Contrasting Phenological Patterns and Reproductive Strategies in Closely Related Monoecious Fig Tree Species
Abstract
1. Introduction
2. Results
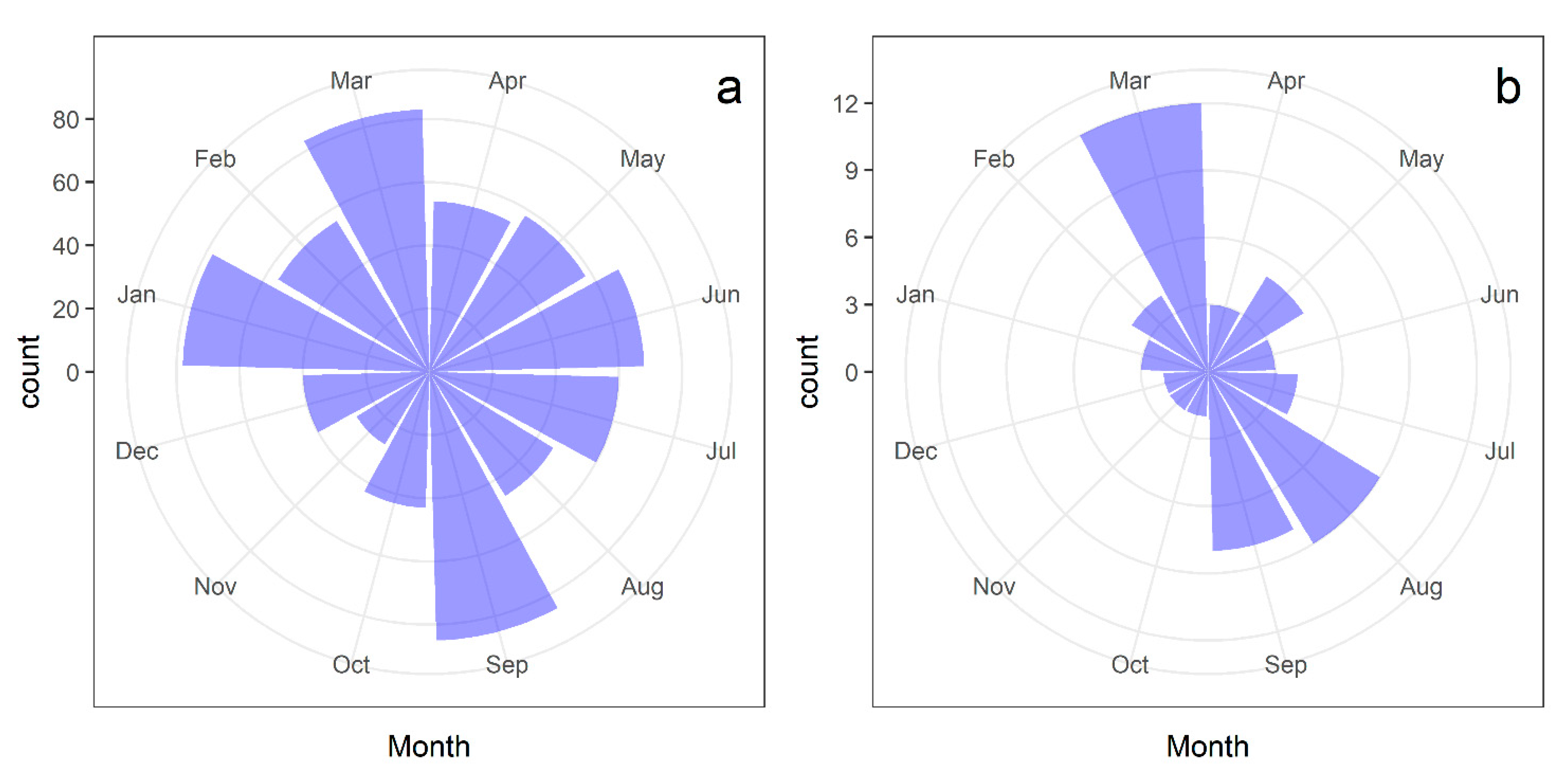
2.1. Flowering Patterns
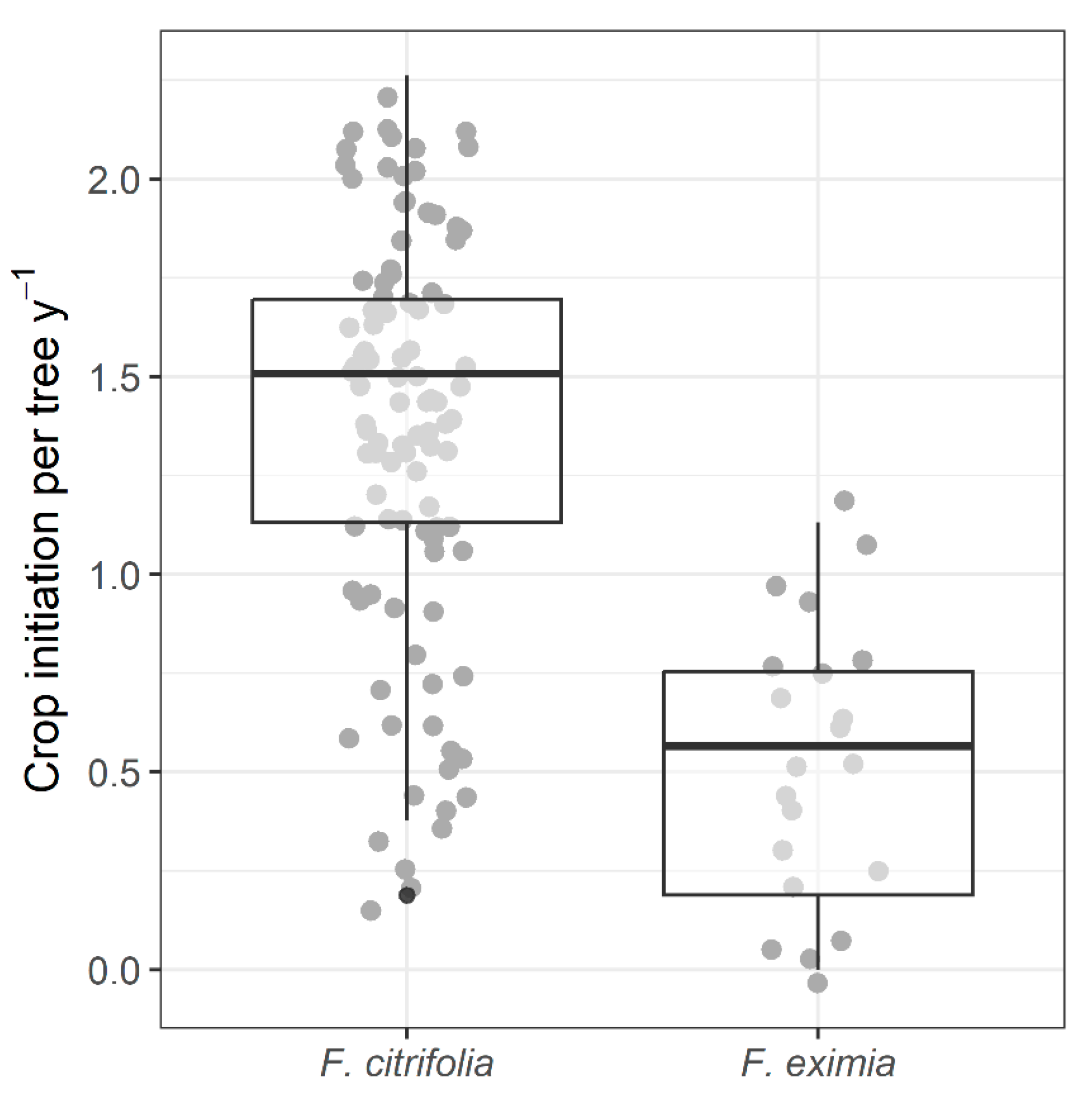
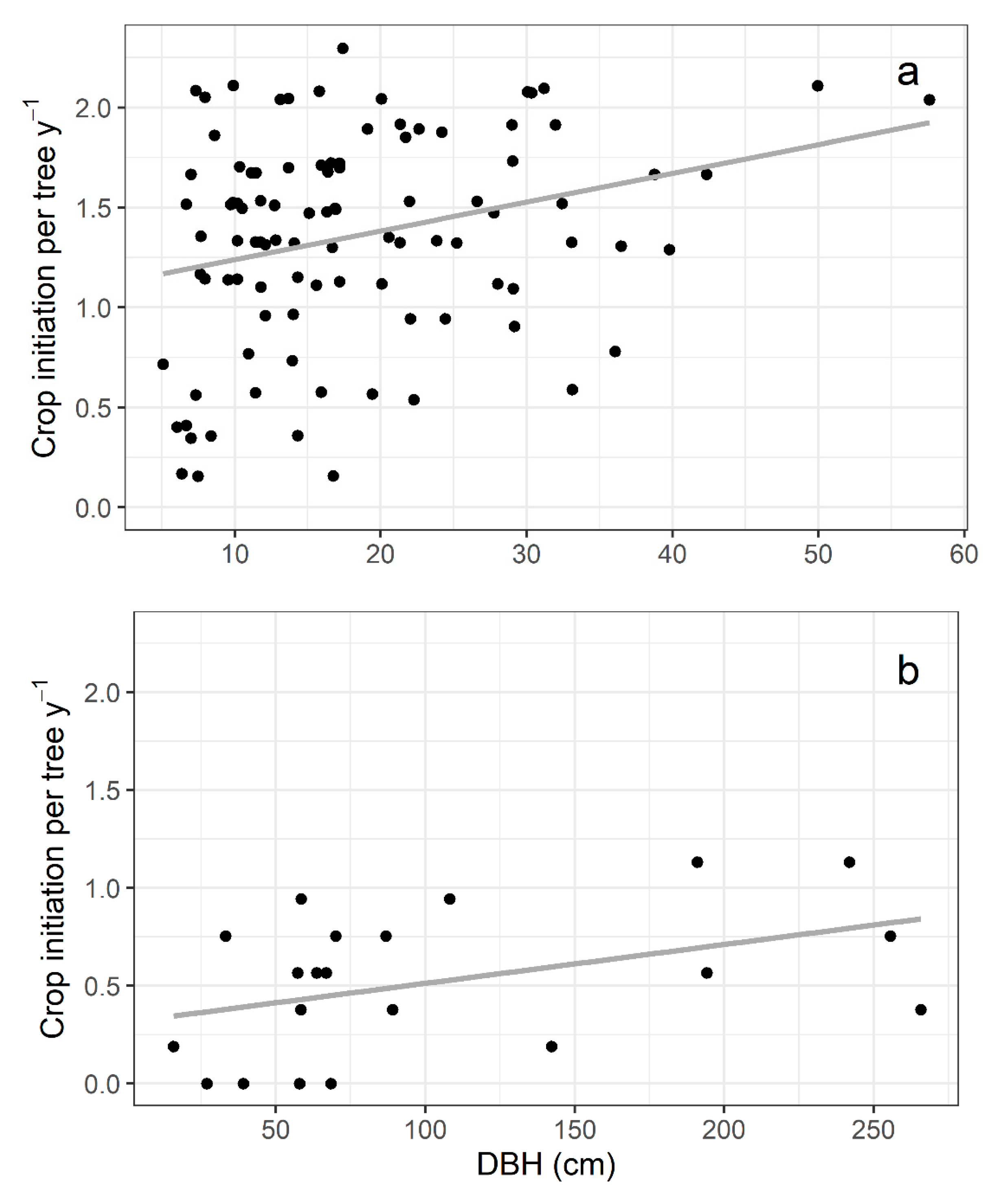
2.2. Frequency of Fig Crop Initiation
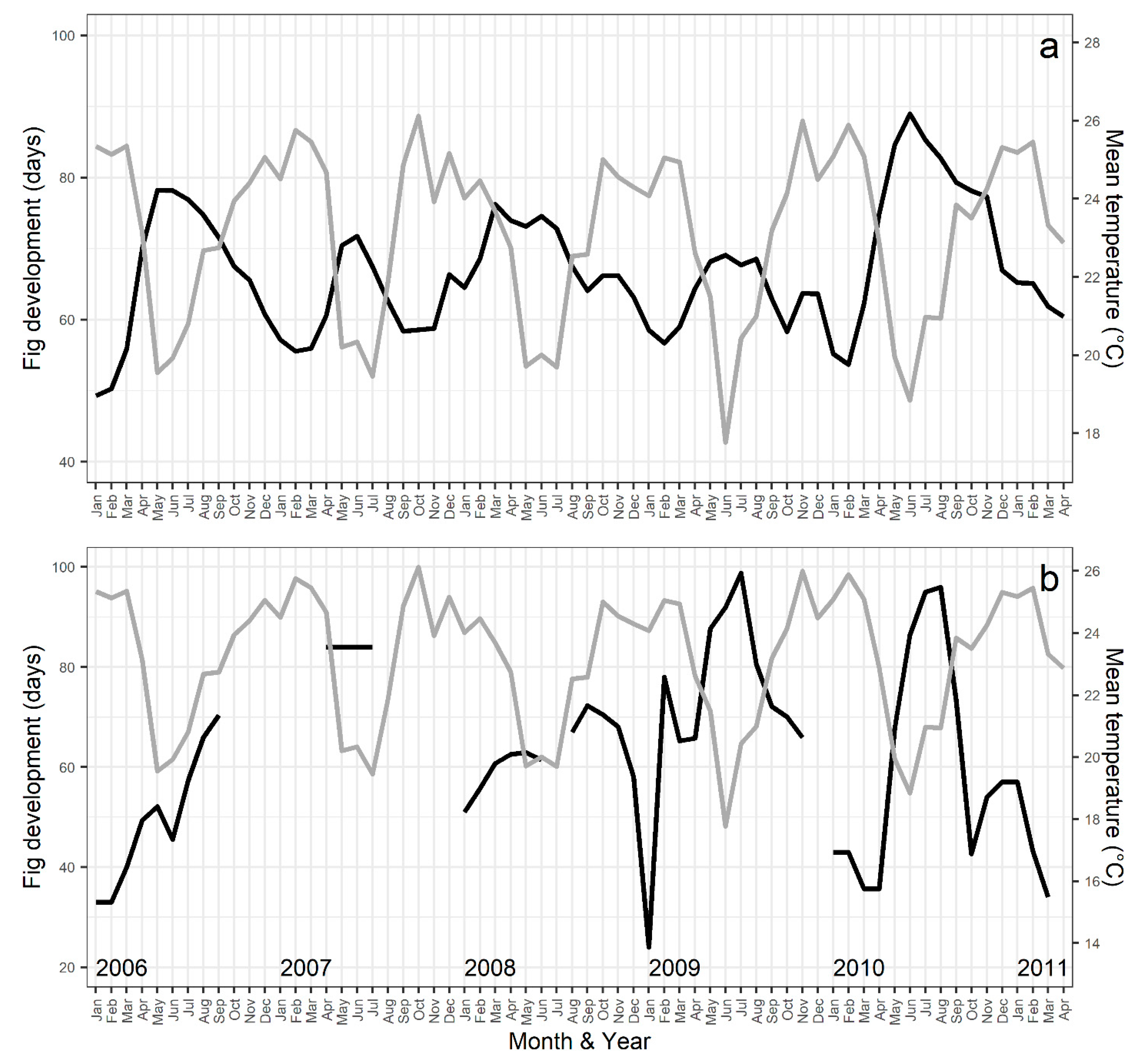
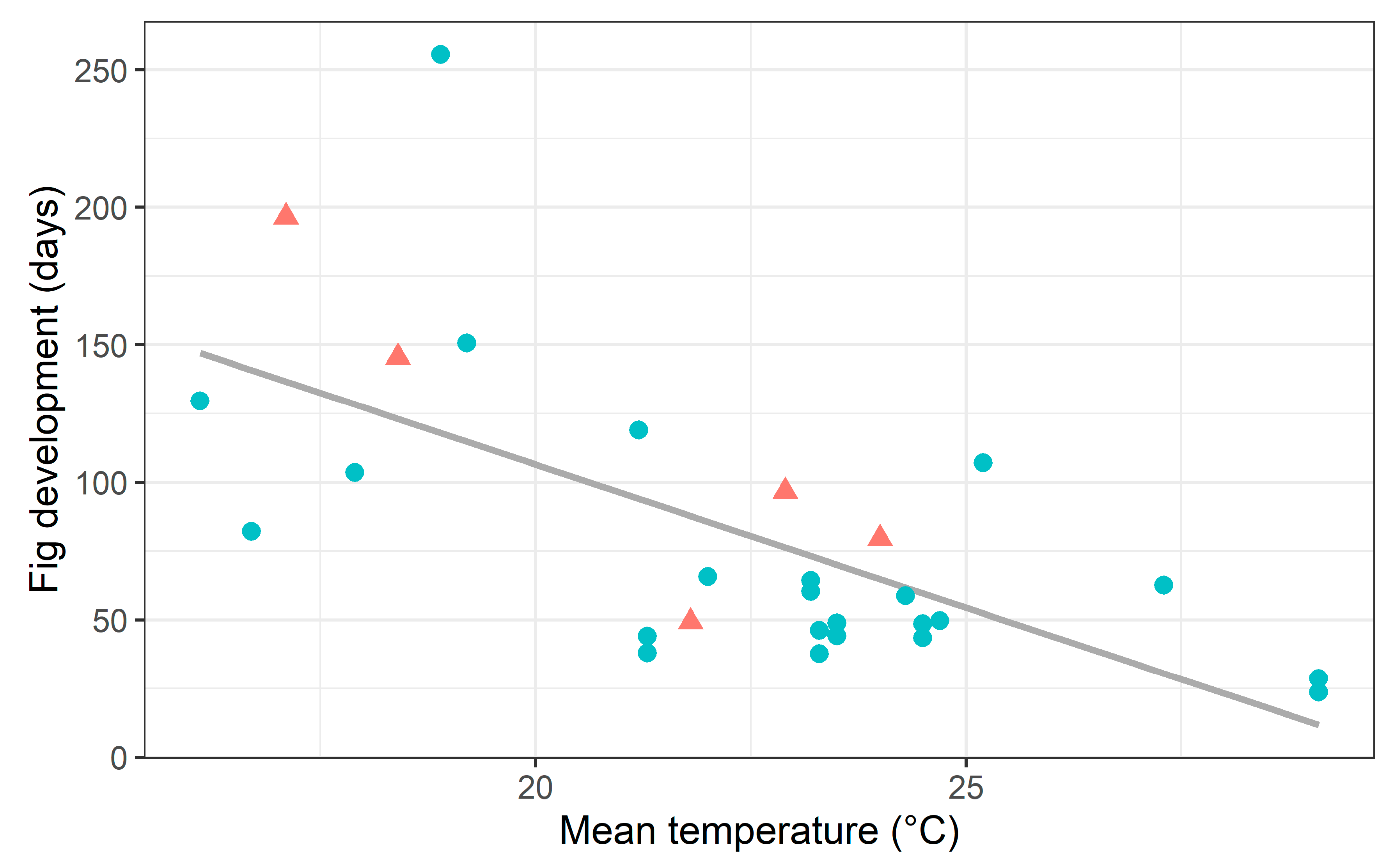
2.3. Fig Development Time
2.4. Reproductive Characteristics and Wasp Community
3. Discussion
3.1. Phenological Patterns
3.2. Reproductive Strategies and Genetic Structure
3.3. Vegetative and Reproductive Growth Rhythms
3.4. Fig Structure and Mutualism Maintenance
3.5. Coordination of Fig and Wasp Development
3.6. Environmental Influences on Flowering Patterns
4. Materials and Methods
4.1. Study Area and Species
4.2. Data Sampling
4.3. Data Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Influences of species, latitudes, and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Alberton, B.; Alvarado, S.T.; Borges, L.A.C.; Buisson, E.; Camargo, M.G.G.; Cancian, L.F.; Carstensen, D.W.; Escobar, D.F.E.; Leite, P.T.P.; et al. Linking plant phenology to conservation biology. Biol. Conserv. 2016, 195, 60–72. [Google Scholar] [CrossRef]
- Khanduri, V.P.; Sharma, C.M.; Singh, S.P. The effects of climate change on plant phenology. Environmentalist 2008, 28, 143–147. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Love, N.L.; Mazer, S.J. Region-specific phenological sensitivities and rates of climate warming generate divergent temporal shifts in flowering date across a species’ range. Am. J. Bot. 2021, 108, 1873–1888. [Google Scholar] [CrossRef]
- Snow, D.W. A Possible Selective Factor in the Evolution of Fruiting Seasons in Tropical Forest. Oikos 1965, 15, 274–281. [Google Scholar] [CrossRef]
- Rathcke, B.; Lacey, E.P. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 1985, 16, 179–214. [Google Scholar] [CrossRef]
- van Schaik, C.P.; Terborgh, J.W.; Wright, S.J. The phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annu. Rev. Ecol. Syst. 1993, 24, 353–377. [Google Scholar] [CrossRef]
- Bawa, K.S.; Kang, H.; Grayum, M.H. Relationships among time, frequency, and duration of flowering in tropical rain forest trees. Am. J. Bot. 2003, 90, 877–887. [Google Scholar] [CrossRef]
- Visser, M.E.; Holleman, L.J. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. Lond. B Biol. Sci. 2001, 268, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.F.; Elkinton, J.S. Interaction between phenology and density effects on mortality from natural enemies. J. Anim. Ecol. 1999, 68, 1093–1100. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Romeis, J.; van Rijn, P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, N.E.; CaraDonna, P.J.; Bronstein, J.L. Phenological shifts and the fate of mutualisms. Oikos 2015, 124, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, J.L. The evolution of facilitation and mutualism. J. Ecol. 2009, 97, 1160–1170. [Google Scholar] [CrossRef]
- Pereira, R.A.S.; Kjellberg, F. Mutualism as a Source of Evolutionary Innovation: Insights from Insect-Plant Interactions. In Plant-Animal Interactions; Del-Claro, K., Torezan-Silingardi, H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 307–332. [Google Scholar] [CrossRef]
- Corlett, R.T. The phenology of Ficus benjamina and Ficus microcarpa in Singapore. J. Singap. Natl. Acad. Sci. 1984, 13, 30–31. [Google Scholar]
- Windsor, D.M.; Morrison, D.W.; Estribi, M.A.; De Leon, B. Phenology of fruit and leaf production by ‘strangler’ figs on Barro Colorado Island, Panama. Exp. Basel 1989, 45, 647–653. [Google Scholar] [CrossRef]
- Milton, K. Leaf change and fruit production in six neotropical Moraceae species. J. Ecol. 1991, 79, 1–26. [Google Scholar] [CrossRef]
- Figueiredo, R.A. Fenologia e Polinização De Três Espécies De Figueiras Em Mata Semidecídua Na Região De Campinas, SP. Master’s Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 1994. [Google Scholar]
- Bianchini, E.; Emmerick, J.M.; Messetti, A.V.L.; Pimenta, J.A. Phenology of two Ficus species in seasonal semi-deciduous forest in Southern Brazil. Braz. J. Biol. 2015, 75, 206–214. [Google Scholar] [CrossRef]
- Harrison, R.D. Figs and the diversity of tropical rainforests. Bioscience 2005, 55, 1053–1064. [Google Scholar] [CrossRef]
- Pereira, R.A.S.; Rodrigues, E.; Menezes, A.O., Jr. Phenological patterns of Ficus citrifolia (Moraceae) in a seasonal humid-subtropical region in Southern Brazil. Plant Ecol. 2007, 188, 265–275. [Google Scholar] [CrossRef]
- Pellmyr, O. Yuccas, Yucca Moths, and Coevolution: A Review. Ann. Mo. Bot. Gard. 2003, 90, 35–55. [Google Scholar] [CrossRef]
- Coelho, L.F.M.; Ribeiro, M.C.; Pereira, R.A.S. Water availability determines the richness and density of fig trees within Brazilian semideciduous forest landscapes. Acta Oecol. 2014, 57, 109–116. [Google Scholar] [CrossRef]
- Compton, S.G. One way to be a fig. Afr. Entomol. 1993, 1, 151–158. [Google Scholar]
- Nazareno, A.G.; Alzate-Marin, A.L.; Pereira, R.S. Dioecy, more than monoecy, affects plant spatial genetic structure: The case study of Ficus. Ecol. Evol. 2013, 3, 3495–3508. [Google Scholar] [CrossRef]
- Hamilton, M.B.; Miller, J.R. Comparing relative rates of pollen and seed gene flow in the island model using nuclear and organelle measures of population structure. Genetics 2002, 162, 1897–1909. [Google Scholar] [CrossRef]
- Kang, H.; Bawa, K.S. Effects of successional status, habit, sexual systems, and pollinators on flowering patterns in tropical rain forest trees. Am. J. Bot. 2003, 90, 865–876. [Google Scholar] [CrossRef]
- Pereira, R.A.S.; Semir, J.; Menezes, A.O., Jr. Pollination and other biotic interactions in figs of Ficus eximia Schott (Moraceae). Braz. J. Bot. 2000, 23, 217–224. [Google Scholar] [CrossRef]
- Anstett, M.C. Unbeatable strategy, constraint and coevolution, or how to resolve evolutionary conflicts: The case of the fig/wasp mutualism. Oikos 2001, 95, 476–484. [Google Scholar] [CrossRef]
- Basso-Alves, J.P.; Pereira, R.A.S.; Peng, Y.Q.; Teixeira, S.P. Different ontogenetic processes promote dicliny in Ficus L. (Moraceae). Acta Oecol. 2014, 57, 5–16. [Google Scholar] [CrossRef]
- Teixeira, S.P.; Costa, M.F.B.; Basso-Alves, J.P.; Kjellberg, F.; Pereira, R.A.S. Morphological diversity and function of the stigma in Ficus species (Moraceae). Acta Oecol. 2018, 90, 117–131. [Google Scholar] [CrossRef]
- Galil, J.; Eisikowitch, D. Studies on mutualistic symbiosis between syconia and sycophilous wasps in monoecious figs. New Phytol. 1971, 70, 773–787. [Google Scholar] [CrossRef]
- Bronstein, J.L.; Patel, A. Causes and consequences of within-tree phenological patterns in the Florida strangling fig, Ficus aurea (Moraceae). Am. J. Bot. 1992, 79, 41–48. [Google Scholar] [CrossRef]
- Zhang, G.M.; Song, Q.S.; Yang, D.R. Phenology of Ficus racemosa in Xishuangbanna, Southwest China. Biotropica 2006, 38, 334–341. [Google Scholar] [CrossRef]
- Lin, S.L.; Zhao, N.X.; Chen, Y.Z. Phenology and the production of seeds and wasps in Ficus microcarpa in Guangzhou, China. Symbiosis 2008, 45, 101–105. [Google Scholar]
- Chiang, Y.-P.; Bain, A.; Wu, W.-J.; Chou, L.-S. Adaptive phenology of Ficus subpisocarpa and Ficus caulocarpa in Taipei, Taiwan. Acta Oecol. 2018, 90, 35–45. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Peng, Y.; Corlett, R.T. Latitudinal effects on phenology near the northern limit of figs in China. Sci. Rep. 2018, 8, 4320. [Google Scholar] [CrossRef]
- Bronstein, J.L.; Patel, A. Temperature-sensitive development: Consequences for local persistence of two subtropical fig wasp species. Am. Midl. Nat. 1992, 128, 397–403. [Google Scholar] [CrossRef]
- Corlett, R.T. The phenology of Ficus fistulosa in Singapore. Biotropica 1987, 19, 122–124. [Google Scholar] [CrossRef]
- Corlett, R.T. Sexual dimorphism in the reproductive phenology of Ficus grossularioides Burm. f. in Singapore. Malay. Nat. J. 1993, 46, 149–155. [Google Scholar]
- Yu, H.; Zhang, Y.; Song, Q.S.; Wu, J.; Peng, Y.; Yang, D.R. Phenology and reproductive strategy of a common fig in Guangzhou. Bot. Stud. 2006, 47, 435–441. [Google Scholar]
- Berg, C.C. Proposals for treating four species complexes in Ficus subgenus Urostigma section Americanae (Moraceae). Blumea-Biodivers. Evol. Biogeogr. Plants 2007, 52, 295–312. [Google Scholar] [CrossRef]
- Cerezini, M.T.; Gobbo, S.E.; Felício, D.T.; Barros, L.O.; Pereira, R.A.S. Community of fig trees in the University of Sao Paulo campus, Ribeirao Preto city: Implications on the frugivory fauna maintenance and on the recomposition of permanent preservation areas. Braz. J. Anim. Environ. Res. 2021, 4, 190–195. [Google Scholar] [CrossRef]
- Galil, J.; Eisikowitch, D. On the pollination ecology of Ficus sycomorus in east Africa. Ecology 1968, 49, 259–269. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org (accessed on 8 July 2024).
- Huang, Y.-T.; Lee, Y.-F.; Kuo, Y.-M.; Chang, S.-Y.; Wu, C.-L. Fruiting Phenology and Nutrient Content Variation among Sympatric Figs and the Ecological Correlates. Bot. Stud. 2019, 60. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.C.; Yao, J.Y.; Chen, Y.Z.; Cook, J.M.; Crozier, R.H. The Phenology and Potential for Self-Pollination of Two Australian Monoecious Fig Species. Symbiosis 2008, 45, 91–96. [Google Scholar]
- Zhang, L.-S.; Compton, S.G.; Xiao, H.; Lu, Q.; Chen, Y. Living on the Edge: Fig Tree Phenology at the Northern Range Limit of Monoecious Ficus in China. Acta Oecol. 2014, 57, 135–141. [Google Scholar] [CrossRef]
| Variables | F. citrifolia | F. eximia | Prob. | ||
|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | ||
| No of pistillate flowers | 51 | 276.0 ± 102.2 | 30 | 331.0 ± 141.6 | 0.047 |
| No of wasps (total) | 51 | 136.3 ± 51.2 | 30 | 159.2 ± 72.8 | 0.101 |
| No of PolFW | 51 | 71.4 ± 67.0 | 30 | 114.2 ± 86.0 | 0.014 |
| No of NPFW | 51 | 64.9 ± 39.5 | 30 | 45.0 ± 29.3 | 0.019 |
| No of Seeds | 51 | 112.0 ± 63.7 | 30 | 140.8 ± 90.4 | 0.022 |
| Fig diameter (cm) | 51 | 1.41 ± 0.19 | 30 | 1.40 ± 0.15 | 0.886 |
| Mesosoma length (mm) | 30 | 0.66 ± 0.05 | 30 | 0.67 ± 0.05 | 0.699 |
| Seed length (mm) | 30 | 1.59 ± 0.30 | 30 | 1.43 ± 0.13 | 0.006 |
| Seed width (mm) | 30 | 1.13 ± 0.12 | 30 | 1.13 ± 0.11 | 0.883 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerezini, M.T.; Rattis, L.; Furini, P.R.; Pereira, R.A.S. Contrasting Phenological Patterns and Reproductive Strategies in Closely Related Monoecious Fig Tree Species. Plants 2024, 13, 1889. https://doi.org/10.3390/plants13141889
Cerezini MT, Rattis L, Furini PR, Pereira RAS. Contrasting Phenological Patterns and Reproductive Strategies in Closely Related Monoecious Fig Tree Species. Plants. 2024; 13(14):1889. https://doi.org/10.3390/plants13141889
Chicago/Turabian StyleCerezini, Monise T., Ludmila Rattis, Paulo R. Furini, and Rodrigo A. S. Pereira. 2024. "Contrasting Phenological Patterns and Reproductive Strategies in Closely Related Monoecious Fig Tree Species" Plants 13, no. 14: 1889. https://doi.org/10.3390/plants13141889
APA StyleCerezini, M. T., Rattis, L., Furini, P. R., & Pereira, R. A. S. (2024). Contrasting Phenological Patterns and Reproductive Strategies in Closely Related Monoecious Fig Tree Species. Plants, 13(14), 1889. https://doi.org/10.3390/plants13141889






