Phytochemical Characterization and Bioactivity Evaluation of Extracts Obtained via Ultrasound-Assisted Extraction of Medicinal Plant Phedimus aizoon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Ultrasound-Assisted Extraction (UAE) of Bioactive Compounds from Phedimus aizoon
2.4. Phytochemical Analysis
2.4.1. Estimation of Phenolic Content and Flavonoid Content
2.4.2. GC-MS Analysis
2.4.3. HPLC Analysis
2.5. Assessment of Antioxidant and Antibacterial Activity
2.5.1. Antioxidant Activity
2.5.2. Antibacterial Activity
2.6. Statistical Analysis
3. Results
3.1. Analysis of Phytochemicals in Extracts from Phedimus aizoon
3.1.1. GC-MS Analysis
3.1.2. HPLC-DAD Analysis
3.2. Bioactivities of Extracts from Phedimus aizoon
3.2.1. Antioxidant Activity
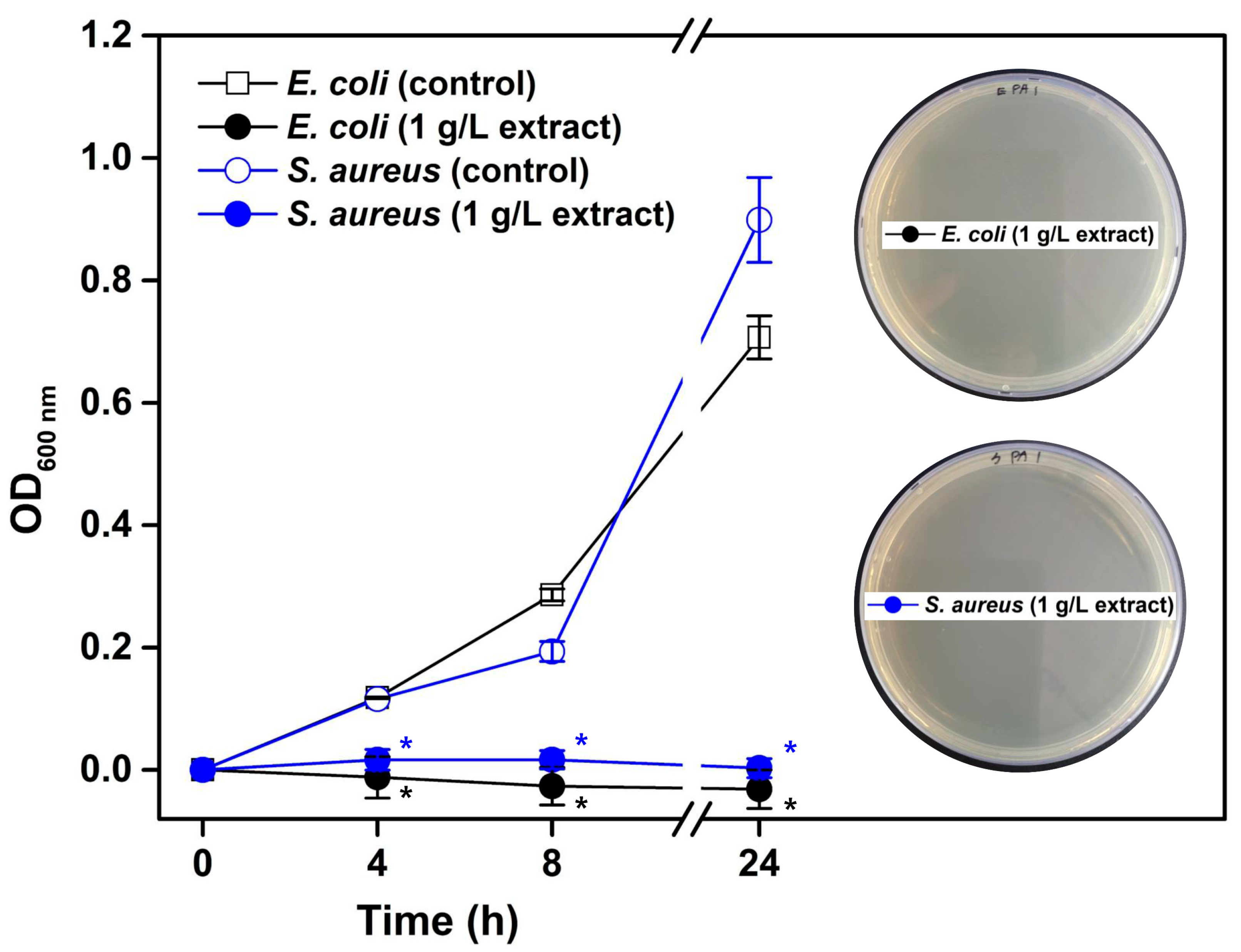
3.2.2. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Kim, Y.; Kim, S.H.; Yang, J.; Cho, M.S.; Koldaeva, M.; Ito, T.; Maki, M.; Kim, S.C. Plastome-based backbone phylogeny of East Asian Phedimus (Subgenus Aizoon: Crassulaceae), with special emphasis on Korean endemics. Front. Plant Sci. 2023, 14, 1089165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Z.; Xie, Y.; Su, L.; Zhang, R.; Wang, H.; Li, C.; Long, S. Drought resistance mechanisms of Phedimus aizoon L. Sci. Rep. 2021, 11, 13600. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Ge, Z.K.; Qiu, J.R.; Luan, S.Q.; Hao, X.C.; Zhao, Y.H. Sedum aizoon L.: A review of its history, traditional uses, nutritional value, botany, phytochemistry, pharmacology, toxicology, and quality control. Front. Pharmacol. 2024, 15, 1349032. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, Z.; Lei, T.; Lv, C.; Wang, J.; Lu, J. New flavonoid glycosides from Sedum aizoon L. Fitoterapia 2015, 101, 125. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, F.; Zhang, X.; Shao, X.; Wei, Y.; Wang, H. Transcriptomic analysis reveals antibacterial mechanism of flavonoids from Sedum aizoon L. against Pseudomonas fragi. Food Control 2021, 134, 108755. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, J.-H. Effect of Gas Bubbles on the Recovery Efficiency of Paclitaxel from Biomass of Taxus chinensis in Ultrasonic Extraction. Biotechnol. Bioprocess Eng. 2023, 28, 545–553. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J.H. Development of an ultrasound-negative pressure cavitation fractional precipitation for the purification of (+)-dihydromyricetin from biomass. Korean J. Chem. Eng. 2023, 40, 1133–1140. [Google Scholar] [CrossRef]
- Tran, T.K.; Ha, P.T.T.; Henry, R.J.; Nguyen, D.N.T.; Tuyen, P.T.; Liem, N.T. Polyphenol Contents, Gas Chromatography-Mass Spectrometry (GC–MS) and Antibacterial Activity of Methanol Extract and Fractions of Sonneratia caseolaris Fruits from Ben Tre Province in Vietnam. J. Microbiol. Biotechnol. 2024, 34, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Batbatan, C.G. Optimization of enzymatic-assisted ultrasonic extraction process of total flavonoids from Sedum aizoon L. and its antioxidant activity. In Proceedings of the 2023 International Conference on Food Science and Bio-Medicine, Guangzhou, China, 15–17 September 2023; BIO Web of Conferences. EDP Sciences: Les Ulis, France, 2023; p. 02004. [Google Scholar] [CrossRef]
- Jin, C.; Wei, X.; Yang, S.; Yao, L.; Gong, G. Microwave-assisted Extraction and Antioxidant Activity of Flavonoids from Sedum aizoon Leaves. Food Sci. Technol. Res. 2017, 23, 111–118. [Google Scholar] [CrossRef]
- Odeh, A.A.; Al-Jaber, H.I.; Barhoumi, L.M.; Al-Fawares, O.L.; Shakya, A.K.; Al-Qudah, M.A.; Al-Sanabra, O.M. Phytochemical and bioactivity evaluation of secondary metabolites and essential oils of Sedum rubens growing wild in Jordan. Arab. J. Chem. 2023, 16, 104712. [Google Scholar] [CrossRef]
- Lee, J.; Song, Y.; Son, H.; Kim, S.; Lee, K.H.; Bazarragchaa, B.; Lee, C.; Yoo, H.Y. Phytochemical and Antioxidant Characterization of Extracts from Unexplored Medicinal Plants Salix schwerinii and Salix kochiana. Horticulturae 2023, 9, 955. [Google Scholar] [CrossRef]
- Kusar, S.; Saddiqe, Z.; Ali, F.; Bashir, S.; Zubairi, T. GCMS and HPLC profiling, antioxidant and anti-inflammatory activities of Crotalaria medicaginea Lamk. S. Afr. J. Bot. 2024, 168, 196–208. [Google Scholar] [CrossRef]
- Dinore, J.M.; Patil, H.S.; Farooqui, S.; Pradhan, V.; Farooqui, M. GC/MS and LC/MS phytochemical analysis of Vigna unguiculata L. Walp pod. Chem. Biodivers. 2023, 20, e202200048. [Google Scholar] [CrossRef]
- Akhter, P.; Bhatti, T.Y.; Shafiq, I.; Jamil, F.; Nazar, R.; Nazir, M.S.; Hassan, S.U.; Hussain, M.; Park, Y. Antioxidant activity of sea buckthorn (Hippophae rhamnoides) seed oil extracted using various organic solvents. Korean J. Chem. Eng. 2023, 40, 2914–2920. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef]
- Rasyid, A.; Putra, M.Y. Antibacterial and antioxidant activity of sea cucumber extracts collected from Lampung waters, Indonesia. Kuwait J. Sci. 2023, 50, 615–621. [Google Scholar] [CrossRef]
- Khan, I.H.; Javaid, A. Antifungal, antibacterial and antioxidant components of ethyl acetate extract of quinoa stem. Plant Prot. 2019, 3, 125–130. [Google Scholar] [CrossRef]
- Roopa, M.S.; Shubharani, R.; Rhetso, T.; Sivaram, V. Comparative analysis of phytochemical constituents, free radical scavenging activity and GC-MS analysis of leaf and flower extract of Tithonia diversifolia (Hemsl.) A. Gray. Int. J. Pharm. Sci. Res. 2020, 11, 5081–5090. [Google Scholar]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Britto, D.; Leslee, C.; Kuppannan, S.B.; Seedevj, P. Structural characterization of n-hexadecanoic acid 382 from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activity. Biomass Convers. Biorefin. 2022; early access. [Google Scholar] [CrossRef]
- Lin, Z.C.; Fang, Y.J.; Huang, A.Y.; Chen, L.Y.; Guo, S.H.; Chen, J.W. Chemical constituents from Sedum aizoon and their hemostatic activity. Pharm. Biol. 2014, 52, 1429–1434. [Google Scholar] [CrossRef]
- Xu, T.; Lv, C.; Lu, J. The fingerprint and flavonoids contents by HPLC and the UPLC-DAD-Q-TOF-MS analysis of Sedum aizoon L. J. Polyphen. 2019, 1, 23–32. [Google Scholar]
- Kim, S.; Lee, K.H.; Lee, J.; Lee, S.K.; Chun, Y.; Lee, J.H.; Yoo, H.Y. Efficient Recovery Strategy of Luteolin from Agricultural Waste Peanut Shells and Activity Evaluation of Its Functional Biomolecules. Int. J. Mol. Sci. 2023, 24, 12366. [Google Scholar] [CrossRef]
- Qi, X.; Lu, X.T.; Sun, X.H.; Lin, C.Q.; Cui, C.B. The regulatory effect of total flavonoids of Sedum aizoon L. on oxidative stress in type 1 diabetic mice. Curr. Res. Food Sci. 2022, 5, 1140–1147. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Wang, C.; Wang, K.; Wei, Y.; Shao, X.; Wang, H. Antimicrobial activity of flavonoids from Sedum aizoon L. against Aeromonas in culture medium and in frozen pork. Food Sci. Nutr. 2019, 7, 3224–3232. [Google Scholar] [CrossRef]
- Wang, J.; Chi, Z.; Zhao, K.; Wang, H.; Zhang, X.; Xu, F.; Shao, X.; Wei, Y. A transcriptome analysis of the antibacterial mechanism of flavonoids from Sedum aizoon L. against Shewanella putrefaciens. World J. Microbiol. Biotechnol. 2020, 36, 94. [Google Scholar] [CrossRef]
- Oyungerel, S.; Yim, J.H. Antimicrobial Activity of Some Mongolian Plants. Mong. J. Biol. Sci. 2023, 21, 3–13. [Google Scholar] [CrossRef]
- Shin, H.; Lee, J.; Bae, J.; Lee, K.H.; Yoo, H.Y.; Park, C. Enhancement of dieckol extraction yield from Ecklonia cava through optimization of major variables in generally recognized as safe solvent-based process. Front. Mar. Sci. 2023, 10, 1287047. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Alhajaim, W.; Fatima, A.; Yasir, S.; Kamal, T.; Abbas, Y.; Khan, S.; Khan, A.H.; Manan, S.; Ullah, M.W.; et al. Development of low-cost bacterial cellulose-pomegranate peel extract-based antibacterial composite for potential biomedical applications. Int. J. Biol. Macromol. 2023, 231, 123269. [Google Scholar] [CrossRef]
- Yousefinia, A.; Khodadadi, M.; Mortazavi-Derazkola, S. An efficient biosynthesis of novel ZnO/CuO nanocomposites using berberis vulgaris extract (ZnO/CuO@ BVENCs) for enhanced photocatalytic degradation of pollution, antibacterial and antifungal activity. Environ. Technol. Innov. 2023, 32, 103340. [Google Scholar] [CrossRef]

| Extraction Yield | Total Phenol Content | Total Flavonoid Content |
|---|---|---|
| 16.56 ± 0.39% | 126.3 ± 2.2 mg CAE/g extracts | 31.0 ± 0.5 mg CE/g extracts |
| No. | Compound 1 | RT (min) | Molecular Weight | Formula | Quality |
|---|---|---|---|---|---|
| 1 | Decane | 6.290 | 142 | C10H22 | 91 |
| 2 | Undecane | 7.886 | 156 | C11H24 | 90 |
| 3 | Dodecane | 9.423 | 170 | C12H26 | 94 |
| 4 | Benzene, 1,3-bis(1,1-dimethylethyl)- | 10.192 | 190 | C14H22 | 95 |
| 5 | Dodecane, 1-iodo- | 10.522 | 296 | C12H25I | 80 |
| 6 | Cyclohexasiloxane, dodecamethyl- | 10.807 | 444 | C12H36O6Si6 | 91 |
| 7 | Tetradecane | 12.242 | 198 | C14H30 | 97 |
| 8 | Cycloheptasiloxane, tetradecamethyl- | 13.026 | 519 | C14H42O7Si7 | 87 |
| 9 | Pentacosane | 13.377 | 352 | C25H52 | 86 |
| 10 | 2,4-Di-tert-butylphenol | 13.611 | 206 | C14H22O | 93 |
| 11 | 10-Methylnonadecane | 13.948 | 282 | C20H42 | 80 |
| 12 | Hentriacontane | 15.896 | 437 | C31H64 | 86 |
| 13 | Hexadecanoic acid, methyl ester | 18.276 | 270 | C17H34O2 | 97 |
| Compound | Caffeic acid | Gallic acid | Vanillic acid | Luteolin | Quercetin | Kaempferol |
| Content (mg/g Extracts) | 0.13 | 2.75 | 0.50 | 0.12 | 0.19 | 0.06 |
| Sample | Radical Scavenging Activity (IC50, µg/mL) | |
|---|---|---|
| ABTS Cation Radical | DPPH Free Radical | |
| Phedimus aizoon extracts | 260.0 ± 2.6 * | 41.0 ± 2.3 * |
| Ascorbic acid (reference) | 72.9 ± 1.0 | 9.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, M.; Son, H.; Kim, S.; Jo, S.; Janchiv, A.; Kim, S.-Y.; Lee, T.; Yoo, H.Y. Phytochemical Characterization and Bioactivity Evaluation of Extracts Obtained via Ultrasound-Assisted Extraction of Medicinal Plant Phedimus aizoon. Plants 2024, 13, 1915. https://doi.org/10.3390/plants13141915
Lee J, Kim M, Son H, Kim S, Jo S, Janchiv A, Kim S-Y, Lee T, Yoo HY. Phytochemical Characterization and Bioactivity Evaluation of Extracts Obtained via Ultrasound-Assisted Extraction of Medicinal Plant Phedimus aizoon. Plants. 2024; 13(14):1915. https://doi.org/10.3390/plants13141915
Chicago/Turabian StyleLee, Jeongho, Minji Kim, Hyerim Son, Seunghee Kim, Sangjin Jo, Agiimaa Janchiv, Soo-Yong Kim, Taek Lee, and Hah Young Yoo. 2024. "Phytochemical Characterization and Bioactivity Evaluation of Extracts Obtained via Ultrasound-Assisted Extraction of Medicinal Plant Phedimus aizoon" Plants 13, no. 14: 1915. https://doi.org/10.3390/plants13141915






