Metabolic Response of the Lycium barbarum Variety ‘Ningqi No. 7′ to Drought Stress
Abstract
1. Introduction
2. Results
2.1. Changes in the Physiological Indices of L. barbarum under Drought Stress
2.2. Metabolite Statistics and Quality Control
2.3. Principal Component Analysis
2.4. Orthogonal Partial Least Squares Discriminant Analysis
2.5. Screening of Differential Metabolites
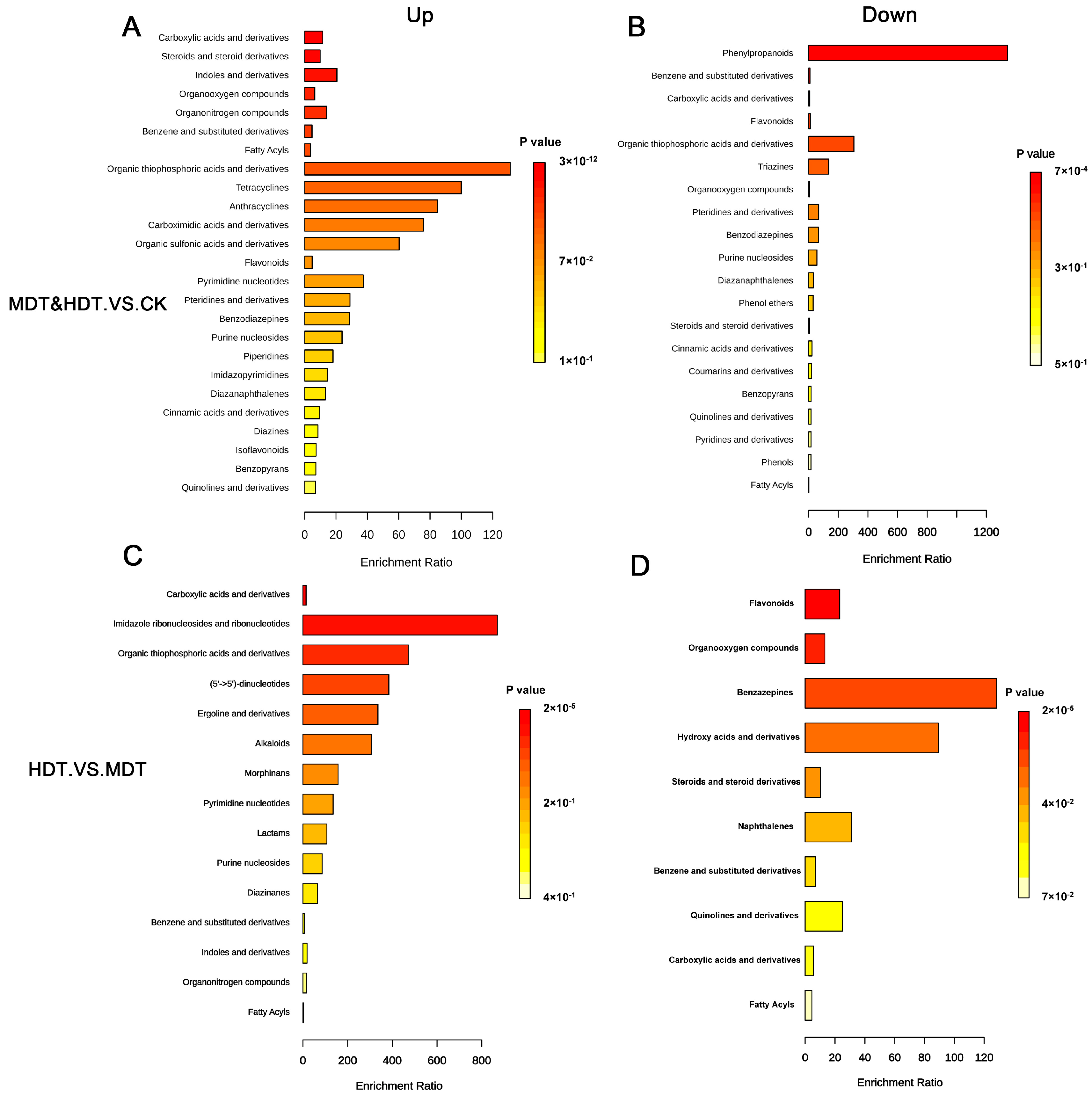
2.6. KEGG Pathway Enrichment Analysis of Differential Metabolites
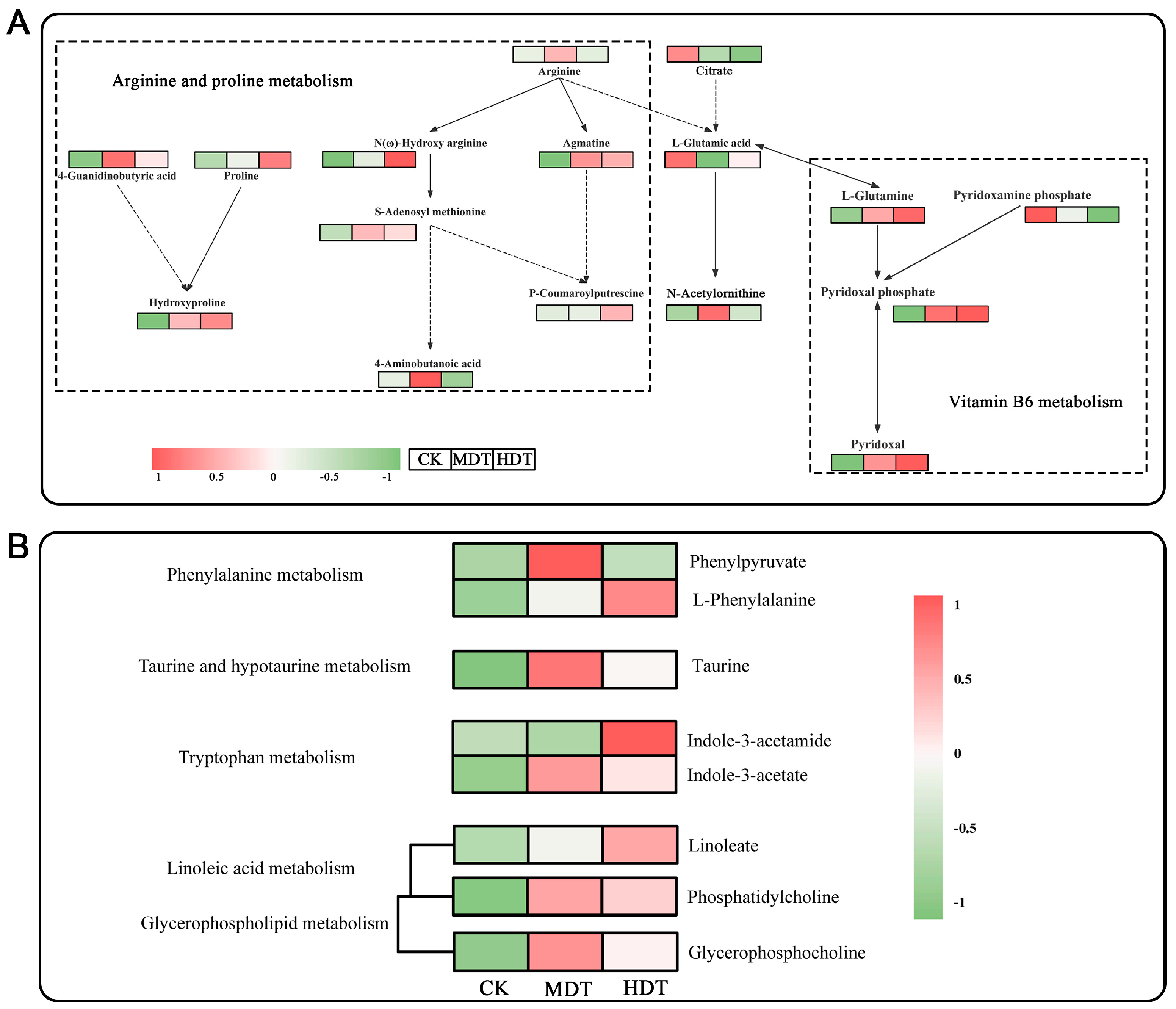
2.7. Analysis of Key Metabolic Pathways
3. Discussion
4. Materials and Methods
4.1. Source of the Tested L. barbarum Plant Specimens
4.2. Drought Stress Treatment
4.3. Determination of Physiological Parameters of L. barbarum Leaves
4.4. UPLC-MS Detection of L. barbarum Leaves
4.5. Metabolomics Data Analysis
4.6. Data Visualization and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.L.; Li, Y.; Fan, Y.F.; Li, Z.; Yoshida, K.; Wang, J.Y.; Liu, Z.J. Wolfberry genomes and the evolution of Lycium (Solanaceae). Commun. Biol. 2021, 4, 671. [Google Scholar] [CrossRef]
- Liang, X.; An, W.; Li, Y.; Qin, X.; Zhao, J.; Su, S. Effects of different nitrogen application rates and picking batches on the nutritional components of Lycium barbarum L. fruits. Front. Plant Sci. 2024, 15, 1355832. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhao, L.; Zhang, Y.; Sun, L.; Yu, X.; Yu, Y. Can climate change influence agricultural GTFP in arid and semi-arid regions of Northwest China? J. Arid Land 2020, 12, 837–853. [Google Scholar] [CrossRef]
- Wang, C.; Qi, G.; Ma, Y.; Yin, M.; Wang, J.; Kang, Y.; Jia, Q.; Gao, Y.; Tian, R.; Zhang, R.; et al. Effects of Water and Nitrogen Control on the Growth Physiology, Yields, and Economic Benefits of Lycium barbarum Plants in a Lycium barbarum+ Alfalfa System. Plants 2024, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Yokoyama, J.; Ohashi, H. Phylogeny and biogeography of the genus Lycium (Solanaceae): Inferences from chloroplast DNA sequences. Mol. Phylogenetics Evol. 2001, 19, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. Economic effects of climate change on global agricultural production. Nat. Conserv. 2021, 44, 117–139. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Liu, K.; Zhang, W.; Zhou, Y.; Tang, M. Synergistic effect of extra potassium application and AM fungi on drought tolerance of Lycium barbarum. New For. 2024, 55, 101–117. [Google Scholar] [CrossRef]
- Xu, S.; Ma, R.; Zhang, E.; Chen, Y.; Wang, Q.; Liu, Q. Drought stress, mercuric chloride, and β-mercaptoethanol effects on hydraulic characteristics of three cultivars of wolfberry (Lycium chinense). J. For. Res. 2021, 32, 233–240. [Google Scholar] [CrossRef]
- Tezara, W.; Martínez, D.; Rengifo, E.; Herrera, A.N. Photosynthetic responses of the tropical spiny shrub Lycium nodosum (Solanaceae) to drought, soil salinity and saline spray. Ann. Bot. 2003, 92, 757–765. [Google Scholar] [CrossRef]
- Xu, S.; Ma, R.; Nan, X.; Yang, S.; Zhang, E.; Zhang, Y. Effects of Drought and Nitrogen Treatments on Water Storage and Transportation in Lycium barbarum L. Gesunde Pflanz. 2023, 75, 861–870. [Google Scholar] [CrossRef]
- Breniere, T.; Fanciullino, A.L.; Dumont, D.; Le Bourvellec, C.; Riva, C.; Borel, P.; Landrier, J.F.; Bertin, N. Effect of long-term deficit irrigation on tomato and goji berry quality: From fruit composition to in vitro bioaccessibility of carotenoids. Front. Plant Sci. 2024, 15, 1339536. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Yan, Y.; Liu, J. Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum Murr: A review. Carbohydr. Polym. 2022, 291, 119618. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Yin, Y.; An, W.; Qin, X.; Wang, Y.; Li, Y.; Fan, Y.; Cao, Y. Transcriptomic and metabolomic analyses of Lycium ruthenicum and Lycium barbarum fruits during ripening. Sci. Rep. 2020, 10, 4354. [Google Scholar] [CrossRef]
- Snyder, F.; Yi, L.; Yazdani, G. Traditional Chinese medicine and European Union law: Cultural logics, product identities, market competition, legal rechanneling, and the need for global legal and medical pluralism. Peking Univ. Law J. 2014, 2, 129–200. [Google Scholar] [CrossRef]
- Liu, J.; Shi, X.; Lin, H.; He, C.; Li, Q.; Shen, G.; Feng, J. Geographical origin identification and quality comparison of Ningxia goji berries (Lycium barbarum L.) by NMR-based techniques. J. Food Compos. Anal. 2023, 119, 105258. [Google Scholar] [CrossRef]
- Pezzatti, J.; Boccard, J.; Codesido, S.; Gagnebin, Y.; Joshi, A.; Picard, D.; González-Ruiz, V.; Rudaz, S. Implementation of liquid chromatography–high resolution mass spectrometry methods for untargeted metabolomic analyses of biological samples: A tutorial. Anal. Chim. Acta 2020, 1105, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Rangani, J.; Panda, A.; Parida, A.K. Metabolomic study reveals key metabolic adjustments in the xerohalophyte Salvadora persica L. during adaptation to water deficit and subsequent recovery conditions. Plant Physiol. Biochem. 2020, 150, 180–195. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Espelie, K.E. Chemistry, biochemistry, and function of suberin and associated waxes. In Natural Products of Woody Plants: Chemicals Extraneous to the Lignocellulosic Cell Wall; Springer: Berlin/Heidelberg, Germany, 1989; pp. 304–367. [Google Scholar]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia AYuan, Z.C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Hanes, J.; Tews, I.; Ealick, S.E.; Begley, T.P. Pyridoxal phosphate: Biosynthesis and catabolism. Biochim. Biophys. Acta-Proteins Proteom. 2011, 1814, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, E.M.; Xu, L.Y. Guide to metabolomics analysis: A bioinformatics workflow. Metabolites 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Li, H.X.; Zhang, C.Z.; Wei, A.N.; Yue, Y.I.N.; Wang YJCao, Y.L. Physiological response of four wolfberry (Lycium Linn.) species under drought stress. J. Integr. Agric. 2018, 17, 603–612. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Zhang, Z.; He, K. The physiological and biochemical photosynthetic properties of Lycium ruthenicum Murr in response to salinity and drought. Sci. Hortic. 2019, 256, 108530. [Google Scholar] [CrossRef]
- Kriedemann, P.E.; Barrs, H.D. Photosynthetic adaptation to water stress and implications for drought resistance. In Crop Reactions to Water and Temperature Stresses in Humid, Temperate Climates; CRC Press: Boca Raton, FL, USA, 2019; pp. 201–230. [Google Scholar]
- Guo, Y.Y.; Yu, H.Y.; Yang, M.M.; Kong, D.S.; Zhang, Y.J. Effect of drought stress on lipid peroxidation, osmotic adjustment and antioxidant enzyme activity of leaves and roots of Lycium ruthenicum Murr. seedling. Russ. J. Plant Physiol. 2018, 65, 244–250. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Faroux, J.M.; Ureta, M.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A. An overview of peroxidation reactions using liposomes as model systems and analytical methods as monitoring tools. Colloids Surf. B Biointerfaces 2020, 195, 111254. [Google Scholar] [CrossRef]
- Becana, M.; Moran, J.F.; Iturbe-Ormaetxe, I. Iron-dependent oxygen free radical generation in plants subjected to environmental stress: Toxicity and antioxidant protection. Plant Soil 1998, 201, 137–147. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 165102. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth–metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Slocum, R.D. Genes, enzymes and regulation of arginine biosynthesis in plants. Plant Physiol. Biochem. 2005, 43, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Pardo-Hernández, M.; López-Delacalle, M.; Rivero, R.M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 2020, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, T.; Hu, J.; Zhao, L.; Yu, C.; Ma, F. Research advances in function and regulation mechanisms of plant small heat shock proteins (sHSPs) under environmental stresses. Sci. Total Environ. 2022, 825, 154054. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hortic. Res. 2020, 7, 40. [Google Scholar] [CrossRef]
- Huang, S.; Zeng, H.; Zhang, J.; Wei, S.; Huang, L. Characterization of enzymes involved in the interconversions of different forms of vitamin B6 in tobacco leaves. Plant Physiol. Biochem. 2011, 49, 1299–1305. [Google Scholar] [CrossRef]
- Geng, R.; Li, X.; Huang, J.; Zhou, W. The chloroplast singlet oxygen-triggered biosynthesis of salicylic acid and jasmonic acid is mediated by EX1 and GUN1 in Arabidopsis. Plant Cell Environ. 2024, 47, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Tian, Y.; Hou, X.; Hou, X.; Jia, Z.; Li, M.; Liu, Y. Multiple forms of vitamin B6 regulate salt tolerance by balancing ROS and abscisic acid levels in maize root. Stress Biol. 2022, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.J.; Schmidt, M.B. Vitamin B6, a growth substance for excised tomato roots. Proc. Natl. Acad. Sci. USA 1939, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Maniero, R.A.; Giehl, R.F.; Melzer, M.; Steensma, P.; Krouk, G.; Wirén, N. PDX1. 1-dependent biosynthesis of vitamin B6 protects roots from ammonium-induced oxidative stress. Mol. Plant 2022, 15, 820–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Q.N.; Lu, J.; Zhang, C.H.; Zhang, L.; Wu, Y. The effects of exogenous pyridoxal-5-phosphate on seedling growth and development of wheat under salt stress. Cereal Res. Commun. 2019, 47, 442–454. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Lycium barbarum berries (Solanaceae) as source of bioactive compounds for healthy purposes: A review. Int. J. Mol. Sci. 2023, 24, 4777. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, Q.; Sun, Y. Black goji berry (Lycium ruthenicum) tea has higher phytochemical contents and in vitro antioxidant properties than red goji berry (Lycium barbarum) tea. Food Qual. Saf. 2020, 4, 193–201. [Google Scholar] [CrossRef]
- Surai, P.F.; Earle-Payne, K.; Kidd, M.T. Taurine as a natural antioxidant: From direct antioxidant effects to protective action in various toxicological models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, V.; Ovadia, R.; Oren-Shamir, M. Phenylalanine in motion: A tale of an essential molecule with many faces. Biotechnol. Adv. 2023, 68, 108246. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Kim, S.; Jeong, E.; Shim, J.S. Modulation of lignin biosynthesis for drought tolerance in plants. Front. Plant Sci. 2023, 14, 1116426. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y. Plant microbiomes with phytohormones: Attribute for plant growth and adaptation under the stress conditions. In Advances in Plant Microbiome and Sustainable Agriculture Functional Annotation and Future Challenges; Springer: Singapore, 2020; pp. 85–103. [Google Scholar] [CrossRef]
- Kamtsikakis, A.; Baales, J.; Zeisler-Diehl, V.V.; Vanhecke, D.; Zoppe, J.O.; Schreiber, L.; Weder, C. Asymmetric water transport in dense leaf cuticles and cuticle-inspired compositionally graded membranes. Nat. Commun. 2021, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Torres-Franklin, M.L.; Repellin, A.; Huynh, V.B.; d’Arcy-Lameta, A.; Zuily-Fodil, Y.; Pham-Thi, A.T. Omega-3 fatty acid desaturase (FAD3, FAD7, FAD8) gene expression and linolenic acid content in cowpea leaves submitted to drought and after rehydration. Environ. Exp. Bot. 2009, 65, 162–169. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 258957. [Google Scholar] [CrossRef] [PubMed]
- Barr, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Du, H.; Guo, Z.L.; Xiong, L.Z. Determination of Relative Rice Leaf Water Content. Bio-protocol 2018, 101, e1010157. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, G.; Jiang, S.; Liu, Y.X. Wekemo Bioincloud: A user-friendly platform for meta-omics data analyses. iMeta 2024, 3, e175. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, C.; Xu, Q.; Zhao, D.; Liu, F.; Han, B. Metabolic Response of the Lycium barbarum Variety ‘Ningqi No. 7′ to Drought Stress. Plants 2024, 13, 1935. https://doi.org/10.3390/plants13141935
Liu X, Wang C, Xu Q, Zhao D, Liu F, Han B. Metabolic Response of the Lycium barbarum Variety ‘Ningqi No. 7′ to Drought Stress. Plants. 2024; 13(14):1935. https://doi.org/10.3390/plants13141935
Chicago/Turabian StyleLiu, Xiao, Chuanzhe Wang, Qiao Xu, Dan Zhao, Fei Liu, and Beibei Han. 2024. "Metabolic Response of the Lycium barbarum Variety ‘Ningqi No. 7′ to Drought Stress" Plants 13, no. 14: 1935. https://doi.org/10.3390/plants13141935
APA StyleLiu, X., Wang, C., Xu, Q., Zhao, D., Liu, F., & Han, B. (2024). Metabolic Response of the Lycium barbarum Variety ‘Ningqi No. 7′ to Drought Stress. Plants, 13(14), 1935. https://doi.org/10.3390/plants13141935







