; Zhou, B. Relationship of soybean trypsin inhibitor and soybean resistance to soybean cyst nematode. Soybean Sci. 2008, 27, 487–489, 495. (In Chinese) [Google Scholar]
- Srinivasan, T.; Kumar, K.R.R.; Kirti, P.B. Constitutive expression of a trypsin protease inhibitor confers multiple stress tolerance in transgenic tobacco. Plant Cell Physiol. 2009, 50, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Song, N.; Chen, S.Y.; Wu, J.S. NaKTI2, a Kunitz trypsin inhibitor transcriptionally regulated by NaWRKY3 and NaWRKY6, is required for herbivore resistance in Nicotiana attenuate. Plant Cell Rep. 2021, 40, 97–109. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. Kunitz trypsin inhibitor: An antagonist of cell death triggered by phytopathogens and fumonisin B1 in Arabidopsis. Mol. Plant 2008, 1, 482–495. [Google Scholar] [CrossRef]
- Xiang, M. Study on the Function of COTI1 and COTI2 of Cassia obtusifolia in Resistance to Pests, Drought and Salt Stress. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2018. (In Chinese). [Google Scholar]
- Chan, S.N.; Bakar, N.A.; Mahmood, M.; Ho, C.L.; Dzaki, N.M.; Shaharuddin, N.A. Identification and expression profiling of a novel Kunitz trypsin inhibitor (KTI) gene from turmeric, Curcuma longa, by real-time quantitative PCR (RT-qPCR). Acta Physiol. Plant 2017, 39, 12. [Google Scholar] [CrossRef]
- Guerra, F.P.; Reyes, L.; Vergara-Jaque, A.; Campos-Hernández, C.; Gutiérrez, A.; Pérez-Díaz, J.; Pérez-Díaz, R.; Blaudez, D.; Ruíz-Lara, S. Populus deltoides Kunitz trypsin inhibitor 3 confers metal tolerance and binds copper, revealing a new defensive role against heavy metal stress. Environ. Exp. Bot. 2015, 115, 28–37. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Z.P.; Ma, J.; Yang, L.F.; Wei, Y. The effects of lead stress on photosynthetic function and chloroplast ultrastructure of Robinia pseudoacacia seedlings. Environ. Sci. Pollut. Res. 2017, 24, 10718–10726. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.D. Physiological Response and Screening and Cloning of Tolerance Genes under Pb and Cd Stress in Robinia pseudoacacia. Master’s Thesis, Henan Institute of Science and Technology, Xinxiang, China, 2021. (In Chinese). [Google Scholar]
- Zhang, X.J.; Dong, Q.D.; Wang, X.D.; Zhang, X.N.; Zhou, J. Cloning and expression analysis of kuntiz trypsin inhibitor gene (RpKTI) in Robinia pseudoacacia. Shandong Agric. Sci. 2022, 54, 10–19. (In Chinese) [Google Scholar]
- Du, W.M. Studies on Transformation of Descurainia sophia Trypsin Inhibitor Gene into Brassica napus L. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2007. (In Chinese). [Google Scholar]
- Xu, S.S.; Tang, Y.; Zhong, M.H.; Li, L.Y.; Wu, L.H.; Lin, K.M.; Cao, G.Q.; Ye, Y.Q. Effects of shading on the growth, photosynthetic characteristics and nutrient content of Aglaonema commutatum. Pratacult. Sci. 2022, 39, 2083–2094. (In Chinese) [Google Scholar]
- Badri, M.A.; Rivard, D.; Coenen, K.; Michaud, D. Unintended molecular interactions in transgenic plants expressing clinically useful proteins: The case of bovine protinin traveling the potato leaf cell secretory pathway. Proteomics 2009, 9, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, F.; Cloutier, C.; Michaud, D. Colorado Potato beetles compensate for tomato cathepsin d inhibitor expressed in transgenic potato. Arch. Insect Biochem. Physiol. 2004, 55, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Rivard, D.; Anguenot, R.; Brunelle, F.; Le, V.Q.; Vézina, L.P.; Trépanier, S.; Michaud, D. An in-built proteinase inhibitor system for the protection of recombinant proteins recovered from transgenic plants. Plant Biotechnol. J. 2006, 4, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Hartl, M.; Giri, A.P.; Kaur, H.; Baldwin, I.T. Serine protease inhibitors specifically defend solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 2010, 22, 4158–4175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Zhao, Y.; Zhao, Y.X.; Cao, H.; Long, J.L. Effects of drought and rewatering on photosynthetic characteristics and chlorophyll ffuorescence of Trachycarpus fortunei seedlings. J. Cent. South. Univ. Forest. Technol. 2021, 41, 45–52. (In Chinese) [Google Scholar]
- Dąbrowski, P.; Pawluśkiewicz, B.; Baczewska, A.H.; Oglęcki, P.; Kalaji, H.M. Chlorophyll a fluorescence of perennial ryegrass (Lolium perenne L.) varieties under long term exposure to shade. Zemdirbyste 2015, 102, 305–312. [Google Scholar] [CrossRef]
- Adams, W.W., 3rd; Demmig-Adams, B.; Rosenstiel, T.N.; Ebbert, V. Dependence of photosynthesis and energy dissipation activity upon growth form and light environment during the winter. Photosynth. Res. 2001, 67, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Tuba, Z.; Saxena, D.K.; Srivastava, K.; Singh, S.; Czebol, S.; Kalaji, M.H. Chlorophyll a fluorescence measurements for validating the tolerant bryophytes for heavy metal (Pb) biomapping. Curr. Sci. India 2010, 98, 1505–1508. [Google Scholar]
- Yao, X.Z.; Lv, L.T.; Zhao, D.G. Improving photosynthetic characteristics of tobacco plants with BAS1 gene in transgenic Arabidopsis thaliana. J. Mount. Agric. Biol. 2016, 35, 30–34+62. (In Chinese) [Google Scholar]
- Caemmerer, S.V.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, L.; Lei, J.J. Photosynthetic responses of Arundinella hirta populations to light intensity and CO2 concentration. Acta Pratacult. Sin. 2016, 25, 254–261. (In Chinese) [Google Scholar]
- Gui, H.Y.; Fang, F.Z.; Mai, Y.Z.; Wu, E.H.; Zhang, X.F. Effects of pruning intensity on growth and physiological characteristics of Hopea hainanensis seedlings and comprehensive evaluation. J. West China Forest. Sci. 2022, 51, 79–85+100. (In Chinese) [Google Scholar]
- Fan, X.R.; Shen, Q.R. Effects of ABA and IAA on the behavior of stomata of rice crop cultivated in aerobic soil condition. Sci. Agric. Sin. 2003, 36, 1450–1455. (In Chinese) [Google Scholar]
- Li, Y.L.; Liu, L.; Li, C.A.; Wang, H.; Liu, H.T.; Miao, G.P. Analysis of carbon fixation, oxygen release characteristics and leaf stomatal micromorphology of 10 Rosaceae greening plants. J. Cent. South. Univ. Forest. Technol. 2023, 43, 102–112. (In Chinese) [Google Scholar]
- Ha, Y.; Shang, Y.; Nam, K.H. Brassinosteroids modulate ABA-induced stomatal closure in Arabidopsis. J. Exp. Bot. 2016, 67, 6297–6308. [Google Scholar] [CrossRef]
- Xia, X.J.; Gao, C.J.; Song, L.X.; Zhou, Y.H.; Shi, K.; Yu, J.Q. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 2014, 37, 2036–2050. [Google Scholar] [CrossRef]
- Isoda, R.; Yoshinari, A.; Ishikawa, Y.; Sasoine, M.; Simon, R.; Frommer, W.B.; Nakamura, M. Sensors for the quantification, localization and analysis of the dynamics of plant hormones. Plant J. 2021, 105, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Peivastegan, B.; Hadizadeh, I.; Nykyri, J.; Nielsen, K.L.; Somervuo, P.; Sipari, N.; Tran, C.; Pirhonen, M. Effect of wet storage conditions on potato tuber transcriptome, phytohormones and growth. BMC Plant Biol. 2019, 19, 262. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of endogenous plant hormones on physiological and growth attributes of Kinnow mandarin grafted on nine rootstocks. J. Plant Growth Regul. 2022, 41, 1254–1264. [Google Scholar] [CrossRef]
- Yan, B.B.; Hou, J.L.; Cui, J.; He, C.; Li, W.B.; Chen, X.Y.; Li, M.; Wang, W.Q. The effects of endogenous hormones on the flowering and fruiting of Glycyrrhiza uralensis. Plants 2019, 8, 519. [Google Scholar] [CrossRef]
- Li, J.R.; Wang, Y.B.; Zhang, Y.; Wu, X.L.; Gong, B.B.; Lu, G.Y.; Gao, H.B. Effects of GR24 and IAA and its interaction on growth of melon radicle and adventitious roots. Acta Agric. Boreali-Occident. Sin. 2021, 30, 1484–1494. (In Chinese) [Google Scholar]
- Li, Y.N.; Li, X.H.; Wang, G.H.; Dong, H. Signaling pathway of brassinosteroids and their roles in induction of plant virus defense response. Acta Agric. Jiangxi 2022, 34, 41–46. (In Chinese) [Google Scholar]
- Zhu, T.; Deng, X.J.; Zhou, X.; Zhu, L.S.; Zou, L.J.; Li, P.X.; Zhang, D.W.; Lin, H.H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinović, N.; Liu, D.R.; Russinov, E.; Yin, Y.H. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Lijith, K.P.; Rinky, K.M.; Devi, D.G. Purification, characterization and antioxidant efficacy testing of trypsin inhibitor protein from Anonna squamosa seeds. J. Plant Biochem. Biotechnol. 2023, 32, 145–152. [Google Scholar] [CrossRef]
- Fan, Y.P. Study on Extraction, Purification of Mung Bean Trypsin Inhibitor and Mechanism of Resistance to Callosobruchus chinensis (L.). Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2018. (In Chinese). [Google Scholar]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain Filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef]
- Yang, Y.M.; Xu, C.N.; Wang, B.M.; Jia, J.Z. Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regul. 2001, 35, 233–237. [Google Scholar]
- Zhao, J.; Li, G.; Yi, G.X.; Wang, B.M.; Deng, A.X.; Nan, T.G.; Li, Z.H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef] [PubMed]







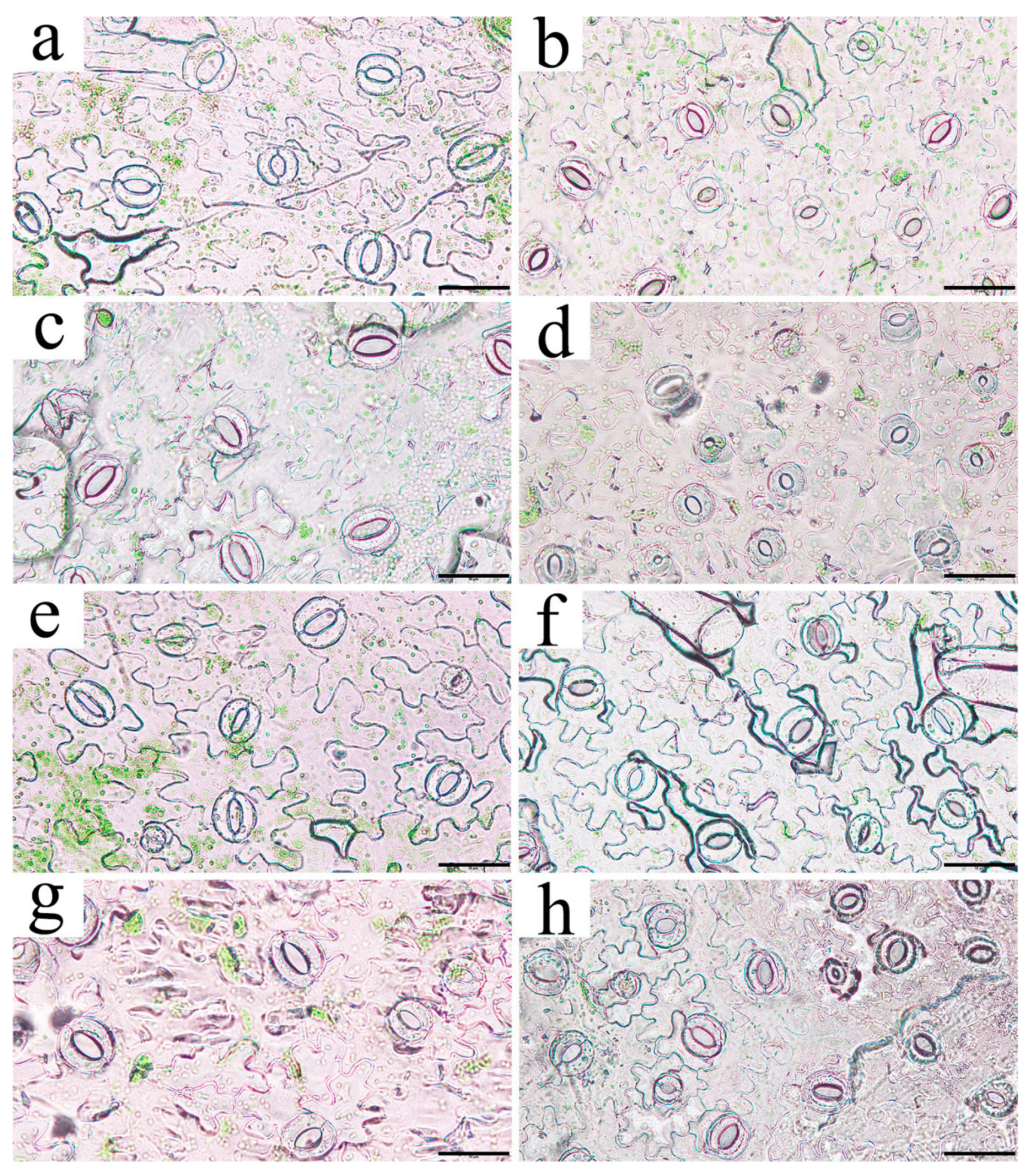
| Lines | Length/μm | Width/μm | Area/μm2 | Density/mm2 |
|---|---|---|---|---|
| WT | 22.22 ± 0.26 ab | 10.11 ± 0.76 ab | 162.00 ± 14.60 ab | 108.78 ± 10.19 bc |
| K1 | 18.24 ± 0.45 c | 9.80 ± 0.22 bc | 122.61 ± 6.28 c | 169.39 ± 6.77 a |
| K2 | 22.92 ± 1.02 a | 11.08 ± 0.14 a | 190.65 ± 14.17 a | 101.01 ± 4.11 c |
| K3 | 16.37 ± 0.70 c | 8.89 ± 0.51 bc | 116.28 ± 12.15 c | 144.52 ± 13.99 a |
| K4 | 17.43 ± 0.55 c | 8.60 ± 0.21 c | 122.99 ± 9.16 c | 136.75 ± 6.22 ab |
| K5 | 20.63 ± 0.51 b | 9.46 ± 0.14 bc | 166.93 ± 7.12 ab | 104.12 ± 13.28 c |
| K8 | 18.26 ± 0.07 c | 9.64 ± 0.25 bc | 138.58 ± 6.85 bc | 163.17 ± 11.73 a |
| Function | Primer Name | Sequence (5′–3′) |
|---|---|---|
| Identification of RpKTI2 transgenic Nicotiana tabacum positive seedlings | OE-RpKTI2 | F: GAGAACACGGGGGACTCTAGAATGAAGCCTGCATTTATTACCCTC R: CGATCGGGGAAATTCGAGCTCTCAAACTACTGAGTTTCCAGTGGC |
| RT-qPCR | NtActin | F: ACCTCTATGGCAACATTGTGCTCAG R: CTGGGAGCCAAAGCGGTGATT |
| qRpKTI2 | F: CCAAGTGGGTAGTGGTTGCT R: ACTGAGTTTCCAGTGGCGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Die, P.; Zhang, S.; Han, X.; Wang, C.; Wang, P. Overexpression of RpKTI2 from Robinia pseudoacacia Affects the Photosynthetic Physiology and Endogenous Hormones of Tobacco. Plants 2024, 13, 1867. https://doi.org/10.3390/plants13131867
Zhou J, Die P, Zhang S, Han X, Wang C, Wang P. Overexpression of RpKTI2 from Robinia pseudoacacia Affects the Photosynthetic Physiology and Endogenous Hormones of Tobacco. Plants. 2024; 13(13):1867. https://doi.org/10.3390/plants13131867
Chicago/Turabian StyleZhou, Jian, Pengxiang Die, Songyan Zhang, Xiaoya Han, Chenguang Wang, and Peipei Wang. 2024. "Overexpression of RpKTI2 from Robinia pseudoacacia Affects the Photosynthetic Physiology and Endogenous Hormones of Tobacco" Plants 13, no. 13: 1867. https://doi.org/10.3390/plants13131867
APA StyleZhou, J., Die, P., Zhang, S., Han, X., Wang, C., & Wang, P. (2024). Overexpression of RpKTI2 from Robinia pseudoacacia Affects the Photosynthetic Physiology and Endogenous Hormones of Tobacco. Plants, 13(13), 1867. https://doi.org/10.3390/plants13131867






