Genetic Control of Tolerance to Drought Stress in Wild Soybean (Glycine soja) at the Vegetative and the Germination Stages
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Distribution
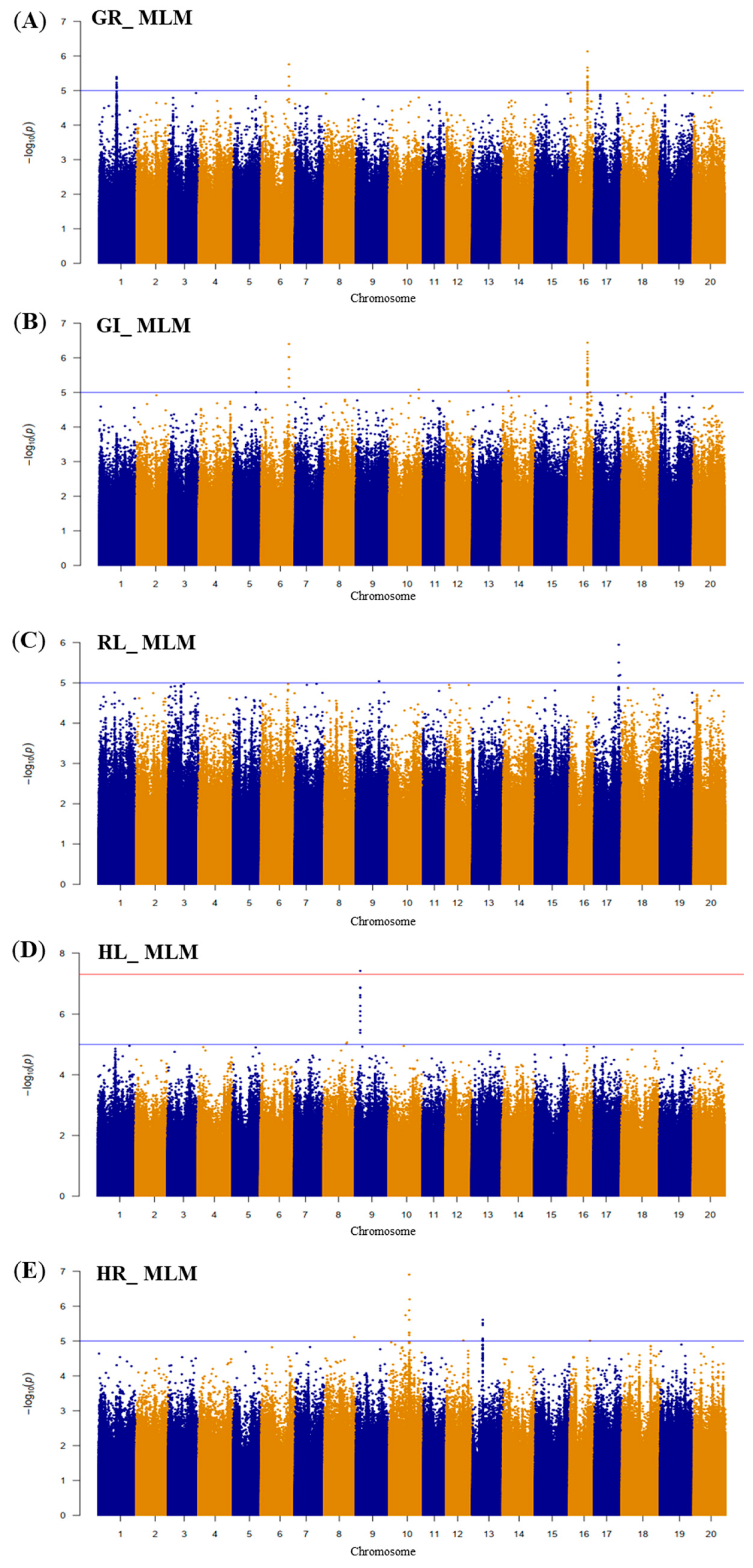
2.2. GWAS Results
2.2.1. SNPs Associated with Drought Tolerance in Wild Soybean at the Vegetative Stage
2.2.2. SNPs Associated with Drought Tolerance in Wild Soybean at the Germination Stage
2.3. Putative Genes Associated with the Significant SNPs for Drought Tolerance
2.3.1. Putative Genes Associated with the Significant SNPs for LWS
2.3.2. Candidate Genes Associated with the Significant SNPs for Germination-Stage Drought-Related Traits
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phenotypic Evaluations
4.2.1. Leaf Wilting Scores (LWSs) of Wild Soybean at the Vegetative Stage
4.2.2. Drought-Related Traits in Wild Soybean at the Germination Stage
4.3. GWAS Analysis
4.4. Putative Candidate Gene Identification
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ravelombola, W.S.; Qin, J.; Shi, A.; Nice, L.; Bao, Y.; Lorenz, A.; Chen, S. Genome-wide association study and genomic selection for tolerance of soybean biomass to soybean cyst nematode infestation. PLoS ONE 2020, 15, e0235089. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.S.; Feng, J.Y.; Ni, Z.J.; Ma, R.H.; Thakur, K.; Wang, S.; Wei, Z.J. An update on the nutritional, functional, sensory characteristics of soy products, and applications of new processing strategies. Trends Food Sci. Tech. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- Frías, E.; Iglesias, Y.; Alvarez-Ordóñez, A.; Prieto, M.; González-Raurich, M.; López, M. Evaluation of cold atmospheric pressure plasma (CAPP) and plasma-activated water (PAW) as alternative non-thermal decontamination technologies for tofu: Impact on microbiological, sensorial and functional quality attributes. Food Res. Int. 2020, 129, 108859. [Google Scholar] [CrossRef] [PubMed]
- Ayman, E.S.; Sorour, S.; Morsi, A.; Islam, M.S.; Ueda, A.; Barutçular, C.; Saneoka, H. Role of osmoprotectants and compost application in improving water stress tolerance in soybean (Glycine max L.). Int. J. Curr. Res. 2016, 8, 25949–25954. [Google Scholar]
- Waqar, A.; Bano, A.; Ajmal, M. Effects of PGPR bioinoculants, hydrogel and biochar on growth and physiology of soybean under drought stress. Commun. Soil Sci. Plant Anal. 2022, 53, 826–847. [Google Scholar] [CrossRef]
- Sadeghi, L.; Rafiee, M.; Daneshian, J. Effect of drought stress and aerosols on yield and some physiological traits of soybean (Glycine max L.). J. Plant Process Function 2021, 10, 263–278. Available online: http://jispp.iut.ac.ir/article-1-1431-en.html (accessed on 1 February 2024).
- Basal, O.; Szabó, A.; Veres, S. Physiology of soybean as affected by PEG-induced drought. Curr. Plant Biol. 2020, 22, 100135. [Google Scholar] [CrossRef]
- Cui, Y.; Ning, S.; Jin, J.; Jiang, S.; Zhou, Y.; Wu, C. Quantitative lasting effects of drought stress at a growth stage on soybean evapotranspiration and aboveground biomass. Water 2020, 13, 18. [Google Scholar] [CrossRef]
- Bazzer, S.K.; Purcell, L.C. Identification of quantitative trait loci associated with canopy temperature in soybean. Sci. Rep. 2020, 10, 17604. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, L.; Du, P.; Xing, X.; Gu, Z.; Yu, Z.; Tao, Y.; Jiang, H. Appropriate Drought Training Induces Optimal Drought Tolerance by Inducing Stepwise H2O2 Homeostasis in Soybean. Plants 2024, 13, 1202. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Purcell, L.C.; Brye, K.R. Differential wilting among soybean genotypes in response to water deficit. Crop Sci. 2009, 49, 290–298. [Google Scholar] [CrossRef]
- Ries, L.L.; Purcell, L.C.; Carter, T.E., Jr.; Edwards, J.T.; King, C.A. Physiological traits contributing to differential canopy wilting in soybean under drought. Crop Sci. 2012, 52, 272–281. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Zwieniecki, M.A.; Holbrook, N.M. Holbrook. Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiol. Plant. 2008, 132, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.J.; Sinclair, T.R. Nitrogen Fixation Drought Tolerance of the Slow-Wilting Soybean PI 471938. Crop Sci. 2013, 53, 2072–2078. [Google Scholar] [CrossRef]
- Du, W.; Wang, M.; Fu, S.; Yu, D. Mapping QTLs for seed yield and drought susceptibility index in soybean (Glycine max L.) across different environments. J. Genet. Genom. 2009, 36, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yu, D.; Fu, S. Detection of quantitative trait loci for yield and drought tolerance traits in soybean using a recombinant inbred line population. J. Integr. Plant Biol. 2009, 51, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, H.; Lee, G.J.; Boerma, R.H. Identification of QTL for increased fibrous roots in soybean. Theor. Appl. Genet. 2011, 122, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.A.R.; Bailey, M.A.; Ashley, D.A.; Wells, R.; Carter, T.E., Jr.; Parrott, W.A.; Boerma, H.R. Molecular markers associated with water use efficiency and leaf ash in soybean. Crop Sci. 1996, 36, 1252–1257. [Google Scholar] [CrossRef]
- Specht, J.E.; Chase, K.; Macrander, M.; Graef, G.L.; Chung, J.; Markwell, J.P.; Germann, M.; Orf, J.H.; Lark, K.G. Soybean response to water. A QTL analysis of drought tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Abdel-Haleem, H.; Carter, T.E.; Purcell, L.C.; King, C.A.; Ries, L.L.; Chen, P.; Schapaugh, W., Jr.; Sinclair, T.R.; Boerma, H.R. Mapping of quantitative trait loci for canopy-wilting trait in soybean (Glycine max L. Merr). Theor. Appl. Genet. 2012, 125, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Charlson, D.V.; Bhatnagar, S.; King, C.A.; Ray, J.D.; Sneller, C.H.; Carter, T.E.; Purcell, L.C. Polygenic inheritance of canopy wilting in soybean [Glycine max (L.) Merr.]. Theor. Appl. Genet. 2009, 119, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; King, C.A.; Chen, P.; Ray, J.D.; Cregan, P.B.; Carter, T.E., Jr.; Li, Z.; Abdel-Haleem, H.; Matson, K.; Schapaugh, W., Jr.; et al. Confirmation of delayed canopy wilting QTLs from multiple soybean mapping populations. Theor. Appl. Genet. 2015, 128, 2047–2065. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; King, C.A.; Chen, P.; Ray, J.D.; Cregan, P.B.; Carter, T.E.; Purcell, L.C. Meta-analysis to refine map position and reduce confidence intervals for delayed-canopy-wilting QTLs in soybean. Mol. Breed. 2016, 36, 91. [Google Scholar] [CrossRef]
- Kaler, A.S.; Ray, J.D.; Schapaugh, W.T.; King, C.A.; Purcell, L.C. Genome-wide association mapping of canopy wilting in diverse soybean genotypes. Theor. Appl. Genet. 2017, 130, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Steketee, C.J.; Schapaugh, W.T.; Carter, T.E., Jr.; Li, Z. Genome-wide association analyses reveal genomic regions controlling canopy wilting in soybean. G3 Genes Genomes Genet. 2020, 10, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Gou, Z.; Zhang, Y.; Wang, X.; Ren, H.; Wen, H.; Kang, B.K.; Li, Y.; Yu, L.; et al. Genome-wide association study of soybean seed germination under drought stress. Mol. Genet. Genom. 2020, 295, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Roldán-Ruiz, I.; Aper, J.; Muylle, H. Genetic control of tolerance to drought stress in soybean. BMC Plant Biol. 2022, 22, 615. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Li, H.; Zhang, Y.; Yu, L.; Qi, X.; Gao, H.; Li, Y.; Qiu, L. Identification of Drought-Tolerance Genes in the Germination Stage of Soybean. Biology 2022, 11, 1812. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Li, S.; Zhang, H.; Liu, X.; Cui, X.; Song, L.; Zhu, Y.; Chen, X.; Chen, H. GmAOC4 modulates seed germination by regulating JA biosynthesis in soybean. Theor. Appl. Genet. 2022, 135, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, Y.; Zheng, J.; Wu, D.; Li, C.; Li, Z.; Zang, Z.; Zhang, Y.; Fang, Q.; Li, W.; et al. A nuclear factor YB transcription factor, GmNFYB17, regulates resistance to drought stress in soybean. Int. J. Mol. Sci. 2022, 23, 7242. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; Fritschi, F.B. Genome-wide association analysis of diverse soybean genotypes reveals novel markers for nitrogen traits. Plant Genome. 2015, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaler, A.S.; Ray, J.D.; Schapaugh, W.T.; Asebedo, A.R.; King, C.A.; Gbur, E.E.; Purcell, L.C. Association mapping identifies loci for canopy temperature under drought in diverse soybean genotypes. Euphytica 2018, 214, 135. [Google Scholar] [CrossRef]
- Chamarthi, S.K.; Kaler, A.S.; Abdel-Haleem, H.; Fritschi, F.B.; Gillman, J.D.; Ray, J.D.; Smith, J.R.; Purcell, L.C. Identification of genomic regions associated with the plasticity of carbon 13 ratio in soybean. Plant Genome 2023, 16, e20284. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.C.; Jo, H.; Tran, H.A.; Lee, J.; Lee, J.-D.; Kim, J.H.; Seo, H.S.; Song, J.T. Assessment of Drought Responses of Wild Soybean Accessions at Different Growth Stages. Agronomy 2024, 14, 471. [Google Scholar] [CrossRef]
- Sloane, R.J.; Patterson, R.P.; Carter, T.E., Jr. Field drought tolerance of a soybean plant introduction. Crop Sci. 1990, 30, 118–123. [Google Scholar] [CrossRef]
- Kunert, K.; Vorster, B.J. In search for drought-tolerant soybean: Is the slow-wilting phenotype more than just a curiosity? J. Exp. Bot. 2020, 71, 457–460. [Google Scholar] [CrossRef]
- Kim, W.J.; Kang, B.H.; Moon, C.Y.; Kang, S.; Shin, S.; Chowdhury, S.; Jeong, S.-C.; Choi, M.-S.; Park, S.-K.; Moon, J.-K.; et al. Genome-Wide Association Study for Agronomic Traits in Wild Soybean (Glycine soja). Agronomy 2023, 13, 739. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, M.Y.; Yang, X.; Lee, S.H. Unveiling synergistic QTLs associated with slow wilting in soybean (Glycine max [L.] Merr.). Theor. Appl. Genet. 2024, 137, 85. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Shekoofa, A.; McClure, A.; Gillman, J.D. Phenotyping and quantitative trait locus analysis for the limited transpiration trait in an upper-mid south soybean recombinant inbred line population (“Jackson” × “KS4895”): High throughput aquaporin inhibitor screening. Front. Plant Sci. 2022, 12, 779834. [Google Scholar] [CrossRef] [PubMed]
- Chamarthi, S.K.; Kaler, A.S.; Abdel-Haleem, H.; Fritschi, F.B.; Gillman, J.D.; Ray, J.D.; Smith, J.R.; Dhanapal, A.P.; King, C.A.; Purcell, L.C. Identification and Confirmation of Loci Associated with Canopy Wilting in Soybean Using Genome-Wide Association Mapping. Front. Plant Sci. 2021, 12, 698116. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Feng, R.; He, Y.; Cao, F.; Zhao, Y.; Zhou, J.; Zhai, H.; Bai, X. Genome-Wide Identification and Characterization of Copper Chaperone for Superoxide Dismutase (CCS) Gene Family in Response to Abiotic Stress in Soybean. Int. J. Mol. Sci. 2023, 24, 5154. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Wang, Y.; Sun, W.; Lou, Q.; Mei, H.; Shen, S.; Chen, H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Evaristo de Deus, K.; Lanna, A.C.; Abreu, F.R.M.; Dias Silveira, R.D.; Jacinto Pereira, W.; Brondani, C.; Pereira Vianello, R. Molecular and biochemical characterization of superoxide dismutase (SOD) in upland rice under drought. Aust. J. Crop Sci. 2015, 9, 744–753. [Google Scholar]
- Lu, Y.Y.; Deng, X.P.; Kwak, S.S. Over expression of CuZn superoxide dismutase (CuZnSOD) and ascorbate peroxidase (APX) in transgenic sweet potato enhances tolerance and recovery from drought stress. Afr. J. Biotechnol. 2010, 9, 8378–8391. [Google Scholar]
- Wang, H.; Liu, S.; Fan, F.; Yu, Q.; Zhang, P.A. Moss 2-oxoglutarate/Fe(ii)-dependent dioxygenases (2-ODD) gene of flavonoids biosynthesis positively regulates plants abiotic stress tolerance. Front. Plant Sci. 2022, 13, 850062. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, A.; Arumugam, C.; Punchakkara, P.M.; Suthanthiram, B.; Raman, T.; Subbaraya, U. Genome-wide characterization of 2OGD superfamily for mining of susceptibility factors responding to various biotic stresses in Musa spp. Physiol. Mol. Biol. Plants 2023, 29, 1319–1338. [Google Scholar] [CrossRef]
- Zang, D.; Li, H.; Xu, H.; Zhang, W.; Zhang, Y.; Shi, X.; Wang, Y. An Arabidopsis zinc finger protein increases abiotic stress tolerance by regulating sodium and potassium homeostasis, reactive oxygen species scavenging and osmotic potential. Front. Plant Sci. 2016, 7, 1272. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Cao, X.Y.; Chen, M.; Zhang, X.K.; Liu, Y.N.; Xu, Z.S.; Li, L.C.; Ma, Y.Z. Isolation and expression pattern assay of a C3HC4-type RING zinc finger protein gene GmRZFP1 in Glycine max (L.). Plant Genet. Res. 2010, 11, 343–348. [Google Scholar]
- Moursi, Y.S.; Thabet, S.G.; Amro, A.; Dawood, M.F.A.; Baenziger, P.S.; Sallam, A. Detailed Genetic Analysis for Identifying QTLs Associated with Drought Tolerance at Seed Germination and Seedling Stages in Barley. Plants 2020, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Moon, J.K.; Park, S.K.; Kim, M.S.; Lee, K.; Lee, S.R.; Park, E. Genetic diversity patterns and domestication origin of soybean. Theor. Appl. Genet. 2019, 132, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lozano, R.; Kim, J.H.; Bae, D.N.; Kim, S.T.; Park, J.H.; Choi, M.S.; Kim, J.; Ok, H.C.; Park, S.K.; et al. The patterns of deleterious mutations during the domestication of soybean. Nat. Commun. 2021, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.D. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. Biorxiv 2008, 3, 731. [Google Scholar] [CrossRef]
- Talebi, R.; Fayaz, F.; Naji, N. Effective selection criteria for assessing drought stress tolerance in durum wheat (Triticum durum Desf.). Gen. App. Plant Physiol. 2009, 35, 64–74. [Google Scholar]


| Traits | Source of Variation | Degree of Freedom | Sum of Squares | Mean Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|---|---|
| LWS | Accession | 186 | 501.810 | 2.700 | 195.9 | <0.0001 |
| Replication | 2 | 0.040 | 0.020 | 1.6 | 0.206 | |
| GR | Accession | 134 | 142,396.100 | 1062.657 | 1.4 | <0.0001 |
| Replication | 2 | 2359.717 | 1179.858 | 1.5 | 0.321 | |
| GI | Accession | 134 | 11.916 | 0.089 | 0.7 | <0.0001 |
| Replication | 2 | 0.309 | 0.154 | 1.2 | 0.389 | |
| RL | Accession | 134 | 351.931 | 2.646 | 34.7 | <0.0001 |
| Replication | 2 | 0.360 | 0.180 | 2.4 | 0.299 | |
| HL | Accession | 134 | 53.692 | 0.407 | 16.0 | <0.0001 |
| Replication | 2 | 0.035 | 0.018 | 0.7 | 0.504 | |
| HR | Accession | 134 | 7.041 | 0.053 | 15.8 | <0.0001 |
| Replication | 2 | 0.007 | 0.004 | 1.1 | 0.346 |
| GR | GI | RL | HL | HR | |
|---|---|---|---|---|---|
| GR | 1 | ||||
| GI | 0.956 ** | 1 | |||
| RL | 0.280 ** | 0.257 ** | 1 | ||
| HL | 0.245 ** | 0.236 ** | 0.450 ** | 1 | |
| HR | 0.024 | 0.045 | −0.403 ** | 0.438 ** | 1 |
| Trait | Chr | Physical Position | −log10(p) | Mean LWS Associated with the SNP Allele | t-Test | MAF | Allelic Effect | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A (n) | T (n) | C (n) | G (n) | |||||||
| LWS | 10 | 11,361,356 | 7.4 | 4.46 (136) | 3.63 (35) | <0.0001 | 0.23 | −0.47 | ||
| 10 | 11,383,213 | 7.11 | 4.5 (129) | 3.5 (22) | <0.0001 | 0.21 | 0.51 | |||
| 11 | 26,601,868 | 26.26 | 2.71 (7) | 4.38 (172) | <0.0001 | 0.06 | 1.04 | |||
| 19 | 34,790,292 | 7.65 | 3.64 (42) | 4.48 (134) | <0.0001 | 0.25 | 0.44 | |||
| 19 | 34,790,013 | 7.39 | 3.69 (45) | 4.47 (133) | <0.0001 | 0.26 | 0.42 | |||
| 19 | 34,789,961 | 7.39 | 4.48 (131) | 3.72 (46) | <0.0001 | 0.15 | −0.78 | |||
| 19 | 34,797,069 | 7.34 | 4.50 (129) | 3.73 (44) | <0.0001 | 0.16 | −0.74 | |||
| 19 | 34,790,351 | 7.28 | 3.73 (44) | 4.50 (129) | <0.0001 | 0.27 | 0.42 | |||
| SNP | Genotypes | |||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 (Reference) | |
| D10_11361356 | T | T | A | A | T | T | A | A |
| D11_26601868 | A | A | A | A | G | G | G | G |
| D19_34790292 | G | C | G | C | C | G | G | C |
| Number of accessions | 1 | 3 | 2 | 3 | 10 | 20 | 98 | 18 |
| LWS ± SD | 2.00 ± 0.00 nd | 2.00 ± 0.87 *** | 2.50 ± 0.00 *** | 2.33 ± 0.57 *** | 3.42 ± 0.74 *** | 3.80 ± 1.11 *** | 4.72 ± 0.46 ns | 4.61 ± 0.50 |
| Trait | Chr | Physical Position | −log10(p) | Mean Trait Score Associated with the SNP Allele | t-Test | MAF | Allelic Effect | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A (n) | T (n) | C (n) | G (n) | |||||||
| GR | 16 | 28,071,218 | 8.04 | 65.96 (76) | 45.84 (37) | <0.0001 | 0.35 | −10.60 | ||
| 16 | 34,049,144 | 7.29 | 22.71 (7) | 61.56 (123) | <0.0001 | 0.07 | −19.53 | |||
| GI | 6 | 39,541,088 | 14.75 | 0.77 (124) | 0.23 (6) | <0.0001 | 0.06 | −0.19 | ||
| 6 | 40,697,687 | 9.65 | 0.77 (13) | 0.72 (95) | 0.69 | 0.20 | 0.08 | |||
| 16 | 28,071,218 | 7.99 | 0.86 (76) | 0.46 (37) | <0.0001 | 0.35 | −0.11 | |||
| RL | 17 | 36,893,010 | 7.42 | 2.93 (126) | 5.33 (6) | <0.0001 | 0.05 | 1.30 | ||
| HL | 9 | 7,627,321 | 25.66 | 1.35 (124) | 1.88 (8) | 0.009 | 0.06 | 0.70 | ||
| HR | 10 | 30,512,307 | 11.81 | 0.39 (87) | 1.00 (4) | <0.0001 | 0.49 | −0.30 | ||
| 13 | 15,434,946 | 6.99 | 0.37 (122) | 0.78 (9) | 0.025 | 0.08 | 0.14 | |||
| Trait | Chr | SNP | Gene | Start | End | Function (PFAM) |
|---|---|---|---|---|---|---|
| LWS | 10 | D10_11361356 | Glyma.10g087500 | 11,350,383 | 11,356,511 | Alpha/beta hydrolase fold |
| Glyma.10g087600 | 11,364,689 | 11,366,137 | hAT family C-terminal dimerization region | |||
| 11 | D11_26601868 | Glyma.11g192700 | 26,591,332 | 26,595,068 | Copper/zinc superoxide dismutase (SODC) | |
| Glyma.11g192800 | 26,596,271 | 26,599,470 | Helix–loop–helix DNA-binding domain | |||
| Glyma.11g192900 | 26,604,592 | 26,608,357 | Zinc finger, C3HC4 type (RING finger) | |||
| 19 | D19_34797069 | Glyma.19g100900 | 34,806,060 | 34,810,057 | B3 DNA binding domain | |
| GI | 6 | D06_39541088 | Glyma.06G239900 | 39,530,793 | 39,532,182 | Plastocyanin-like domain |
| GR, GI | 16 | D16_28071218 | Glyma.16g128600 | 28,057,444 | 28,062,790 | Protein kinase domain |
| Glyma.16g128700 | 28,077,595 | 28,080,215 | 2OG-Fe (II) oxygenase superfamily | |||
| GR | 16 | D16_34049144 | Glyma.16g179900 | 34,036,942 | 34,040,157 | GRAS domain family |
| RL | 17 | D17_36893010 | Glyma.17g218300 | 36,878,303 | 36,882,265 | Zn-finger in ubiquitin-hydrolases and other protein |
| Glyma.17g218400 | 36,900,654 | 36,906,204 | BT1 family | |||
| HL | 9 | D09_7627321 | Glyma.09g072700 | 7,619,132 | 7,620,402 | Plant invertase/pectin methylesterase inhibitor |
| Glyma.09g072800 | 7,633,793 | 7,634,182 | Unknown | |||
| HR | 10 | D10_30512307 | Glyma.10g119600 | 30,488,462 | 30,491,514 | K+ potassium transporter/DNA polymerase alpha/epsilon subunit B |
| Glyma.10g119700 | 30,491,587 | 30,494,187 | Microtubule-associated protein (MAP65/ASE1 family) | |||
| Glyma.10g119800 | 30,514,616 | 30,515,487 | Homeobox-leucine zipper protein | |||
| Glyma.10g119900 | 3,516,480 | 30,519,657 | Acyltransferase | |||
| Glyma.10g120000 | 30,522,225 | 30,525,686 | LSM domain | |||
| 13 | D13_15434946 | Glyma.13g056700 | 15,423,364 | 15,424,333 | Unknown | |
| Glyma.13g056800 | 15,431,070 | 15,433,820 | UDP-glucoronosyl and UDP-glucosyl transferase | |||
| Glyma.13g05690 | 15,439,351 | 15,446,633 | WD domain, G-beta repeat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.C.; Tran, H.A.; Lee, J.-D.; Seo, H.S.; Jo, H.; Song, J.T. Genetic Control of Tolerance to Drought Stress in Wild Soybean (Glycine soja) at the Vegetative and the Germination Stages. Plants 2024, 13, 1894. https://doi.org/10.3390/plants13141894
Nguyen TC, Tran HA, Lee J-D, Seo HS, Jo H, Song JT. Genetic Control of Tolerance to Drought Stress in Wild Soybean (Glycine soja) at the Vegetative and the Germination Stages. Plants. 2024; 13(14):1894. https://doi.org/10.3390/plants13141894
Chicago/Turabian StyleNguyen, Thi Cuc, Hai Anh Tran, Jeong-Dong Lee, Hak Soo Seo, Hyun Jo, and Jong Tae Song. 2024. "Genetic Control of Tolerance to Drought Stress in Wild Soybean (Glycine soja) at the Vegetative and the Germination Stages" Plants 13, no. 14: 1894. https://doi.org/10.3390/plants13141894





