Treatments with Diquat Reveal the Relationship between Protein Phosphatases (PP2A) and Oxidative Stress during Mitosis in Arabidopsis thaliana Root Meristems
Abstract
1. Introduction
2. Results
2.1. Abnormal Chromatin Organization Due to the Herbicide DQ Treatment
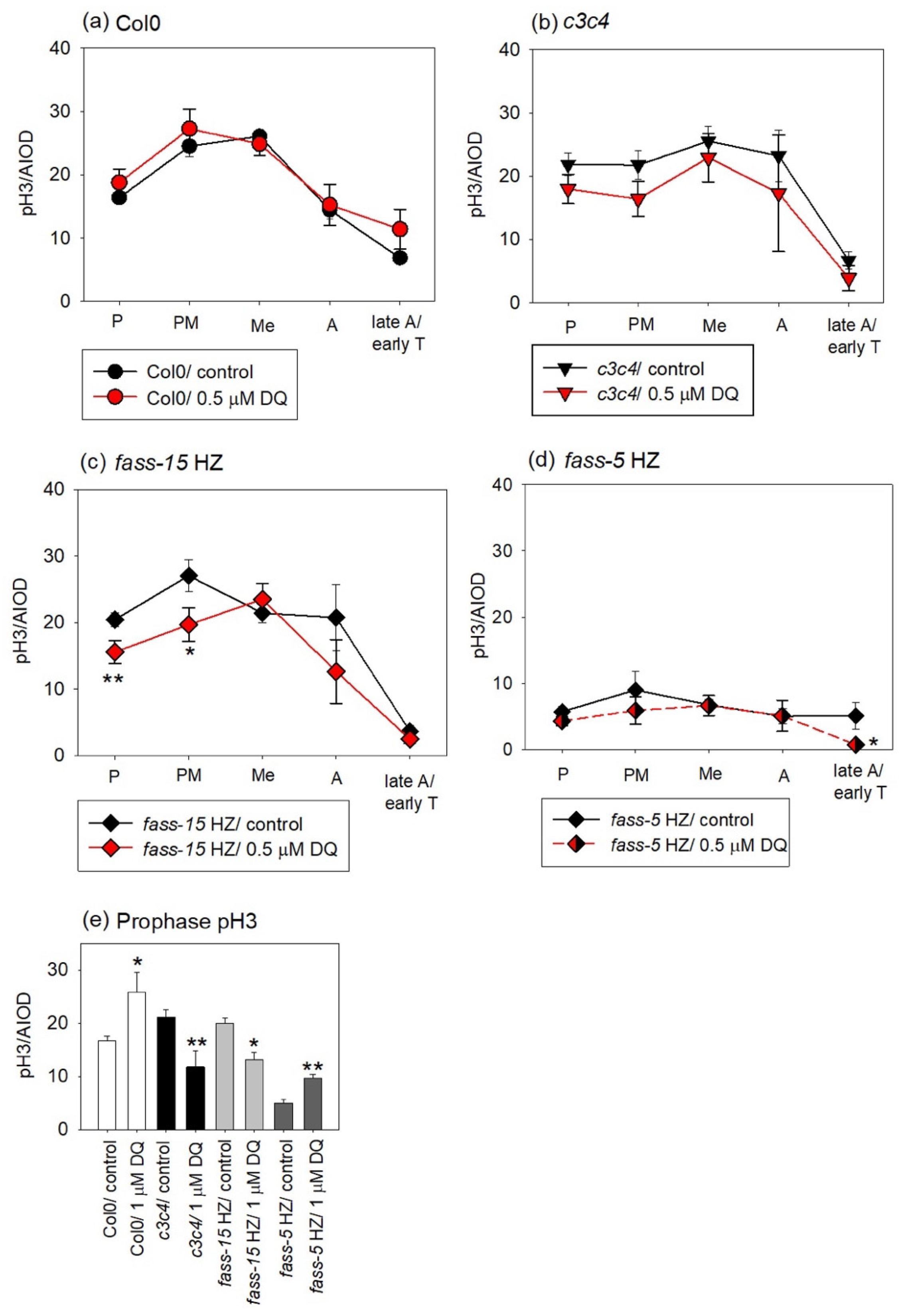
2.2. DQ Treatments Modulate Mitotic Activities in All Genotypes
2.3. Evaluation of the Phosphorylation Level of Mitotic Histone H3 in Col0 and Phosphatase Mutants and Examining the Impact of DQ Treatment
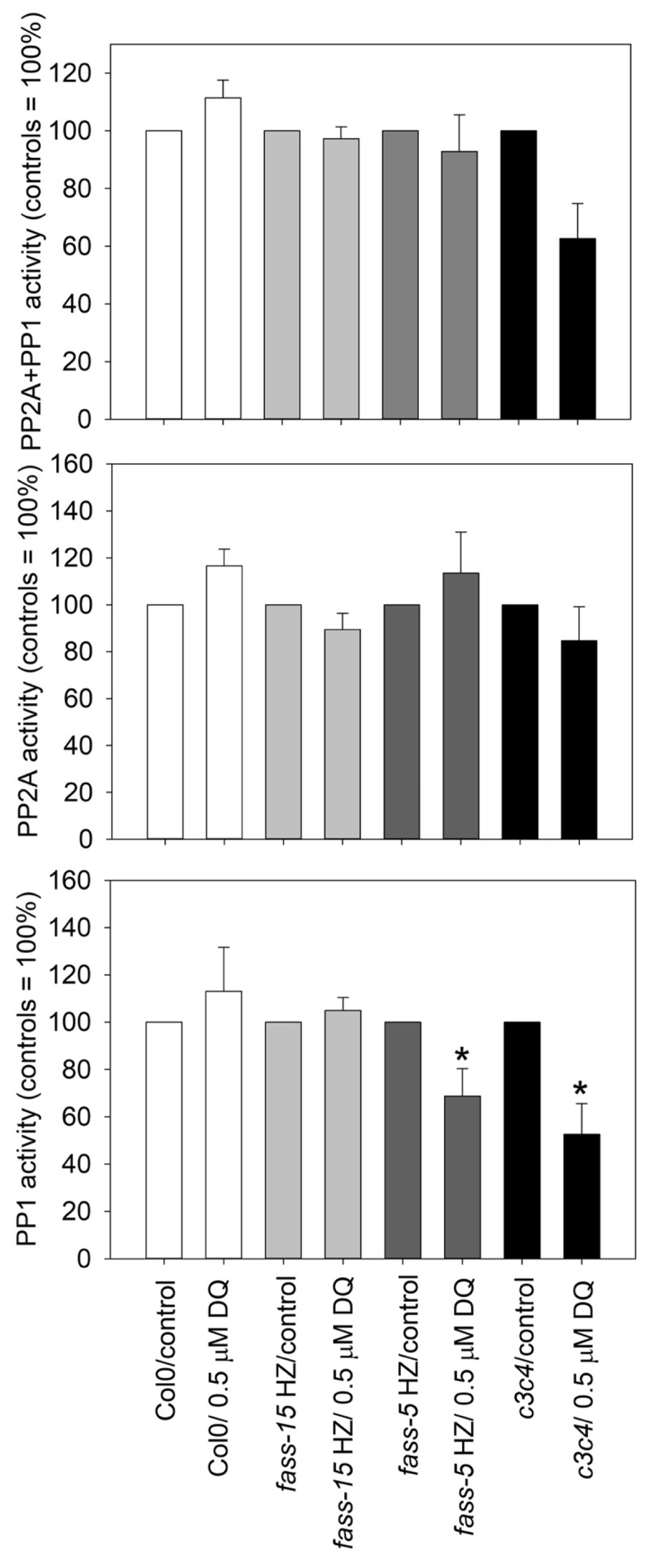
2.4. DQ Has Differential Effects on PP2A and PP1 Phosphatase Activities in Different Genotypes
3. Discussion
4. Materials and Methods
4.1. Plant Material and DQ Treatments
4.2. Immunofluorescence and Histochemical Labeling Procedures
4.3. The Quantification of Mitotic Activities and Histone H3 Phosphorylation Signal
4.4. The Assay of Protein Phosphatase Activities
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.D.; Walker, J.C. Plant Protein Phosphatases. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Luan, S. Protein Phosphatases in Plants. Annu. Rev. Plant. Biol. 2003, 54, 63–92. [Google Scholar] [CrossRef] [PubMed]
- Farkas, I.; Dombrádi, V.; Miskei, M.; Szabados, L.; Koncz, C. Arabidopsis PPP Family of Serine/Threonine Phosphatases. Trends Plant Sci. 2007, 12, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Máthé, C.; Garda, T.; Freytag, C.; M-Hamvas, M. The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling—Facts and Hypotheses. Int. J. Mol. Sci. 2019, 20, 3028. [Google Scholar] [CrossRef] [PubMed]
- Wera, S.; Hemmingst, B.A. Serine/Threonine Protein Phosphatases. Biochem. J. 1995, 311, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, Q.; Su, H.; Birchler, J.A.; Han, F. Histone Phosphorylation: Its Role during Cell Cycle and Centromere Identity in Plants. Cytogenet. Genome Res. 2014, 143, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.; Tándor, I.; Kónya, Z.; Bátori, R.; Roszik, J.; Vereb, G.; Erdődi, F.; Vasas, G.; M-Hamvas, M.; Jambrovics, K.; et al. Microcystin-LR, a Protein Phosphatase Inhibitor, Induces Alterations in Mitotic Chromatin and Microtubule Organization Leading to the Formation of Micronuclei in Vicia faba. Ann. Bot. 2012, 110, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Ujvárosi, A.Z.; Riba, M.; Garda, T.; Gyémánt, G.; Vereb, G.; M-Hamvas, M.; Vasas, G.; Máthé, C. Attack of Microcystis aeruginosa Bloom on a Ceratophyllum submersum Field: Ecotoxicological Measurements in Real Environment with Real Microcystin Exposure. Sci. Total Environ. 2019, 662, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.; Wako, T.; Furushima-Shimogawara, R.; Presting, G.; Künzel, G.; Schubert, I.; Fukui, K. The Cell Cycle Dependent Phosphorylation of Histone H3 Is Correlated with the Condensation of Plant Mitotic Chromosomes. Plant J. 1999, 18, 675–679. [Google Scholar] [CrossRef]
- Hans, F.; Dimitrov, S. Histone H3 Phosphorylation and Cell Division. Oncogene 2001, 20, 3021–3027. [Google Scholar] [CrossRef]
- Houben, A.; Demidov, D.; Caperta, A.D.; Karimi, R.; Agueci, F.; Vlasenko, L. Phosphorylation of Histone H3 in Plants—A Dynamic Affair. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2007, 1769, 308–315. [Google Scholar] [CrossRef]
- Manzanero, S.; Rutten, T.; Kotseruba, V.; Houben, A. Alterations in the Distribution of Histone H3 Phosphorylation in Mitotic Plant Chromosomes in Response to Cold Treatment and the Protein Phosphatase Inhibitor Cantharidin. Chromosome Res. 2002, 10, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Livanos, P.; Apostolakos, P.; Galatis, B. Plant Cell Division: ROS Homeostasis Is Required. Plant Signal Behav. 2012, 7, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S. Promotion of Oxidative Stress in the Aquatic Macrophyte Ceratophyllum demersum during Biotransformation of the Cyanobacterial Toxin Microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Máthé, C.; Vasas, G.; Borbély, G.; Erdődi, F.; Beyer, D.; Kiss, A.; Surányi, G.; Gonda, S.; Jámbrik, K.; M-Hamvas, M. Histological, Cytological and Biochemical Alterations Induced by Microcystin-LR and Cylindrospermopsin in White Mustard (Sinapis alba L.) Seedlings. Acta. Biol. Hung. 2013, 64, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Garda, T.; Kónya, Z.; Tándor, I.; Beyer, D.; Vasas, G.; Erdődi, F.; Vereb, G.; Papp, G.; Riba, M.; M-Hamvas, M.; et al. Microcystin-LR Induces Mitotic Spindle Assembly Disorders in Vicia faba by Protein Phosphatase Inhibition and Not Reactive Oxygen Species Induction. J. Plant Physiol. 2016, 199, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Freytag, C.; Garda, T.; Kónya, Z.; M-Hamvas, M.; Tóth-Várady, B.; Juhász, G.P.; Ujlaky-Nagy, L.; Kelemen, A.; Vasas, G.; Máthé, C. B” and C Subunits of PP2A Regulate the Levels of Reactive Oxygen Species and Superoxide Dismutase Activities in Arabidopsis. Plant Physiol. Biochem. 2023, 195, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H. Stress Effects on the Reactive Oxygen Species-Dependent Regulation of Plant Growth and Development. J. Exp. Bot. 2021, 72, 5795–5806. [Google Scholar] [CrossRef]
- Jones, G.M.; Vale, J.A. Mechanisms of Toxicity, Clinical Features, and Management of Diquat Poisoning: A Review. Clin. Toxicol. 2000, 38, 123–128. [Google Scholar] [CrossRef]
- Acar, A. In Vivo Toxicological Assessment of Diquat Dibromide: Cytotoxic, Genotoxic, and Biochemical Approach. Environ. Sci. Pollut. Res. 2021, 28, 47550–47561. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, M.; Wang, W.; Wang, Q.; Huang, M.; Li, C.; Lian, Q.; Xia, J.; Qi, J.; Xiang, C.; et al. Changing Gly311 to an Acidic Amino Acid in the MATE Family Protein DTX6 Enhances Arabidopsis Resistance to the Dihydropyridine Herbicides. Mol. Plant 2021, 14, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Su, Y.; Lin, R.; Lin, F.; Shang, P.; Hussain, R.; Shi, D. Effects of Acute Diquat Poisoning on Liver Mitochondrial Apoptosis and Autophagy in Ducks. Front. Vet. Sci. 2021, 8, 727766. [Google Scholar] [CrossRef]
- Spinner, L.; Gadeyne, A.; Belcram, K.; Goussot, M.; Moison, M.; Duroc, Y.; Eeckhout, D.; De Winne, N.; Schaefer, E.; Van De Slijke, E.; et al. A Protein Phosphatase 2A Complex Spatially Controls Plant Cell Division. Nat. Commun. 2013, 4, 1863. [Google Scholar] [CrossRef]
- Gadjev, I.; Stone, J.M.; Gechev, T.S. Programmed Cell Death in Plants: New Insights into Redox Regulation and the Role of Hydrogen Peroxide. Int. Rev. Cell Mol. Biol. 2008, 270, 87–144. [Google Scholar]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Reichheld, J.P.; Vernoux, T.; Lardon, F.; Van Montagu, M.; Inzé, D. Specific Checkpoints Regulate Plant Cell Cycle Progression in Response to Oxidative Stress. Plant J. 1999, 17, 647–656. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic Regulation in Plant Abiotic Stress Responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Kumar, S.R.M.; Wang, Y.; Zhang, X.; Cheng, H.; Sun, L.; He, S.; Hao, F. Redox Components: Key Regulators of Epigenetic Modifications in Plants. Int. J. Mol. Sci. 2020, 21, 1419. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P. Epigenetic Variation and Environmental Change. J. Exp. Bot. 2015, 66, 3541–3548. [Google Scholar] [CrossRef]
- Yung, W.S.; Li, M.W.; Sze, C.C.; Wang, Q.; Lam, H.M. Histone Modifications and Chromatin Remodelling in Plants in Response to Salt Stress. Physiol. Plant. 2021, 173, 1495–1513. [Google Scholar] [CrossRef]
- Żabka, A.; Winnicki, K.; Polit, J.T.; Wróblewski, M.; Maszewski, J. Cadmium (II)-Induced Oxidative Stress Results in Replication Stress and Epigenetic Modifications in Root Meristem Cell Nuclei of Vicia faba. Cells 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Żabka, A.; Polit, J.T.; Maszewski, J. DNA Replication Stress Induces Deregulation of the Cell Cycle Events in Root Meristems of Allium cepa. Ann. Bot. 2012, 110, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Gautam, V.; Bali, S.; Sharma, A.; Khanna, K.; Arora, S.; Thukral, A.K.; Ohri, P.; Karpets, Y.V.; et al. ROS Signaling in Plants under Heavy Metal Stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2017; pp. 185–214. [Google Scholar]
- Chen, Y.; Zhang, J.B.; Wei, N.; Liu, Z.H.; Li, Y.; Zheng, Y.; Li, X.B. A Type-2C Protein Phosphatase (GhDRP1) Participates in Cotton (Gossypium hirsutum) Response to Drought Stress. Plant Mol. Biol. 2021, 107, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, J.; Qin, Q.; Zhang, J.; Chen, Y.; Zhao, L.; He, K.; Hou, S. Type One Protein Phosphatases (TOPPs) Contribute to the Plant Defense Response in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, J.; Zhang, Y.; Guo, Y.; Ji, W. Genome-wide Characterization and Expression Analysis of TOPP-Type Protein Phosphatases in Soybean (Glycine max L.) Reveal the Role of GmTOPP13 in Drought Tolerance. Genes Genom. 2021, 43, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.D.; Lin, K.H.; Chen, C.C.; Chiang, C.M. Oryza sativa Protein Phosphatase 1a (OsPP1a) Involved in Salt Stress Tolerance in Transgenic Rice. Mol. Breed. 2016, 36, 22. [Google Scholar] [CrossRef]
- Máthé, C.; Freytag, C.; Kelemen, A.; M-Hamvas, M.; Garda, T. “B” Regulatory Subunits of PP2A: Their Roles in Plant Development and Stress Reactions. Int. J. Mol. Sci. 2023, 24, 5147. [Google Scholar] [CrossRef]
- Camilleri, C.; Azimzadeh, J.; Pastuglia, M.; Bellini, C.; Grandjean, O.; Bouchez, D. The Arabidopsis TONNEAU2 Gene Encodes a Putative Novel Protein Phosphatase 2A Regulatory Subunit Essential for the Control of the Cortical Cytoskeleton. Plant Cell 2002, 14, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Kirik, A.; Ehrhardt, D.W.; Kirik, V. TONNEAU2/FASS Regulates the Geometry of Microtubule Nucleation and Cortical Array Organization in Interphase Arabidopsis Cells. Plant Cell 2012, 24, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Domínguez, T.; Sauer, M.; Paredes, P.; Duprat, A.; Rojo, E.; Sanmartín, M.; Sánchez-Serrano, J.J. Specialized Functions of the PP2A Subfamily II Catalytic Subunits PP2A-C3 and PP2A-C4 in the Distribution of Auxin Fluxes and Development in Arabidopsis. Plant J. 2013, 73, 862–872. [Google Scholar] [CrossRef]
- Alonso, J.M.; Stepanova, N.A.; Leisse, J.T.; Kim, J.C.; Chen, H.; Shinn, P.; Stevenson, K.D.; Zimmermann, J.; Barajas, P.; Cheuk, R.; et al. Genome-Wide Insertional Mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1978, 15, 473. [Google Scholar] [CrossRef]
- Pasternak, T.; Tietz, O.; Rapp, K.; Begheldo, M.; Nitschke, R.; Ruperti, B.; Palme, K. Protocol: An Improved and Universal Procedure for Whole-Mount Immunolocalization in Plants. Plant Methods 2015, 11, 50. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nature Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Erdődi, F.; Tóth, B.; Hirano, K.; Hirano, M.; Hartshorne, D.J.; Gergely, P. Endothall Thioanhydride Inhibits Protein Phosphatases-1 and -2A in vivo. Am. J. Physiol. 1995, 269, C1176–C1184. [Google Scholar] [CrossRef] [PubMed]
- Garda, T.; Kónya, Z.; Freytag, C.; Erdődi, F.; Gonda, S.; Vasas, G.; Szücs, B.; M-Hamvas, M.; Kiss-Szikszai, A.; Vámosi, G.; et al. Allyl-Isothiocyanate and Microcystin-LR Reveal the Protein Phosphatase Mediated Regulation of Metaphase-Anaphase Transition in Vicia faba. Front. Plant Sci. 2018, 9, 1823. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dedinszki, D.; Sipos, A.; Kiss, A.; Bátori, R.; Kónya, Z.; Virág, L.; Erdődi, F.; Lontay, B. Protein Phosphatase-1 Is Involved in the Maintenance of Normal Homeostasis and in UVA Irradiation-Induced Pathological Alterations in HaCaT Cells and in Mouse Skin. Biochim. Biophys. Acta. Mol. Basis Dis. 2015, 1852, 22–33. [Google Scholar] [CrossRef]
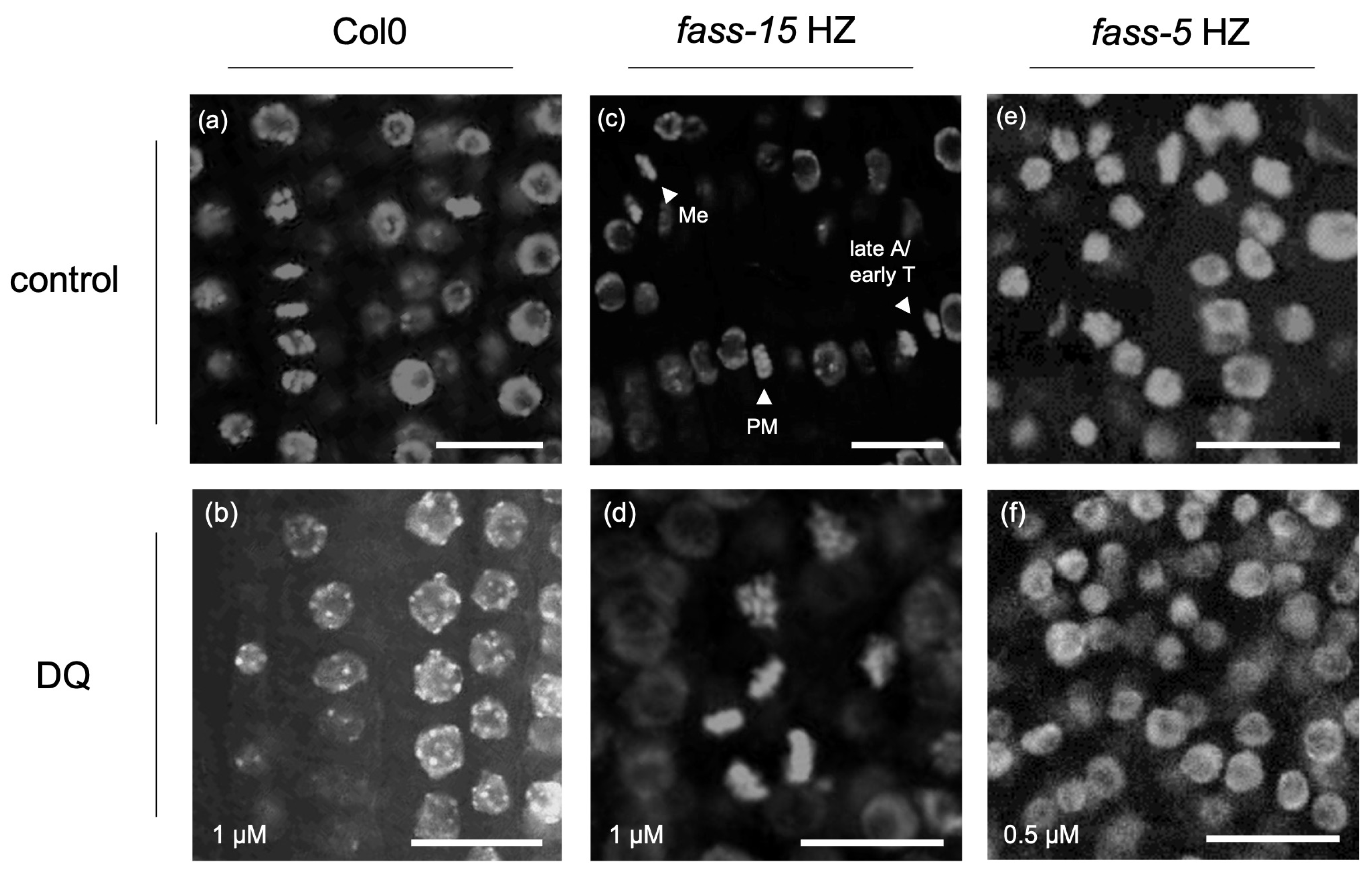

| Col0 | fass-15 HZ | fass-5 HZ | c3c4 | |
|---|---|---|---|---|
| control | 6% | 31.5% | 35% | 16% |
| slight blebbing | slight or frequent blebbing, sporadic chromatin condensation | mostly slight blebbing, frequent chromatin condensation | slight blebbing, some chromatin condensation | |
| 0.5 μM DQ | 35% | 33.5% | 7% | 22% |
| slight or frequent blebbing | mostly slight blebbing, sporadic chromatin condensation | slight blebbing, frequent chromatin condensation | slight or frequent blebbing | |
| 1 μM DQ | 33.5% | 50% | 11% | 14% |
| slight blebbing | mostly slight blebbing | slight blebbing, frequent chromatin condensation | slight blebbing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelemen, A.; Garda, T.; Kónya, Z.; Erdődi, F.; Ujlaky-Nagy, L.; Juhász, G.P.; Freytag, C.; M-Hamvas, M.; Máthé, C. Treatments with Diquat Reveal the Relationship between Protein Phosphatases (PP2A) and Oxidative Stress during Mitosis in Arabidopsis thaliana Root Meristems. Plants 2024, 13, 1896. https://doi.org/10.3390/plants13141896
Kelemen A, Garda T, Kónya Z, Erdődi F, Ujlaky-Nagy L, Juhász GP, Freytag C, M-Hamvas M, Máthé C. Treatments with Diquat Reveal the Relationship between Protein Phosphatases (PP2A) and Oxidative Stress during Mitosis in Arabidopsis thaliana Root Meristems. Plants. 2024; 13(14):1896. https://doi.org/10.3390/plants13141896
Chicago/Turabian StyleKelemen, Adrienn, Tamás Garda, Zoltán Kónya, Ferenc Erdődi, László Ujlaky-Nagy, Gabriella Petra Juhász, Csongor Freytag, Márta M-Hamvas, and Csaba Máthé. 2024. "Treatments with Diquat Reveal the Relationship between Protein Phosphatases (PP2A) and Oxidative Stress during Mitosis in Arabidopsis thaliana Root Meristems" Plants 13, no. 14: 1896. https://doi.org/10.3390/plants13141896
APA StyleKelemen, A., Garda, T., Kónya, Z., Erdődi, F., Ujlaky-Nagy, L., Juhász, G. P., Freytag, C., M-Hamvas, M., & Máthé, C. (2024). Treatments with Diquat Reveal the Relationship between Protein Phosphatases (PP2A) and Oxidative Stress during Mitosis in Arabidopsis thaliana Root Meristems. Plants, 13(14), 1896. https://doi.org/10.3390/plants13141896









