Tolerance Mitigates Gall Effects When Susceptible Plants Fail to Elicit Induced Defense
Abstract
1. Introduction
2. Results
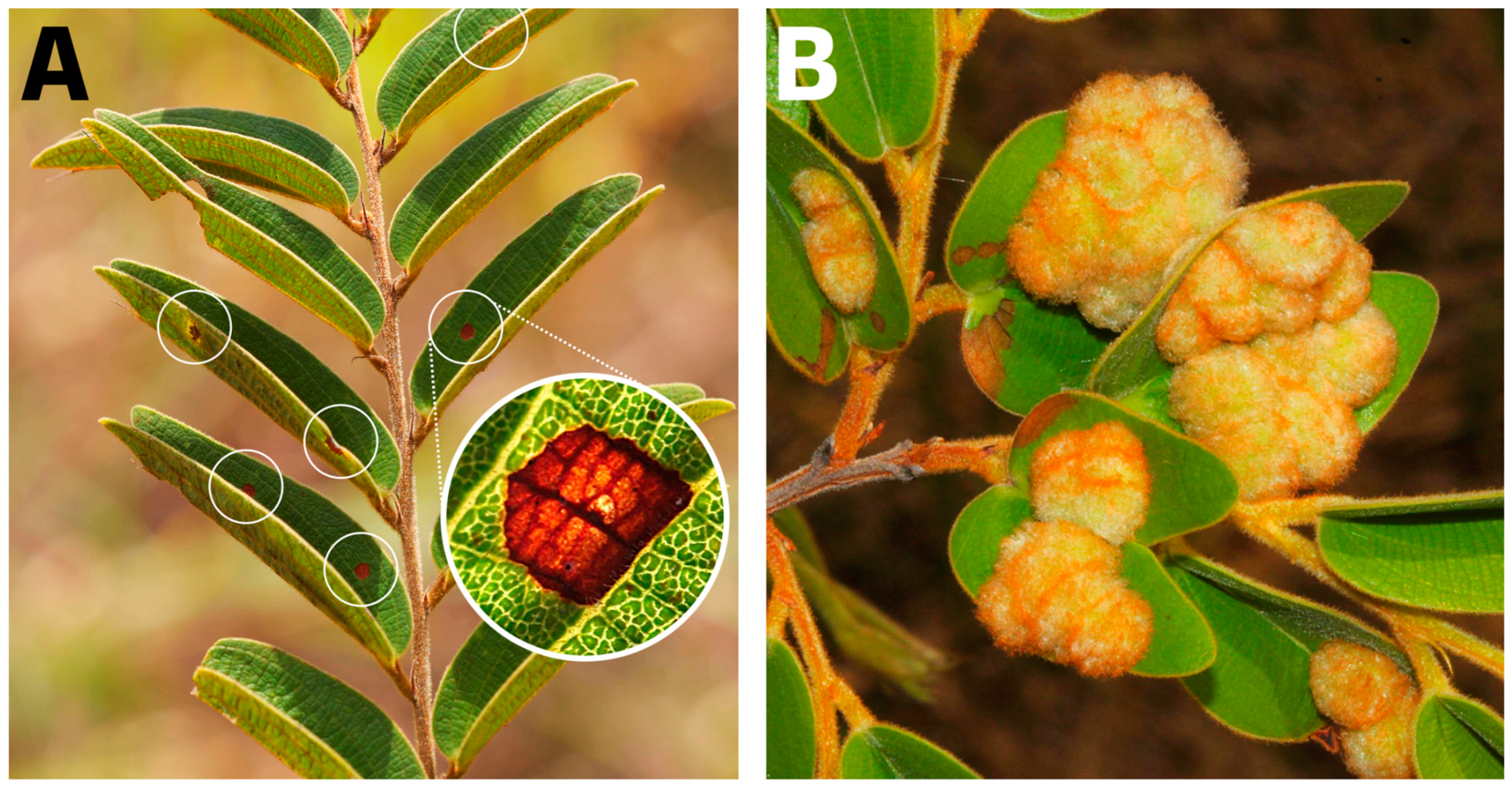
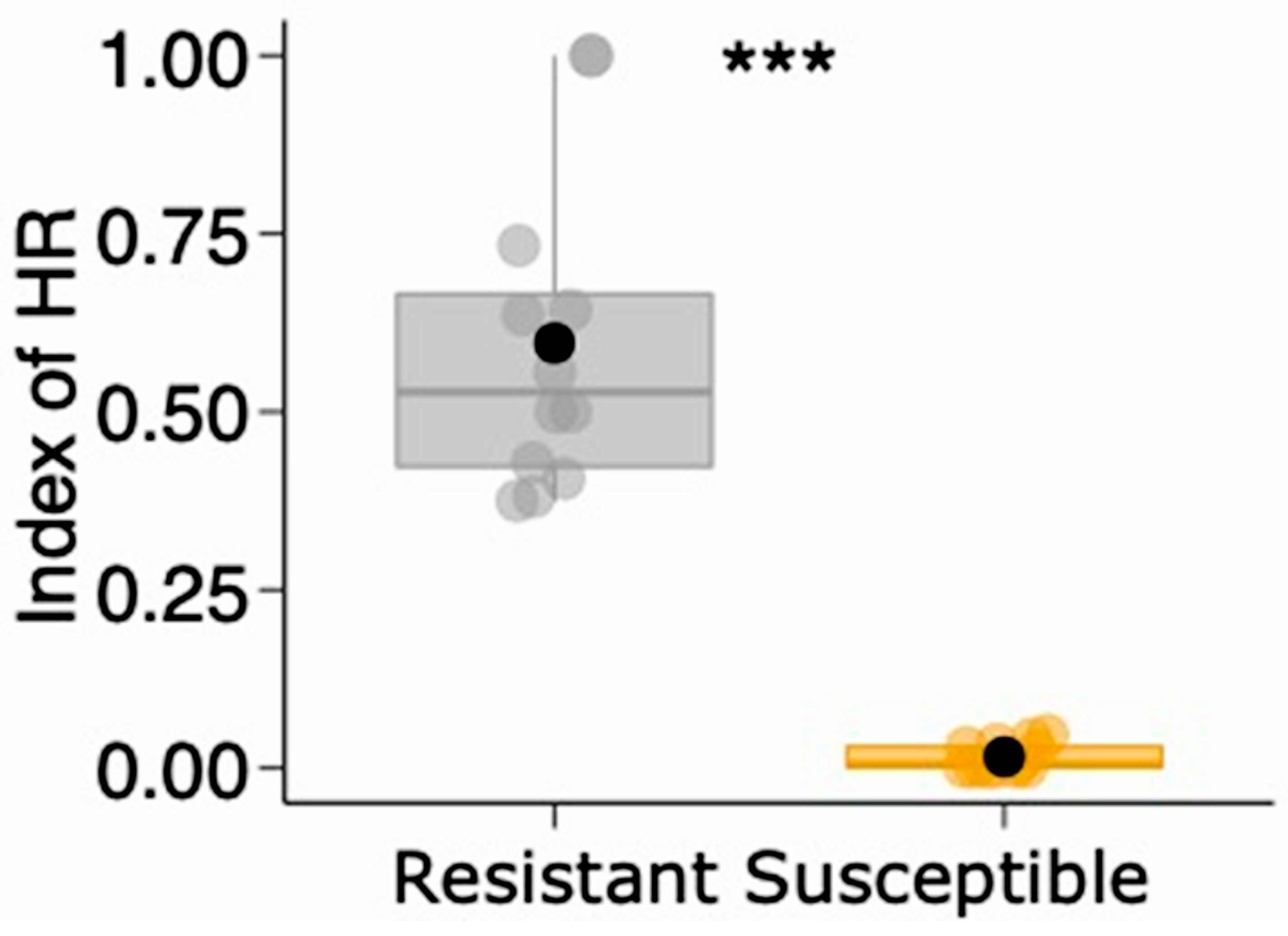
2.1. Hypersensitivity Reaction and Gall-Related Parameters
2.2. Foliar Nutrients
2.3. Plant Vegetative and Reproductive Traits
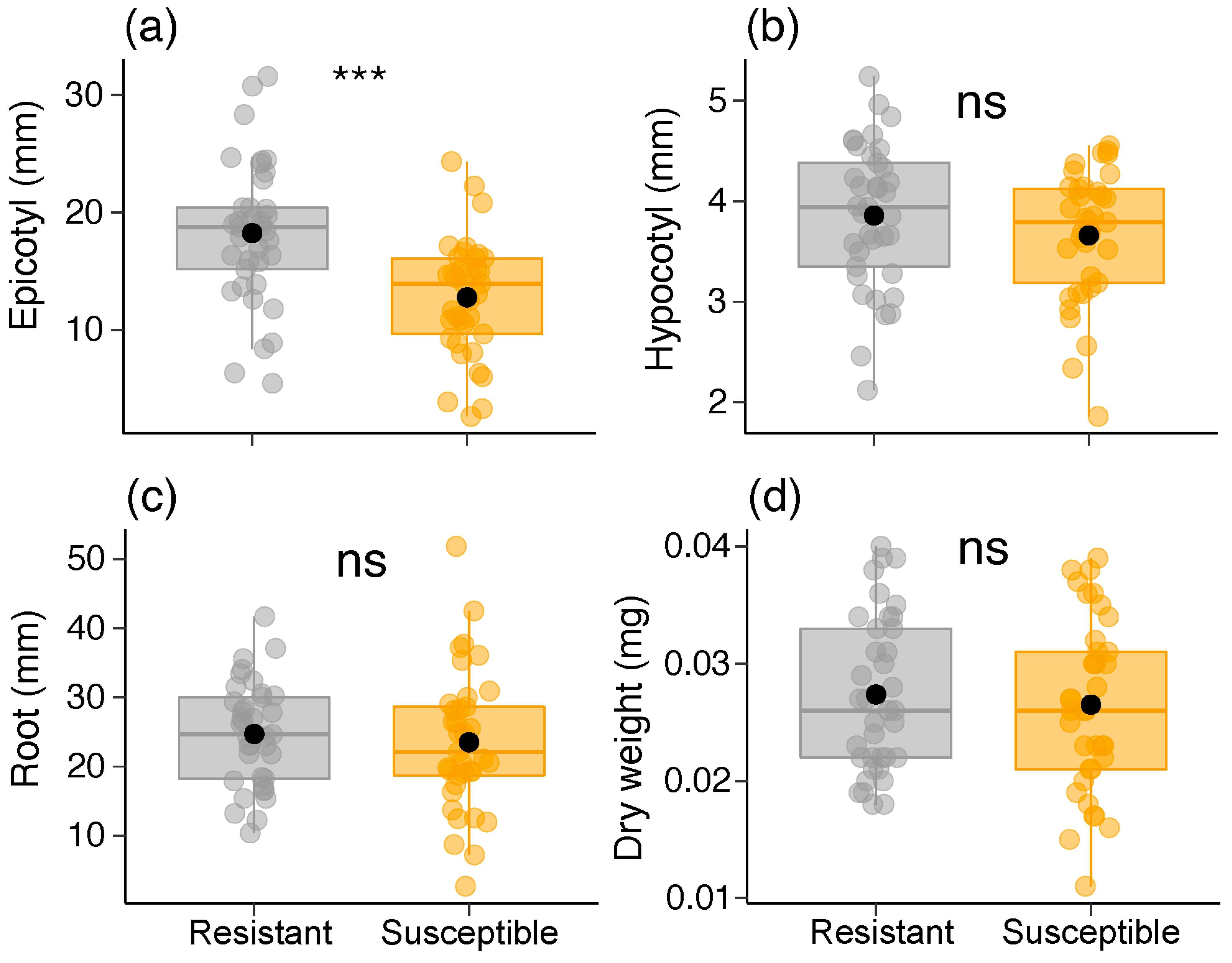
2.4. Germination Rate
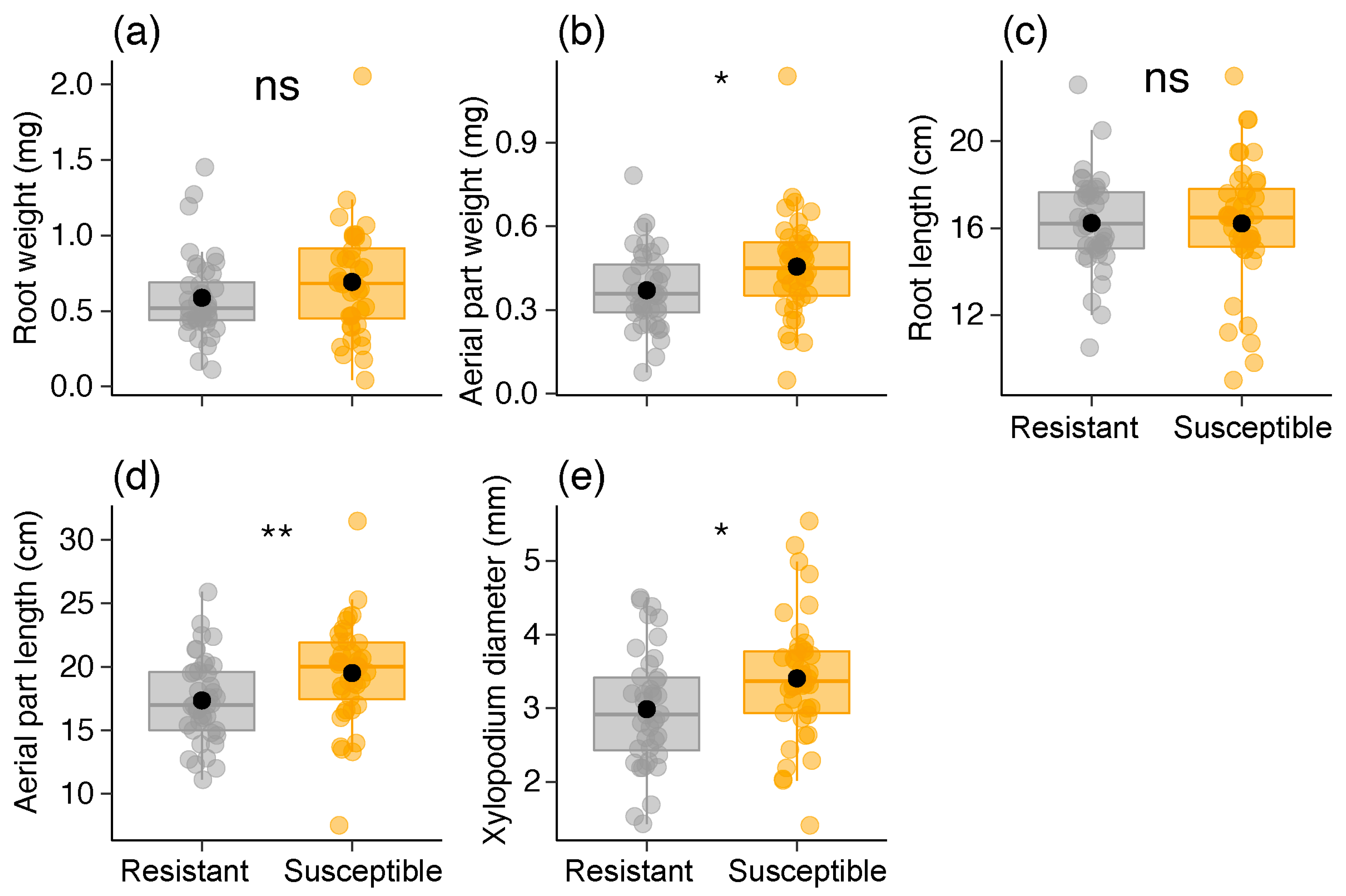
2.5. Seedling and Sapling Parameters
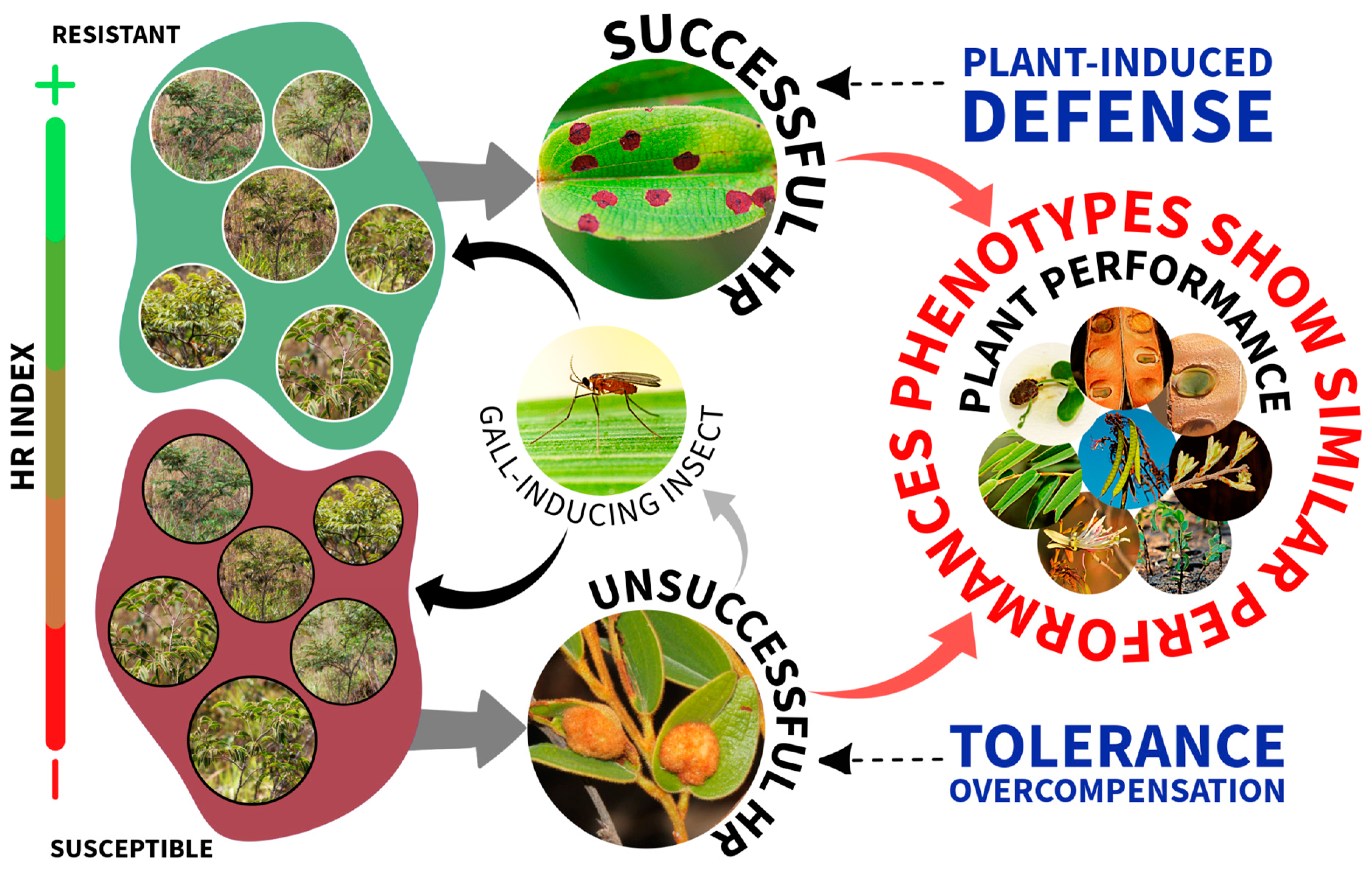
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Study Area
5.2. Study System
5.3. Hypersensitivity Reaction and Gall-Related Parameters
5.4. Nutritional Content
5.5. Vegetative Plant Traits and Fluctuating Asymmetry
5.6. Fluctuating Asymmetry Analyses
5.7. Reproductive Performance beyond a Generation
- (1).
- Germination rate
- (2).
- Seedlings parameters
- (3).
- Sapling traits
6. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewinsohn, T.M.; Prado, P.I.; Jordano, P.; Bascompte, J.; Olesen, J.M. Structure in plant–animal interaction assemblages. Oikos 2006, 113, 174–184. [Google Scholar] [CrossRef]
- Becerra, J.X. The impact of herbivore-plant coevolution on plant community structure. Proc. Natl. Acad. Sci. USA 2007, 104, 7483–7488. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-López, Y.; de Araújo, W.S.; González-Rodríguez, A.; Oyaka, K.; Fagundes, M.; Diaz-Castelazo, C.; Sánchez-Echeverría, K.; Borges, M.; Pérez-López, G.; Cuevas-Reyes, P. Quantitative trophic networks of insect gallers and their parasitoids in the hybrid oak complex Quercus magnoliifolia x Quercus resinosa. Arthropod-Plant Interact. 2022, 16, 631–643. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Quesada, M.; Siebe, C.; Oyama, K. Spatial patterns of herbivory by gall-forming insects: A test of the soil fertility hypothesis in a Mexican tropical dry forest. Oikos 2004, 107, 181–189. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Gilberti, L.; González-Rodríguez, A.; Fernandes, G.W. Patterns of herbivory and fluctuating asymmetry in Solanum lycocarpum St. Hill (Solanaceae) along an urban gradient in Brazil. Ecol. Indic. 2013, 24, 557–561. [Google Scholar] [CrossRef]
- Pérez-Solache, A.; Vaca-Sánchez, M.S.; Maldonado-López, Y.; De Faria, M.L.; Borges, M.A.Z.; Fagundes, M.; Oyama, K.; Méndez-Solórzano, M.I.; Aguilar-Peralta, J.S.; Hernández-Guzmán, R.; et al. Changes in land use of temperate forests associated to avocado production in Mexico: Impacts on soil properties, plant traits and insect-plant interactions. Agric. Syst. 2023, 204, 103556. [Google Scholar] [CrossRef]
- Wetzel, W.C.; Inouye, B.D.; Hahn, P.G.; Whitehead, S.R.; Underwood, N. Variability in Plant-Herbivore Interactions. Ann. Rev. Ecol. Evol. Syst. 2023, 54, 451–474. [Google Scholar] [CrossRef]
- Strong, D.R.; Lawton, J.H.; Southwood, R. Insects on Plants: Community Patterns and Mechanisms; Blackwell: Oxford, UK, 1984. [Google Scholar]
- Harris, M.O.; Pitzschke, A. Plants make galls to accommodate foreigners: Some are friends, most are foes. New Phytol. 2020, 225, 1852–1872. [Google Scholar] [CrossRef]
- Fagundes, M.; Cuevas-Reyes, P.; Ramos Leite, L.F.; Borges, M.A.Z.; De Araújo, W.S.; Fernandes, G.W.; Siqueira, W.K. Diversity of gall-inducing insects associated with a widely distributed tropical tree species: Testing the environmental stress hypothesis. Environ. Entomol. 2020, 49, 838–847. [Google Scholar] [CrossRef]
- García-Jain, S.E.; Maldonado-López, Y.; Oyama, K.; López-Maldonado, M.C.; Fagundes, M.; de Faria, M.L.; Espírito-Santo, M.M.; Vaca-Sánchez, M.S.; Cuevas-Reyes, P. Guild-dependent effects of forest fragmentation in canopy arthropod diversity associated to Quercus deserticola. Eur. J. For. Res. 2023, 142, 217–230. [Google Scholar] [CrossRef]
- Araújo, W.S.; Scareli-Santos, C.; Guilherme, F.A.G.; Cuevas-Reyes, P. Comparing galling insect richness among Neotropical savannas: Effects of plant richness, vegetation structure and super-host presence. Biodivers. Conserv. 2013, 22, 1083–1094. [Google Scholar] [CrossRef]
- Ananthakrishnan, T.N. Biology of Gall Insects; Arnold: London, UK, 1984; 362p. [Google Scholar]
- Dreger-Jauffret, F.; Shorthouse, J.D. Diversity of gall-inducing insects and their galls. In Biology of Insect Induced Galls; Shorthouse, J.D., Rohfritsch, O., Eds.; Oxford University: Oxford, UK, 1992. [Google Scholar]
- Pascual-Alvarado, E.; Cuevas-Reyes, P.; Quesada, M.; Oyama, K. Interactions between galling insects and leaf-feeding insects: The role of plant phenolic compounds and their possible interference with herbivores. J. Trop. Ecol. 2008, 24, 329–336. [Google Scholar] [CrossRef]
- Weis, A.E.; Walton, R.; Crego, C.L. Reactive plant tissue sites and the population biology of gall makers. Ann. Rev. Entomol. 1988, 33, 467–486. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Siebe, C.; Martínez-Ramos, M.; Oyama, K. Species richness of gall-forming insects in a tropical rain forest: Correlations with plant diversity and soil fertility. Biodivers. Conserv. 2003, 12, 411–422. [Google Scholar] [CrossRef]
- Waring, G.L.; Price, P.W. Plant water stress and gall formation (Cecidomyiidae: Asphondylia spp.) on creosote bush. Ecol. Entomol. 1990, 15, 87–95. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Price, P.W. The adaptive significance of insect gall distribution: Survivorship of species in xeric and mesic habitats. Oecologia 1992, 90, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Lawton, J.H. Host-plant manipulation by gall-insects: A test of the nutrition hypothesis. J. Anim. Ecol. 1992, 61, 113–119. [Google Scholar] [CrossRef]
- Schmitz, O.J. Herbivory from individuals to ecosystems. Ann. Rev. Ecol. Evol. Syst. 2006, 39, 133–152. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Quesada, M.; Oyama, K. Abundance and Leaf Damage Caused by Gall-Inducing Insects in a Mexican Tropical Dry Forest. Biotropica 2006, 38, 107–115. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Zangrel, A.R. Plant–insect interactions in terrestrial ecosystems. In Plant–Animal Interactions—An Evolutionary Approach; Herrera, C.M., Pellmyr, O., Eds.; Blackwell Science: Hoboken, NJ, USA, 2002; pp. 77–106. [Google Scholar]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. R. Soc. B 2006, 273, 2575–2584. [Google Scholar] [CrossRef]
- Coutinho, R.D.; Cuevas-Reyes, P.; Fernandes, G.W.; Fagundes, M. Community structure of gall-inducing insects associated with a tropical shrub: Regional, local and individual patterns. Trop. Ecol. 2019, 60, 74–82. [Google Scholar] [CrossRef]
- Calixto, E.S.; Maron, J.L.; Hahn, P.G. Interactions between large-scale and local factors influence seed predation rates and seed loss. Ecol. Evol. 2023, 13, e10208. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S. Life history evolution: Successes, limitations, and prospects. Sci. Nat. 2000, 87, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Campbell, A.S.; Barret, S.C.H. Evolutionary interactions between plant reproduction and defense against herbivores. Ann. Rev. Ecol. Evol. Syst. 2015, 46, 191–213. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Hastings, A.P.; Johnson, M.T.; Maron, J.L.; Salminen, J.P. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 2012, 338, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.G.; Maron, J.L. A framework for predicting intraspecific variation in plant defense. Trends Ecol. Evol. 2016, 31, 646–656. [Google Scholar] [CrossRef] [PubMed]
- López-Goldar, X.; Zas, R.; Sampedro, L. Resource availability drives microevolutionary patterns of plant defences. Funct. Ecol. 2020, 34, 1640–1652. [Google Scholar] [CrossRef]
- Seixas, L.; Demetrio, G.R.; Barão, K.R.; Cornelissen, T.G. Temporal co-occurrence of leaf herbivory by chewers, leaf miners and gall-formers on a tropical tree: Do leaf traits matter? Arthropod-Plant Interact. 2024, 18, 533–546. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- van Velzen, E.; Etienne, R.S. The importance of ecological costs for the evolution of plant defense against herbivory. J. Theor. Biol. 2015, 372, 89–99. [Google Scholar] [CrossRef]
- Foss, L.K.; Rieske, L.K. Stem galls affect oak foliage with potential consequences for herbivory. Ecol. Entomol. 2004, 29, 273–280. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Rupp, M.J. Phytochemical Induction by Herbivores; Wiley-Interscience: New York, NY, USA, 1991; 481p. [Google Scholar]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Dicke, M.; van Loon, J.J.A. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef]
- Fernandes, G.W. Hypersensitivity: A neglected plant resistance mechanism against insect herbivores. Environ. Entomol. 1990, 19, 1173–1182. [Google Scholar] [CrossRef]
- Fernandes, G.W. Hypersensitivity as a phenotypic basis of plant induced resistance against a galling insect (Diptera: Cecidomyiidae). Environ. Entomol. 1998, 27, 260–267. [Google Scholar] [CrossRef]
- Fritig, B.; Kauffmann, S.; Dumas, B.; Geoffroy, P.; Kopp, M.; Legrand, M. Mechanism of the hypersensitivity reaction of plants. In Ciba Foundation Symposium 133-Plant Resistance to Virus; Evered, D., Harnett, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 92–108. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Negreiros, D. The occurrence and effectiveness of hypersensitive reaction against galling herbivores across host taxa. Ecol. Entomol. 2001, 26, 46–55. [Google Scholar] [CrossRef]
- Cornelissen, T.G.; Negreiros, D.; Fernandes, G.W. Plant resistance against gall-forming insects: The role of hypersensitivity. In Mechanisms and Deployment of Resistance in Trees to Insects; Wagner, M.R., Clancy, K.M., Lieutier, F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 137–152. [Google Scholar]
- Santos, J.C.; Fernandes, G.W. Mediation of herbivore attack and induced resistance by plant vigor and ontogeny. Acta Oecol. 2010, 36, 617–625. [Google Scholar] [CrossRef]
- Maldonado-López, Y.; Cuevas-Reyes, P.; Stone, G.N.; Nieves-Aldrey, J.L.; Oyama, K. Gall wasp community response to fragmentation of oak tree species: Importance of fragment size and isolated trees. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Maldonado-López, Y.; Cuevas-Reyes, P.; Oyama, K. Diversity of gall wasps (Hymenoptera: Cynipidae) associated with oak trees (Fagaceae: Quercus) in a fragmented landscape in Mexico. Arthropod Plant Interact. 2016, 10, 29–39. [Google Scholar] [CrossRef]
- Agrawal, A.A. Herbivory and maternal effects: Mechanisms and consequences of transgenerational induced plant resistance. Ecology 2002, 83, 3408–3415. [Google Scholar] [CrossRef]
- Holeski, L.M.; Jander, G.; Agrawal, A.A. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 2012, 27, 618–626. [Google Scholar] [CrossRef]
- Pieterse, C.M.J. Prime Time for Transgenerational Defense. Plant Physiol. 2012, 158, 545. [Google Scholar] [CrossRef]
- Colicchio, J. Transgenerational effects alter plant defence and resistance in nature. J. Evol. Biol. 2017, 30, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.; Sampedro, L.; Neylan, I.; Siemens, D.; Dirzo, R. Phenotypic plasticity in plant defense across life stages: Inducibility, transgenerational induction, and transgenerational priming in wild radish. Proc. Natl. Acad. Sci. USA 2021, 118, e2005865118. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Fishbein, M. Plant defense syndromes. Ecology 2006, 87, S132–S149. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Strong, D.R. Oviposition choice and larval survival of Dasineura marginemtorquens (Diptera, Cecidomyiidae) on resistant and susceptible Salix viminalis. Ecol. Entomol. 1992, 1, 227–232. [Google Scholar] [CrossRef]
- Marini-Filho, O.J.; Fernandes, G.W. Stem galls drain nutrients and decrease shoot performance in Diplusodon orbicularis (Lythraceae). Arthropod-Plant Interact. 2012, 6, 121–128. [Google Scholar] [CrossRef]
- Santos, J.C.; Alves-Silva, E.; Cornelissen, T.G.; Fernandes, G.W. The effect of fluctuating asymmetry and leaf nutrients on gall abundance and survivorship. Basic Appl. Ecol. 2013, 14, 489–495. [Google Scholar] [CrossRef]
- Agrawal, A.A. Induced responses to herbivory and increased plant performance. Science 1998, 279, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Simms, E.L. Defining tolerance as a norm of reaction. Evol. Ecol. 2000, 14, 563–570. [Google Scholar] [CrossRef]
- Fornoni, J.; Valverde, P.L.; Núñez-Farfán, J. Evolutionary ecology of tolerance to herbivory: Advances and perspectives. Comments Theor. Biol. 2003, 8, 643–663. [Google Scholar]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.R.; Silveira FA, O.; Santos, J.C.; Rosa, L.H.; Cares, J.E.; Café-Filho, A.C.; Fernandes, G.W. Nematode-induced galls in Miconia albicans: Effect of host plant density and correlations with performance. Plant Species Biol. 2013, 28, 63–69. [Google Scholar] [CrossRef]
- Giron, D.; Huguet, E.; Stone, G.N.; Body, M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J. Insect Physiol. 2016, 84, 70–89. [Google Scholar] [CrossRef]
- Santos, J.C.; de Araujo NA, V.; Silva, H.V.; Andrade, J.F.; Silva, E.A.; Almeida, W.R.; Carmo-Oliveira, R. How detrimental are seed galls to their hosts? Plant performance, germination, developmental instability, and tolerance to herbivory in Inga laurina, a leguminous tree. Plant Biol. 2016, 18, 962–972. [Google Scholar] [CrossRef]
- Mahlobo, T.; Dube, N.; Zachariades, C.; Munyai, T.C. Impact of the gall-inducing fly Polymorphomyia basilica Snow (Diptera:Tephritidae) on the growth and reproduction of Chromolaena odorata (L.) RM King & H. Rob (Asteraceae) in the laboratory. Arthropod-Plant Interact. 2023, 17, 639–646. [Google Scholar] [CrossRef]
- Shen, C.S.; Bach, C.E. Genetic variation in resistance and tolerance to insect herbivory in Salix Cordata. Ecol. Entomol. 1997, 22, 335–342. [Google Scholar] [CrossRef]
- Bingham, R.A.; Agrawal, A.A. Specificity and trade-offs in the induced plant defence of common milkweed Asclepias syriaca to two lepidopteran herbivores. J. Ecol. 2010, 98, 1014–1022. [Google Scholar] [CrossRef]
- Cornelissen, T.G.; Fernandes, G.W. Patterns of attack by herbivores on the tropical shrub Bauhinia brevipes (Leguminosae): Vigour or chance? Eur. J. Entomol. 2001, 98, 37–40. [Google Scholar] [CrossRef]
- Agrawal, A.A. Future directions in the study of induced plant responses to herbivory. Entomol. Exp. Appl. 2005, 115, 97–105. [Google Scholar] [CrossRef]
- Mundim, F.M.; Vieira-Neto, E.H.M.; Alborn, H.; Bruna, E.M. Disentangling the influence of water limitation and simultaneous above and belowground herbivory on plant tolerance and resistance to stress. J. Ecol. 2021, 109, 2729–2739. [Google Scholar] [CrossRef]
- Garcia, L.C.; Eubanks, M.D. Overcompensation for insect herbivory: A review and meta-analysis of the evidence. Ecology 2019, 100, e02585. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Alves-Silva, E.; Cornelissen, T.G.; Fernandes, G.W. Differences in leaf nutrients and developmental instability in relation to induced resistance to a gall midge. Arthropod-Plant Interact. 2017, 11, 163–170. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Perfect is best: Low leaf fluctuating asymmetry reduces herbivory by leaf miners. Oecologia 2005, 142, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Sullivan, J.J. The impact of herbivory on plants in different resource conditions: A meta-analysis. Ecology 2001, 82, 204–2058. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Calixto, E.S.; Novaes, L.R.; Santos, D.F.B.; Lange, D.; Moreira, X.; Del-Claro, K. Climate seasonality drives ant-plant-herbivore interactions via plant phenology in an extrafloral nectary-bearing plant community. J. Ecol. 2021, 109, 639–651. [Google Scholar] [CrossRef]
- Jacobucci, G.B.; Oliveira, P.E.; Costa, A.N. Aspectos da História Natural da Reserva Ecológica do Panga, 1st ed.; Editora UFU: Uberlândia, Brazil, 2023; 246p. [Google Scholar]
- Vaz, A.M.S.F.; Tozzi, A.M.G.A. Bauhinia ser. Cansenia (Leguminosae: Caesalpinioideae) no Brasil. Rodriguésia 2003, 54, 55–143. [Google Scholar] [CrossRef]
- Silveira, F.A.O.; Santos, J.C.; Franceschinelli, E.V.; Morellato, L.P.C.; Fernandes, G.W. Costs and benefits of reproducing under unfavorable conditions: An integrated view of ecological and physiological constraints in a cerrado shrub. Plant Ecol. 2015, 216, 963–974. [Google Scholar] [CrossRef]
- Maia, V.C.; Fernandes, G.W. Two new species of Asphondyliini (Diptera: Cecidomyiidae) associated with Bauhinia brevipes (Fabaceae) in Brazil. Zootaxa 2005, 40, 27–40. [Google Scholar] [CrossRef]
- Sá, C.E.M.; Silveira, F.A.O.; Santos, J.C.; Isaias, R.M.D.S.; Fernandes, G.W. Anatomical and developmental aspects of leaf galls induced by Schizomyia macrocapillata Maia (Diptera:Cecidomyiidae) on Bauhinia brevipes Vogel (Fabaceae). Braz. J. Bot. 2009, 32, 319–327. [Google Scholar] [CrossRef]
- Santos, J.C.; Silveira, F.A.O.; Fernandes, G.W. Long term oviposition preference and larval performance of Schizomyia macrocapillata (Diptera: Cecidomyiidae) on larger shoots of its host plant Bauhinia brevipes (Fabaceae). Evol. Ecol. 2008, 22, 123–137. [Google Scholar] [CrossRef]
- Pashalidou, F.G.; Lucas-Barbosa, D.; van Loon, J.J.A.; Dicke, M.; Fatouros, N.E. Phenotypic plasticity of plant response to herbivore eggs: Effects on resistance to caterpillars and plant development. Ecology 2013, 94, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Detoni, M.L.; Vasconcelos, E.G.; Maia, A.C.R.G.; Gusmão, M.A.N.; Isaias, R.M.S.; Soares, G.L.G.; Santos, J.C.; Fernandes, G.W. Protein content and electrophoretic profile of insect galls on susceptible and resistant host plants of Bauhinia brevipes Vogel (Fabaceae). Aust. J. Bot. 2011, 59, 509–514. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Leaf-Mining Insects and Fluctuating Asymmetry in Elm Ulmus glabra Leaves. J. Anim. Ecol. 1995, 64, 697–707. [Google Scholar] [CrossRef]
- Yezerinac, S.M.; Lougheed, S.C.; Handford, P. Measurement error and morphometric studies: Statistical power and observer experience. Syst. Biol. 1992, 41, 471–482. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Similar responses of insect herbivores to leaf fluctuating asymmetry. Arthropod Plant Interact. 2011, 5, 59–69. [Google Scholar] [CrossRef]
- Santana, D.G.; Lobo, G.A.; Salomão, A.N.; Pereira, V.J. Robustness of germination analysis methods for Copaifera langsdorffii Desf. (Fabaceae) seeds. Biosci. J. 2016, 32, 160–171. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Zanotti, R.F.; Dias, D.C.F.S.; Barros, R.S.; DaMatta, F.M.; Oliveira, G.L. Germination and biochemical changes in ‘Formosa’ papaya seeds treated with plant hormones. Acta Sci. Agron. 2014, 36, 435–442. [Google Scholar] [CrossRef]
- Bezerra, A.M.E.; Momenté, V.G.; Medeiros Filho, S. Germinação de sementes e desenvolvimento de plântulas de moringa (Moringa oleifera Lam.) em função do peso da semente e do tipo de substrato. Hortic. Bras. 2004, 22, 295–299. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry analyses revisited. Developmental Instability: Causes and Consequences. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 279–319. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. 2020. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 30 April 2024).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Newcastle upon Tyne, UK, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 30 April 2024).






| Analysis | Response Variable | Distribution | Likelihood Ratio Test | p-Value | Resistant (Mean ± SD) | Susceptible (Mean ± SD) |
|---|---|---|---|---|---|---|
| Gall-related parameters | Number of galled leaves (mean) | Gaussian | 4.474 | 0.034 | 0.54 ± 0.45 | 1.14 ± 0.87 |
| Number of galls | Negative Binomial | 15.908 | 0.001 | 8.08 ± 6.77 | 48.6 ± 40.4 | |
| Number of attacks | Negative Binomial | 8.564 | 0.003 | 16.2 ± 10.8 | 49.9 ± 41.8 | |
| Number of HRs | Negative Binomial | 33.857 | 0.001 | 8.16 ± 4.62 | 1.25 ± 1.76 | |
| Fluctuating asymmetry (mm) | Gaussian | 0.759 | 0.383 | 0.02 ± 0.01 | 0.02 ± 0.01 | |
| Foliar nutrients | Boron (mg·kg−1) | Gaussian | 0.362 | 0.547 | 33.5 ± 11.1 | 36.6 ± 11.8 |
| Calcium (mg·kg−1) | Gaussian | 0.605 | 0.436 | 11.1 ± 2.71 | 12.1 ± 2.85 | |
| Copper (mg·kg−1) | Gaussian | 0.470 | 0.492 | 5.9 ± 0.5 | 6.3 ± 1.5 | |
| Iron (mg·kg−1) | Gaussian | 0.527 | 0.467 | 72.4 ± 10.4 | 75.4 ± 7.6 | |
| Magnesium (mg·kg−1) | Gaussian | 8.758 | 0.003 | 2.8 ± 0.4 | 3.4 ± 0.4 | |
| Manganese (mg·kg−1) | Gaussian | 0.154 | 0.694 | 99.9 ± 44.0 | 108.3 ± 50.8 | |
| Nitrogen (mg·kg−1) | Gaussian | 0.009 | 0.921 | 18.8 ± 5.3 | 18.6 ± 5.9 | |
| Phosphorus (mg·kg−1) | Gaussian | 0.101 | 0.750 | 1.0 ± 0.1 | 0.9 ± 0.1 | |
| Potassium (mg·kg−1) | Gaussian | 0.001 | 0.99 | 10.1 ± 1.0 | 10.1 ± 1.6 | |
| Sulfur (mg·kg−1) | Gaussian | 0.164 | 0.685 | 0.76 ± 0.1 | 0.79 ± 0.1 | |
| Zinc (mg·kg−1) | Gaussian | 2.109 | 0.146 | 15.6 ± 2.3 | 17.2 ± 2.8 | |
| Vegetative plant traits | Stem diameter (mm) | Gaussian | 2.519 | 0.112 | 13.3 ± 4.45 | 16.3 ± 4.93 |
| Canopy diameter (m) | Gaussian | 4.533 | 0.033 | 0.78 ± 0.26 | 1.14 ± 0.51 | |
| Number of branches | Negative Binomial | 1.097 | 0.294 | 147.5 ± 62.8 | 179.0 ± 65.8 | |
| Number of leaves | Negative Binomial | 6.013 | 0.014 | 679.1 ± 373.4 | 1452 ± 1300 | |
| Leaf area (cm2) | Gaussian | 0.063 | 0.801 | 7.77 ± 1.96 | 7.53 ± 2.63 | |
| Leaf length (cm) | Gaussian | 0.792 | 0.373 | 3.95 ± 0.94 | 4.28 ± 0.90 | |
| Reproductive plant traits | Number of inflorescences | Negative Binomial | 0.039 | 0.843 | 2.16 ± 2.48 | 1.91 ± 2.81 |
| Number of flowers | Negative Binomial | 0.005 | 0.938 | 5.50 ± 6.68 | 5.16 ± 8.34 | |
| Number of fruits | Negative Binomial | 0.021 | 0.882 | 5.16 ± 6.17 | 4.58 ± 8.46 | |
| Number of seeds | Negative Binomial | 0.678 | 0.410 | 92.0 ± 73.3 | 132.6 ± 115.3 | |
| Seedling parameters | Viable seed ratio | Beta | 1.965 | 0.160 | 0.66 ± 0.21 | 0.54 ± 0.11 |
| Epicotyl (mm) | Gaussian | 18.108 | 0.001 | 18.2 ± 6.0 | 12.7 ± 4.9 | |
| Hypocotyl (mm) | Gaussian | 1.573 | 0.209 | 3.8 ± 0.7 | 3.6 ± 0.6 | |
| Root (mm) | Gaussian | 0.335 | 0.562 | 24.7 ± 7.4 | 23.5 ± 10.1 | |
| Dry weight (mg) | Gaussian | 0.282 | 0.595 | 0.02 ± 0.01 | 0.02 ± 0.01 | |
| Sapling parameters | Root weight (mg) | Gaussian | 2.096 | 0.147 | 0.58 ± 0.2 | 0.69 ± 0.3 |
| Aerial part weight (mg) | Gaussian | 5.407 | 0.020 | 0.37 ± 0.13 | 0.45 ± 0.18 | |
| Root length (cm) | Gamma | 0.001 | 0.976 | 16.2 ± 2.2 | 16.2 ± 2.9 | |
| Aerial part length (cm) | Gaussian | 6.813 | 0.009 | 17.3 ± 3.3 | 19.5 ± 4.0 | |
| Xylopodium diameter (mm) | Gaussian | 5.081 | 0.024 | 2.9 ± 0.7 | 3.4 ± 0.8 | |
| Fluctuating asymmetry (mm) | Gaussian | 3.316 | 0.068 | 0.15 ± 0.07 | 0.19 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, J.F.; Calixto, E.S.; Demetrio, G.R.; Venâncio, H.; Meiado, M.V.; de Santana, D.G.; Cuevas-Reyes, P.; de Almeida, W.R.; Santos, J.C. Tolerance Mitigates Gall Effects When Susceptible Plants Fail to Elicit Induced Defense. Plants 2024, 13, 1472. https://doi.org/10.3390/plants13111472
Andrade JF, Calixto ES, Demetrio GR, Venâncio H, Meiado MV, de Santana DG, Cuevas-Reyes P, de Almeida WR, Santos JC. Tolerance Mitigates Gall Effects When Susceptible Plants Fail to Elicit Induced Defense. Plants. 2024; 13(11):1472. https://doi.org/10.3390/plants13111472
Chicago/Turabian StyleAndrade, Janete Ferreira, Eduardo Soares Calixto, Guilherme Ramos Demetrio, Henrique Venâncio, Marcos Vinicius Meiado, Denise Garcia de Santana, Pablo Cuevas-Reyes, Wanessa Rejane de Almeida, and Jean Carlos Santos. 2024. "Tolerance Mitigates Gall Effects When Susceptible Plants Fail to Elicit Induced Defense" Plants 13, no. 11: 1472. https://doi.org/10.3390/plants13111472
APA StyleAndrade, J. F., Calixto, E. S., Demetrio, G. R., Venâncio, H., Meiado, M. V., de Santana, D. G., Cuevas-Reyes, P., de Almeida, W. R., & Santos, J. C. (2024). Tolerance Mitigates Gall Effects When Susceptible Plants Fail to Elicit Induced Defense. Plants, 13(11), 1472. https://doi.org/10.3390/plants13111472







