Tissue-Specific Natural Synthesis of Galanthaminein Zephyranthes Species and Its Accumulation in Different In Vitro-Grown Organs Following Methyl Jasmonate Treatment
Abstract
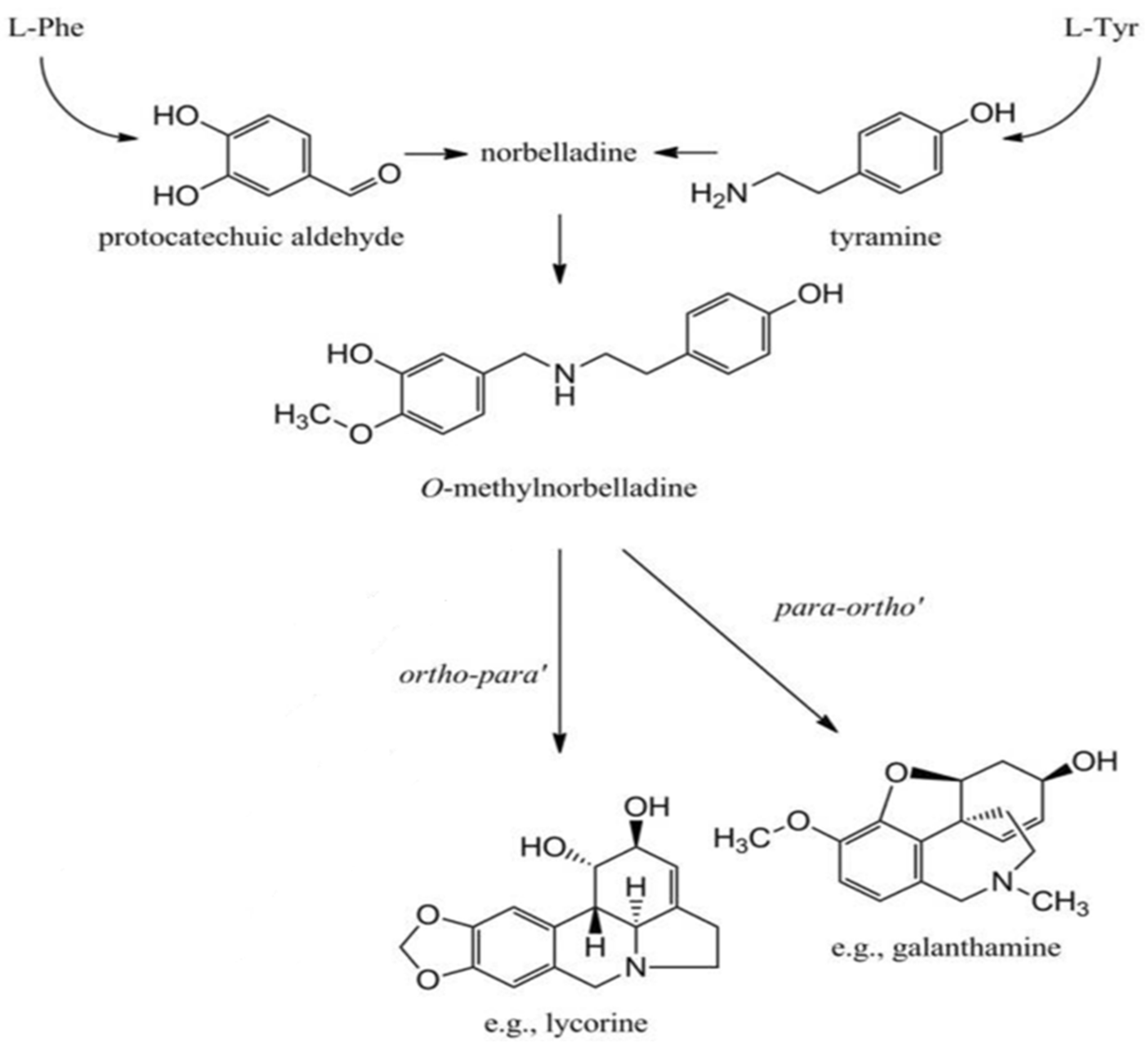
:1. Introduction
2. Results
2.1. GC-MS Study and Bioactive Compounds
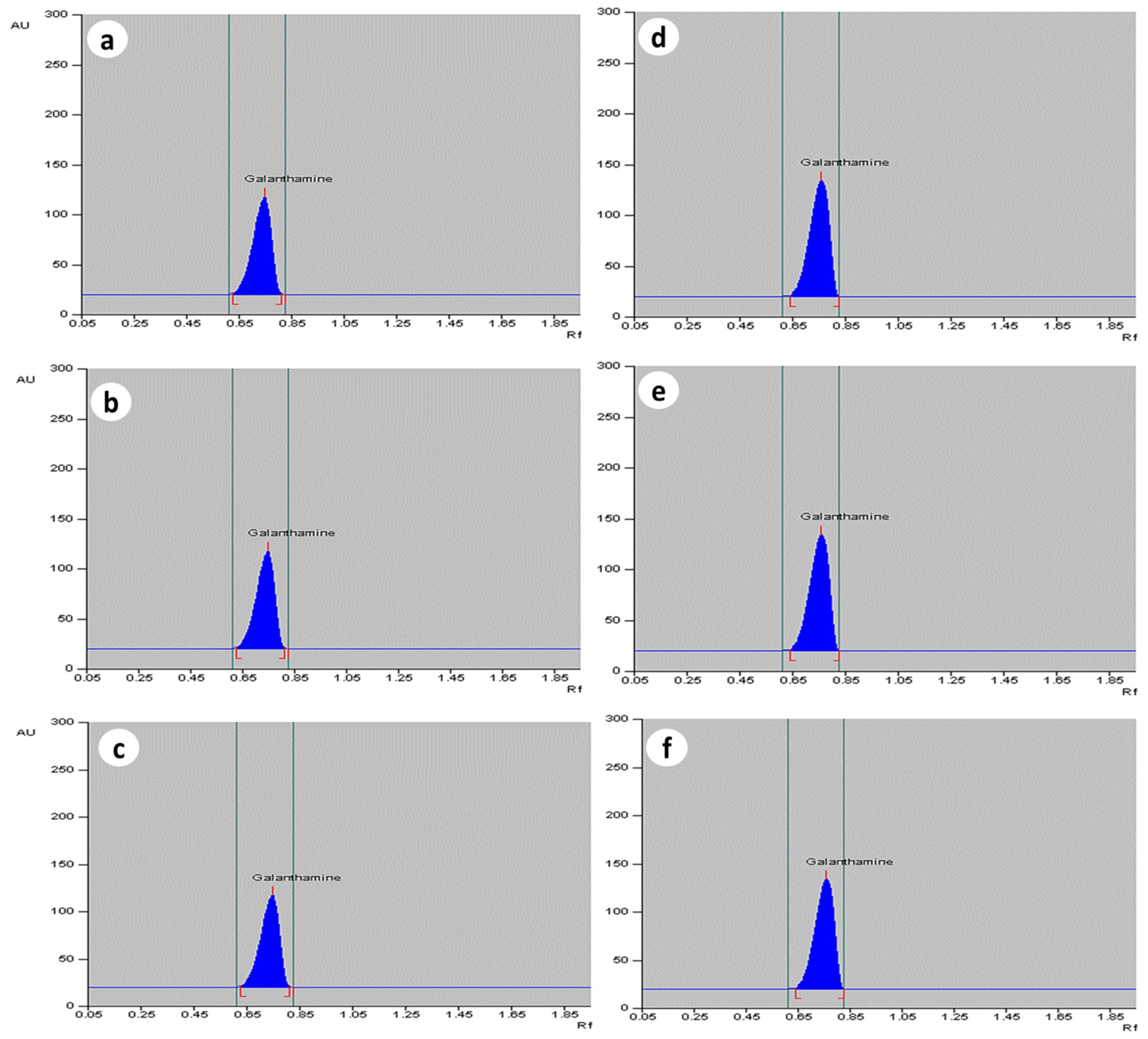
2.2. Quantification of Galanthamine in In Vivo Grown Plants of Zephyranthes spp.
2.3. Effect of MJ Dosage on Accumulation of Galanthamine in In Vitro-Derived Plantlets of Zephyranthes spp.
3. Discussion
4. Materials and Methods
4.1. Plant Material and Surface Sterilization Method
4.2. GC-MS Analysis
4.3. Elicitor Preparation and Dosage
4.4. Extraction Procedure for High Performance Thin Layer Chromatography (HPTLC)
4.4.1. Stock Solution and Sample Extraction Procedure
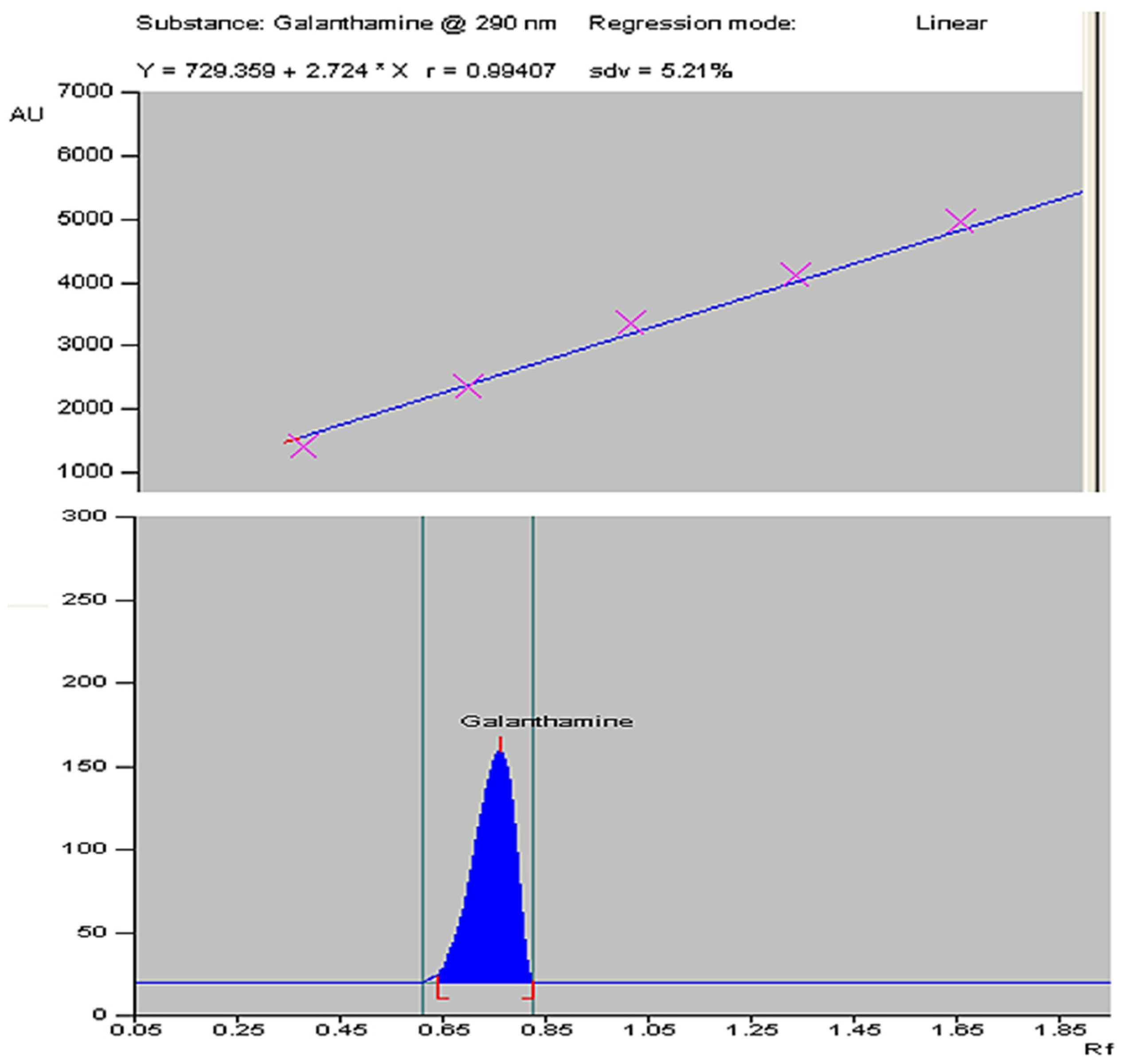
4.4.2. HPTLC Instrumentation and Chromatographic Conditions
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kartesz, J.T. A Synonymized Checklist of the Vascular Flora Ofthe United States, Canada, and Greenland; Timber Press: Portland, OR, USA, 1994; 622p. [Google Scholar]
- Hutchinson, J. The Families of Flowering Plants; Clarendon: Oxford, UK, 2003; 510p. [Google Scholar]
- Katoch, D.; Singh, B. Phytochemistry and Pharmacology of Genus Zephyranthes. Med. Aromat. Plants 2015, 4, 212. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Augustin, M.M.; Starks, C.M.; O’Neil-Johnson, M.; May, G.D.; Crow, J.A.; Kutchan, T.M. Cloning and characterization of a norbelladine 4′-O-methyltransferase involved in the biosynthesis of the Alzheimer’s drug galanthamine in Narcissus sp. aff. pseudonarcissus. PLoS ONE 2014, 9, e103223. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yao, G. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2019, 36, 1462–1488. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.; Verpoorte, R. Alkaloids. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2015; pp. 77–84. [Google Scholar]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Daffodils as potential crops of galanthamine. Assessment of more than 100 ornamental varieties for their alkaloid content and acetylcholinesterase inhibitory activity. Ind. Crops Prod. 2013, 43, 237–244. [Google Scholar] [CrossRef]
- Saliba, S.; Ptak, A.; Boisbrun, M.; Spina, R.; Dupire, F.; Laurain-Mattar, D. Stimulating effect of both 4′-O-methylnorbelladine feeding and temporary immersion conditions on galanthamine and lycorine production by Leucojum aestivum L. bulblets. Eng. Life Sci. 2016, 16, 731–739. [Google Scholar] [CrossRef]
- Singh, A.; Desgagné-Penix, I. Biosynthesis of the Amaryllidaceae alkaloids. Plant Sci. Today 2014, 1, 114–120. [Google Scholar] [CrossRef]
- Thilina, U.; Jayawardena, T.U.; Merindol, N.; Liyanage, N.S.; Desgagne-Penix, I. Unveiling Amaryllidaceae alkaloids: From biosynthesis to antiviral potential—A review. Nat. Prod. Rep. 2023, 41, 721–747. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peskind, E.R.; Wessel, T.; Yuan, W.; Galantamine USA-Study Group. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. Neurology 2000, 54, 2261–2268. [Google Scholar] [CrossRef]
- LaBerge, S.; LaMarca, K.; Baird, B. Pre-sleep treatment with galantamine stimulates lucid dreaming: A double-blind, placebo-controlled, crossover study. PLoS ONE 2018, 13, e0201246. [Google Scholar] [CrossRef]
- Koola, M.M. Galantamine-Memantine combination in the treatment of Alzheimer’s disease and beyond. Psychiatry Res. 2020, 293, 113409. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, T.E. Bioprospecting elicitation with gamma irradiation combine with chitosan to enhance, yield production, bioactive secondary metabolites and antioxidant activity for saffron. J. Plant Sci. 2019, 7, 137–143. [Google Scholar]
- Malik, M.Q.; Mujib, A.; Gulzar, B.; Zafar, N.; Syeed, R.; Mamgain, J.; Ejaz, B. Genome size analysis of field grown and somatic embryo regenerated plants in Allium sativum L. J. Appl. Genet. 2020, 61, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Saiman, M.Z.; Miettinen, K.; Mustafa, N.R.; Choi, Y.H.; Verpoorte, R.; Schulte, A.E. Metabolic alteration of Catharanthus roseus cell suspension cultures overexpressing geraniol synthase in the plastids or cytosol. Plant Cell Tissue Organ Cult. 2018, 134, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNSП2 genes. Plant Physiol. Biochem. 2020, 148, 35–44. [Google Scholar] [CrossRef]
- Fan, S.; Jian, D.; Wei, X.; Chen, J.; Beeson, R.C.; Zhou, Z.; Wang, X. Micropropagation of blueberry ‘Bluejay’and ‘Pink Lemonade’through in vitro shoot culture. Sci. Hortic. 2017, 226, 277–284. [Google Scholar] [CrossRef]
- Mamgain, J.; Mujib, A.; Syeed, R.; Ejaz, B.; Malik, M.Q.; Bansal, Y. Genome size and gas chromatography-mass spectrometry (GC-MS) analysis of field-grown and in vitro regenerated Pluchea lanceolata Plants. J. Appl. Genet. 2023, 64, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Ahmad, N.; Anis, M.; Alatar, A.A. Influence of meta-topolin on in vitro organogenesis in Tecoma stans L., assessment of genetic fidelity and phytochemical profiling of wild and regenerated plants. Plant Cell Tissue Organ Cult. 2019, 138, 339–351. [Google Scholar] [CrossRef]
- Khan, A.; Shah, A.H.; Ali, N. In-vitro propagation and phytochemical profiling of a highly medicinal and endemic plant species of the himalayan region (Saussurea costus). Sci. Rep. 2021, 11, 23575. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Abbasi, B.H.; Zeb, A.; Ali, G.S. Carbohydrate-induced biomass accumulation and elicitation of secondary metabolites in callus cultures of Fagonia indica. Ind. Crops Prod. 2018, 126, 168–176. [Google Scholar] [CrossRef]
- Du, L.; Li, D.; Zhang, J.; Du, J.; Luo, Q.; Xiong, J. Elicitation of Lonicera japonica Thunb suspension cell for enhancement of secondary metabolites and antioxidant activity. Ind. Crops Prod. 2020, 156, 112877. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Zafar, N.; Mujib, A.; Ali, M.; Tonk, D.; Gulzar, B. Aluminum chloride elicitation (amendment) improves callus biomass growth and reserpine yield in Rauvolfia serpentina leaf callus. Plant Cell Tissue Organ Cult. 2017, 130, 357–368. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. J. Pharmacogn. Phytochem. 2018, 7, 750–757. [Google Scholar]
- Sharifzadeh Naeini, M.; Naghavi, M.R.; Bihamta, M.R.; Sabokdast, M.; Salehi, M. Production of some benzylisoquinoline alkaloids in Papaver armeniacum L. hairy root cultures elicited with salicylic acid and methyl jasmonate. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 261–271. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Giménez, M.J.; García-Pastor, M.E.; Serrano, M.; Zapata, P.J. Preharvest application of methyl jasmonate as an elicitor improves the yield and phenolic content of artichoke. J. Agric. Food Chem. 2017, 65, 9247–9254. [Google Scholar] [CrossRef]
- Pesaraklu, A.; Radjabian, T.; Salami, S.A. Methyl jasmonate and Ag+ as effective elicitors for enhancement of phenolic acids contents in Salvia officinalis and Salvia verticillata, as two traditional medicinal plants. S. Afr. J. Bot. 2021, 141, 105–115. [Google Scholar] [CrossRef]
- Paeizi, M.; Karimi, F.; Razavi, K. Changes in medicinal alkaloids production and expression of related regulatory and biosynthetic genes in response to silver nitrate combined with methyl jasmonate in Catharanthus roseus in vitro propagated shoots. Plant Physiol. Biochem. 2018, 132, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Buraphaka, H.; Putalun, W. Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crops Prod. 2020, 146, 112171. [Google Scholar] [CrossRef]
- Andi, S.A.; Gholami, M.; Ford, C.M. The effect of methyl jasmonate and light irradiation treatments on the stilbenoid biosynthetic pathway in Vitis vinifera cell suspension cultures. Nat. Prod. Res. 2018, 32, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Bahabadi, S.E.; Sharifi, M.; Behmanesh, M.; Safaie, N.; Murata, J.; Araki, R.; Yamagaki, T.; Satake, H. Time-course changes in fungal elicitor-induced lignan synthesis and expression of the relevant genes in cell cultures of Linum album. J. Plant Physiol. 2012, 169, 487–491. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, J.; Zhu, J.; He, S.; Zhang, W.; Yu, R.; Zi, J.; Song, L.; Huang, X. Effects of β-cyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl. Microbiol. Biotechnol. 2015, 99, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.V.; Pongkitwitoon, B.; Pathomwichaiwat, T.; Viboonjun, U.; Prathanturarug, S. Effects of methyl jasmonate on the growth and triterpenoid production of diploid and tetraploid Centella asiatica (L.) Urb. hairy root cultures. Sci. Rep. 2019, 9, 18665. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Fan, M.Z.; Li, X.F.; Piao, X.C.; Gao, R.; Lian, M.L. Involvement of putrescine, nitric oxide, and hydrogen peroxide in methyl jasmonate-induced ginsenoside synthesis in adventitious root cultures of Panax ginseng CA Meyer. J. Plant Growth Regul. 2021, 40, 1440–1449. [Google Scholar] [CrossRef]
- Seegers, C.L.; Setroikromo, R.; Tepper, P.G.; Horvatovich, P.; Peters, R.; Quax, W.J. Methyl jasmonate treatment increases podophyllotoxin production in Podophyllum hexandrum roots under glasshouse conditions. Plant Soil 2017, 417, 117–126. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef] [PubMed]
- Saiman, M.Z.; Mustafa, N.R.; Choi, Y.H.; Verpoorte, R.; Schulte, A.E. Metabolic alterations and distribution of five-carbon precursors in jasmonic acid-elicited Catharanthus roseus cell suspension cultures. Plant Cell Tissue Organ Cult. 2015, 122, 351–362. [Google Scholar] [CrossRef]
- Syeed, R.; Mujib, A.; Dewir, Y.H.; Malik, M.Q.; Bansal, Y.; Ejaz, B.; Mamgain, J.; Hakiman, M.; Alsughayyir, A. Methyl jasmonate elicitation for in vitro lycorine accumulation in three Zephyranthes species and comparative analysis of tissue-cultured and field grown plants. Horticulturae 2023, 9, 832. [Google Scholar] [CrossRef]
- Sape, S.T.; Kandukuri, A.V.; Owk, A.K. Direct axillary shoot regeneration with nodal explants of Bacopa monnieri (L.) Pennell—A multi medicinal herb. J. Appl. Biol. Sci. 2020, 14, 190–197. [Google Scholar]
- Bhat, M.S.; Rather, Z.A.; Nazki, I.T.; Banday, N.; Wani, T.; Rafiq, S.; Farooq, I.; Noureldeen, A.; Darwish, H. Standardization of in vitro micropropagation of Winter Jasmine (Jasminum nudiflorum) using nodal explants. Saudi J. Biol. Sci. 2022, 29, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Oseni, O.M.; Nailwal, T.K.; Pande, V. Callus induction and multiple shoot proliferation from nodal explants of Mansonia altissima: Confirmation of genetic stability using ISSR and RAPD markers. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 479–488. [Google Scholar] [CrossRef]
- Buah, J.N.; Danso, E.; Taah, E.A.; Abole, E.A.; Bediako, E.A.; Asiedu, J.; Baidoo, R. The effects of different concentrations cytokinins on the in vitro multiplication of plantain (Musa sp.). Biotechnology 2010, 9, 343–347. [Google Scholar] [CrossRef]
- Singh, M.K.; Yadav, T.; Raman, R.K. A quick method for micro-propagation of Aloe vera L. from leaf explants via callus induction. J. Entomol. Zool. Stud. 2020, 8, 201–206. [Google Scholar]
- Hill, K.; Schaller, G.E. Enhancing plant regeneration in tissue culture: A molecular approach through manipulation of cytokinin sensitivity. Plant Signal. Behav. 2013, 8, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Mujib, A.; Fatima, S.; Malik, M.Q. Gamma ray–induced tissue responses and improved secondary metabolites accumulation in Catharanthus roseus. Appl. Microbiol. Biotechnol. 2022, 106, 6109–6123. [Google Scholar] [CrossRef] [PubMed]
- Dilshad, E.; Asif, A.; Arooj, H.; Khan, S.H.; Bakhtiar, S.M. Impact of BAP on in vitro regeneration of potato (Solanum tuberosum L.). Curr. Trends OMICS 2021, 1, 67–79. [Google Scholar] [CrossRef]
- Mood, K.; Jogam, P.; Sirikonda, A.; Shekhawat, M.S.; Rohela, G.K.; Manokari, M.; Allini, V.R. Micropropagation, morpho-anatomical characterization, and genetic stability studies in Lippia javanica (Burm. f.) Spreng: A multipurpose medicinal plant. Plant Cell Tissue Organ Cult. 2022, 150, 427–437. [Google Scholar] [CrossRef]
- Singh, C.K.; Raj, S.R.; Patil, V.R.; Jaiswal, P.S.; Subhash, N. Plant regeneration from leaf explants of mature sandalwood (Santalum album L.) trees under in vitro conditions. In Vitro Cell. Dev. Biol.-Plant 2013, 49, 216–222. [Google Scholar] [CrossRef]
- Martins, J.P.; Wawrzyniak, M.K.; Ley-López, J.M.; Kalemba, E.M.; Mendes, M.M.; Chmielarz, P. 6-Benzylaminopurine and kinetin modulations during in vitro propagation of Quercus robur (L.): An assessment of anatomical, biochemical, and physiological profiling of shoots. Plant Cell Tissue Organ Cult. 2022, 151, 149–164. [Google Scholar] [CrossRef]
- Talan, A.; Mujib, A.; Ejaz, B.; Bansal, Y.; Dewir, Y.H.; Magyar-Tábori, K. In vitro propagation and phytochemical composition of Centratherum punctatum Cass-a medicinal plant. Horticulturae 2023, 9, 1189. [Google Scholar] [CrossRef]
- Mujib, A.; Pipal, T.; Ali, M.; Tonk, D.; Zafar, N.; Gulzar, B. In vitro propagation of Althaea officinalis: The role of plant growth regulators in morphogenesis. BioTechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2017, 98, 167–173. [Google Scholar] [CrossRef]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Zakaria, S.; Hamzah, Z.; Reddy Pamuru, R.; Gundala, P.B.; Reduan, M.F. Micropropagation of Alocasia longiloba Miq and comparative antioxidant properties of ethanolic extracts of the field-grown plant, in vitro propagated and in vitro-derived callus. Plants 2020, 9, 816. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Mujib, A.; Bansal, Y.; Dewir, Y.H.; Mendler-Drienyovszki, N. An Efficient In Vitro Shoot Organogenesis and Comparative GC-MS Metabolite Profiling of Gaillardia pulchella Foug. Horticulturae 2024, 10, 728. [Google Scholar] [CrossRef]
- Bhat, M.P.; Rudrappa, M.; Hugar, A.; Gunagambhire, P.V.; Suresh Kumar, R.; Nayaka, S.; Almansour, A.I.; Perumal, K. In vitro investigation on the biological activities of squalene derived from the soil fungus Talaromyces pinophilus. Heliyon 2023, 9, e21461. [Google Scholar] [CrossRef] [PubMed]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC-MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep. 2020, 10, 16438. [Google Scholar] [CrossRef]
- Bansal, Y.; Mujib, A.; Mamgain, J.; Dewir, Y.H.; Rihan, H.Z. Phytochemical composition and detection of novel bioactives in anther callus of Catharanthus roseus L. Plants 2023, 12, 2186. [Google Scholar] [CrossRef]
- Abou-Donia, A.H.; Toaima, S.M.; Hammoda, H.M.; Shawky, E. New rapid validated HPTLC method for the determination of lycorine in amaryllidaceae plants extracts. Chromatographia 2007, 65, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Abou-Donia, A.H.; Toaima, S.M.; Hammoda, H.M.; Shawky, E. New rapid validated HPTLC method for the determination of galanthamine in Amaryllidaceae plant extracts. Phytochem. Anal. 2008, 19, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules 2020, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Asao, T.; Asaduzzaman, M. (Eds.) Phytochemicals: Source of Antioxidants and Role in Disease Prevention; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Ptak, A.; Morańska, E.; Saliba, S.; Zieliński, A.; Simlat, M.; Laurain-Mattar, D. Elicitation of galanthamine and lycorine biosynthesis by Leucojum aestivum L. and L. aestivum ‘Gravety Giant’plants cultured in bioreactor RITA®. Plant Cell Tissue Organ Cult. 2017, 128, 335–345. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hahn, E.J.; Murthy, H.N.; Paek, K.Y. Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol. Lett. 2004, 26, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ma, Y.; Hu, P.; Zhang, Y.; Chen, J.; Li, X. Elicitation of furanocoumarins in Changium smyrnioides suspension cells. Plant Cell Tissue Organ Cult. 2017, 130, 1–12. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha× piperita cell suspension cultures. Plant Cell Tissue Organ Cult. 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult. 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Tarakemeh, A.; Azizi, M.; Rowshan, V.; Salehi, H.; Spina, R.; Dupire, F.; Arouie, H.; Laurain-Mattar, D. Screening of Amaryllidaceae alkaloids in bulbs and tissue cultures of Narcissus papyraceus and four varieties of N. tazetta. J. Pharm. Biomed. Anal. 2019, 172, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Akhgari, A.; Laakso, I.; Maaheimo, H.; Choi, Y.H.; Seppänen-Laakso, T.; Oksman-Caldentey, K.M.; Rischer, H. Methyljasmonate elicitation increases terpenoid indole alkaloid accumulation in Rhazya stricta hairy root cultures. Plants 2019, 8, 534. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.R.; Gierczik, K.; Ibrahem, M.M.; Matter, M.A.; Galiba, G. Anticancer compounds production in Catharanthus roseus by methyl jasmonate and UV-B elicitation. S. Afr. J. Bot. 2021, 142, 34–41. [Google Scholar] [CrossRef]
- Ho, T.T.; Murthy, H.N.; Park, S.Y. Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue Organ Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Medicinal plants: Factors of influence on the content of secondary metabolites. Química Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
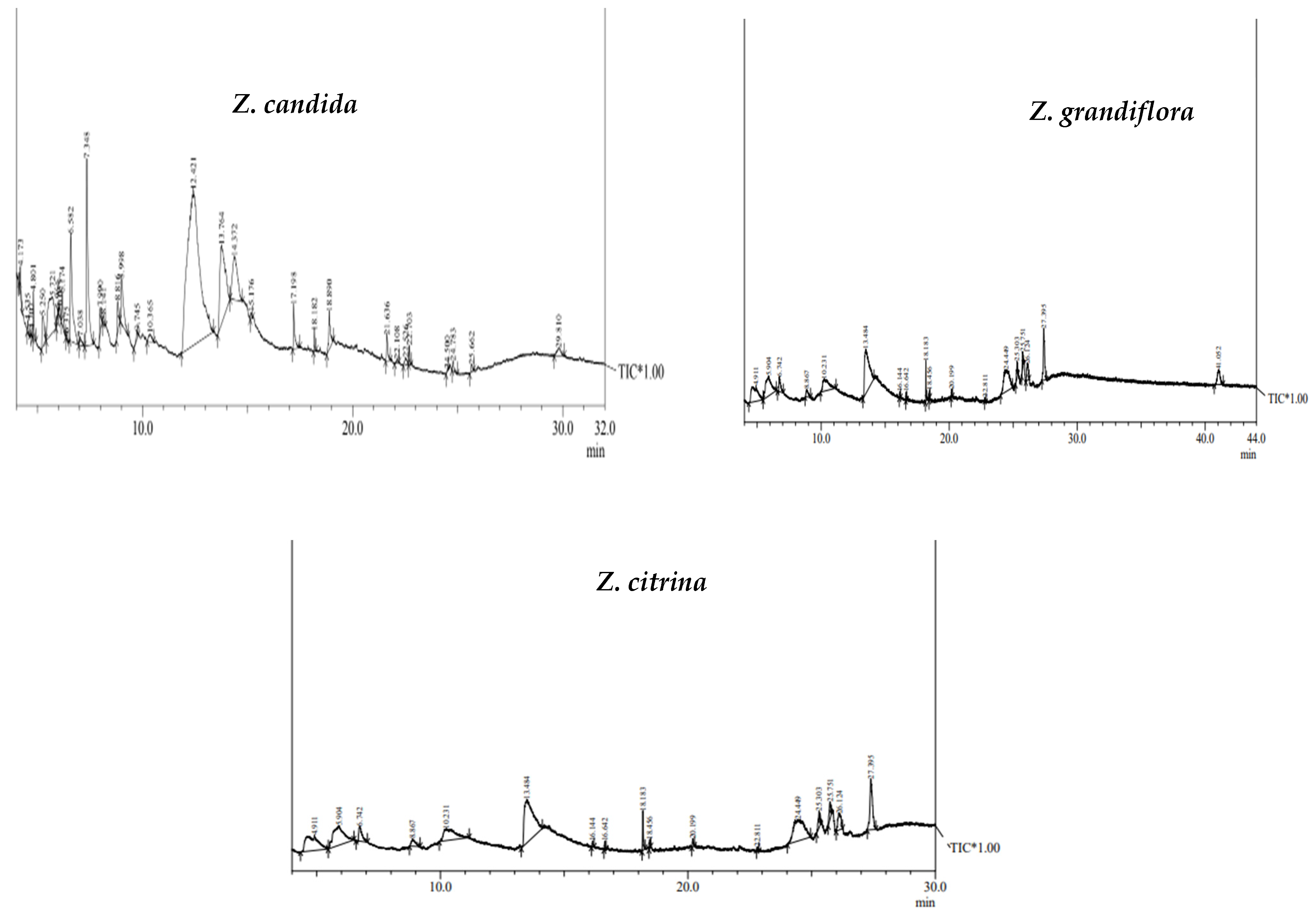

| Peak | R.Time | Area | Area% | Name |
|---|---|---|---|---|
| 1 | 4.173 | 529325 | 0.57 | N-Isopentyl-N-nitroso-pentylamine |
| 2 | 4.535 | 212439 | 0.23 | Methylolacetone |
| 3 | 4.707 | 92871 | 0.10 | N-acetyl-LAsparticacid |
| 4 | 4.801 | 885370 | 0.96 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one |
| 5 | 5.250 | 1473326 | 1.59 | 2-Hydroxy-gamma-butyrolactone |
| 6 | 5.721 | 5266629 | 5.70 | 1,2,3-propanetriol |
| 7 | 5.964 | 197792 | 0.21 | Acetic acid, pentylester |
| 8 | 6.059 | 263065 | 0.28 | 4-Acetylbutyricacid |
| 9 | 6.174 | 1048162 | 1.13 | 3,4-dihydroxy-3-methylbutyl Acetate |
| 10 | 6.375 | 123480 | 0.13 | 1,3-dioxolane-4-methanol,2,2- Dimethyl- |
| 11 | 6.582 | 5097432 | 5.51 | Cyclopropylmethanol |
| 12 | 7.038 | 415911 | 0.45 | 5-amino-6-nitroso-2,4(1h,3h)- Pyrimidinedione |
| 13 | 7.990 | 7141625 | 7.72 | 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6- methyl- |
| 14 | 8.141 | 867823 | 0.94 | 2(3H)-Furanone,dihydro-4-hydroxy-3-methylene- |
| 15 | 8.816 | 237368 | 0.26 | Carbamicacid,(3,4,4-trimethyl-1,2-dioxetan-3- yl)methyl |
| 16 | 8.998 | 935988 | 1.01 | 5-Hydroxymethylfurfural |
| 17 | 9.745 | 2562191 | 2.77 | 1,2,3-propanetriol,diacetate |
| 18 | 10.365 | 296036 | 0.32 | 2-Methoxy-4-vinylphenol |
| 19 | 12.421 | 511122 | 0.55 | .alpha.-D-Galactopyranoside,methyl |
| 20 | 13.764 | 43505913 | 47.05 | guanosine |
| 21 | 15.176 | 9639265 | 10.42 | alpha.-D-Galactopyranoside |
| 22 | 17.198 | 5062691 | 5.48 | Pretazettinealpha.-D-Galactopyranoside,methyl |
| 23 | 15.176 | 165523 | 0.18 | Stevioside |
| 24 | 17.198 | 1307423 | 1.41 | n-Hexadecanoicacid |
| 25 | 18.182 | 311511 | 0.34 | 13-Hexyloxacyclotridec-10-en-2-one |
| 26 | 18.890 | 1369541 | 1.48 | 9-Octadecenoicacid |
| 27 | 21.636 | 789504 | 0.85 | Lycoramine |
| 28 | 22.108 | 100646 | 0.11 | Lycorenan-7-one,2,4-didehydro-2- Deshydroxy-phenanthridin-1-ol |
| 29 | 22.526 | 381154 | 0.41 | 4-[1-(1-hydroxy-ethyl)-1h-indol-4-yl]- 2-meth |
| 30 | 22.703 | 336879 | 0.36 | Dimethyl2,6-dimethyl-4-(2- Nitrophenyl)-1,4- |
| 31 | 24.500 | 78521 | 0.08 | 1,3-cyclohexanedicarboxamide, Trans- |
| 32 | 24.783 | 312808 | 0.34 | Tazettine |
| 33 34 | 25.662 29.810 | 251686 693091 | 0.27 075 | Galanthan-1-ol,9-methoxy-4-methyl-11-oxa-4-azatetracyclotetraen-14-ol Isopropyllinoleate |
| Parts Used | Z. candida | Z. grandiflora | Z. citrina |
|---|---|---|---|
| Bulb | 2.41 ± 0.04a | 2.13 ± 0.04a | 2.02 ± 0.03a |
| Leaf | 1.38 ± 0.02b | 1.23 ± 0.03b | 1.12 ± 0.01b |
| Root | 0.61 ± 0.01c | 0.50 ± 0.01c | 0.42 ± 0.01c |
| Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Bulb | 2.41 ± 0.03c | 2.51 ± 0.04b | 2.58 ± 0.04d | 3.97 ± 0.04a | 2.02 ± 0.02e |
| Leaf | 1.38 ± 0.04d | 1.53 ± 0.03b | 1.87 ± 0.02c | 2.06 ±0.03a | 1.27 ± 0.02d |
| Root | 0.51 ± 0.01c | 0.53 ± 0.01b | 0.67 ± 0.01d | 0.70 ±0.01a | 0.54 ± 0.01c |
| Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Bulb | 2.07 ± 0.04c | 2.26 ± 0.05b | 2.62 ± 0.04c | 2.93 ± 0.06a | 1.64 ± 0.05d |
| Leaf | 1.43 ± 0.02c | 1.52 ± 0.03b | 1.74 ± 0.02d | 1.97 ± 0.04a | 1.01 ± 0.03e |
| Root | 0.60 ± 0.03b | 0.61 ± 0.01a | 0.63 ± 0.01b | 0.65 ± 0.01a | 0.47 ± 0.001b |
| Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Bulb | 2.13 ± 0.03c | 2.45 ± 0.04c | 2.66 ± 0.05b | 2.87 ± 0.02a | 2.06 ± 0.04b |
| Leaf | 1.22 ± 0.01c | 1.43 ± 0.02d | 1.65 ± 0.03b | 1.98 ± 0.003a | 1.32 ± 0.01d |
| Root | 0.34 ± 0.01c | 0.37 ± 0.01d | 0.40 ± 0.01b | 0.42 ± 0.001a | 0.12 ± 0.006e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syeed, R.; Mujib, A.; Bansal, Y.; Mohsin, M.; Nafees, A.; Malik, M.Q.; Mamgain, J.; Ejaz, B.; Dewir, Y.H.; Magyar-Tábori, K. Tissue-Specific Natural Synthesis of Galanthaminein Zephyranthes Species and Its Accumulation in Different In Vitro-Grown Organs Following Methyl Jasmonate Treatment. Plants 2024, 13, 1931. https://doi.org/10.3390/plants13141931
Syeed R, Mujib A, Bansal Y, Mohsin M, Nafees A, Malik MQ, Mamgain J, Ejaz B, Dewir YH, Magyar-Tábori K. Tissue-Specific Natural Synthesis of Galanthaminein Zephyranthes Species and Its Accumulation in Different In Vitro-Grown Organs Following Methyl Jasmonate Treatment. Plants. 2024; 13(14):1931. https://doi.org/10.3390/plants13141931
Chicago/Turabian StyleSyeed, Rukaya, A. Mujib, Yashika Bansal, Mohammad Mohsin, Afeefa Nafees, Moien Qadir Malik, Jyoti Mamgain, Bushra Ejaz, Yaser Hassan Dewir, and Katalin Magyar-Tábori. 2024. "Tissue-Specific Natural Synthesis of Galanthaminein Zephyranthes Species and Its Accumulation in Different In Vitro-Grown Organs Following Methyl Jasmonate Treatment" Plants 13, no. 14: 1931. https://doi.org/10.3390/plants13141931
APA StyleSyeed, R., Mujib, A., Bansal, Y., Mohsin, M., Nafees, A., Malik, M. Q., Mamgain, J., Ejaz, B., Dewir, Y. H., & Magyar-Tábori, K. (2024). Tissue-Specific Natural Synthesis of Galanthaminein Zephyranthes Species and Its Accumulation in Different In Vitro-Grown Organs Following Methyl Jasmonate Treatment. Plants, 13(14), 1931. https://doi.org/10.3390/plants13141931








