Biochar Blended with Alkaline Mineral Can Better Inhibit Lead and Cadmium Uptake and Promote the Growth of Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil, Biochar and Alkaline Mineral
2.2. Pot Experiment
2.3. Assay
2.4. Statistical Analysis
3. Results
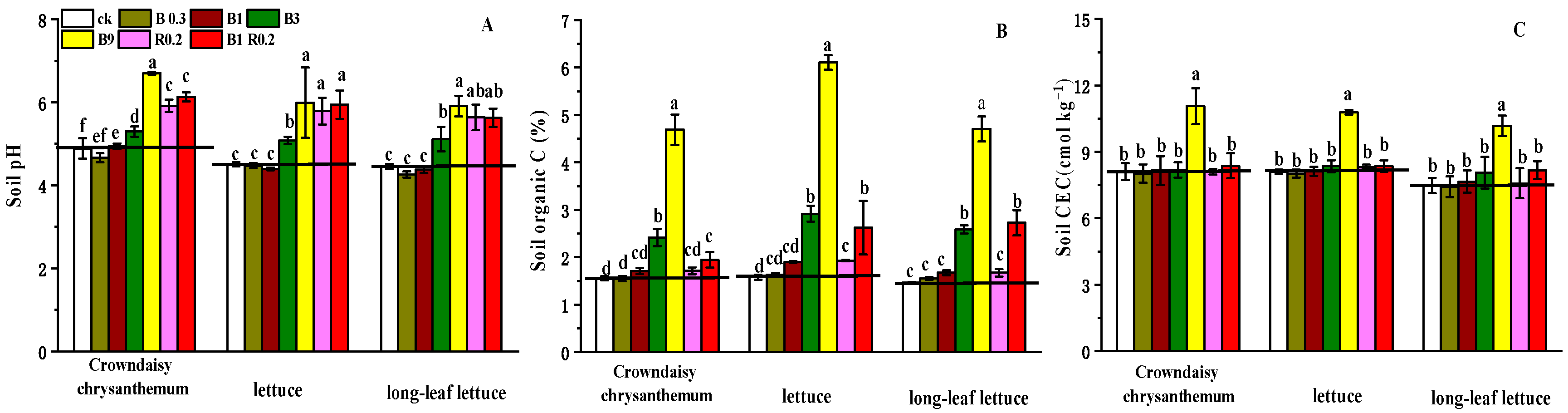
3.1. The Impacts of Amendments with Both Peanut Shell Biochar and Alkaline Mineral on Soil Properties
3.1.1. Soil pH, OC and CEC
3.1.2. Soil Available Cd and Pb
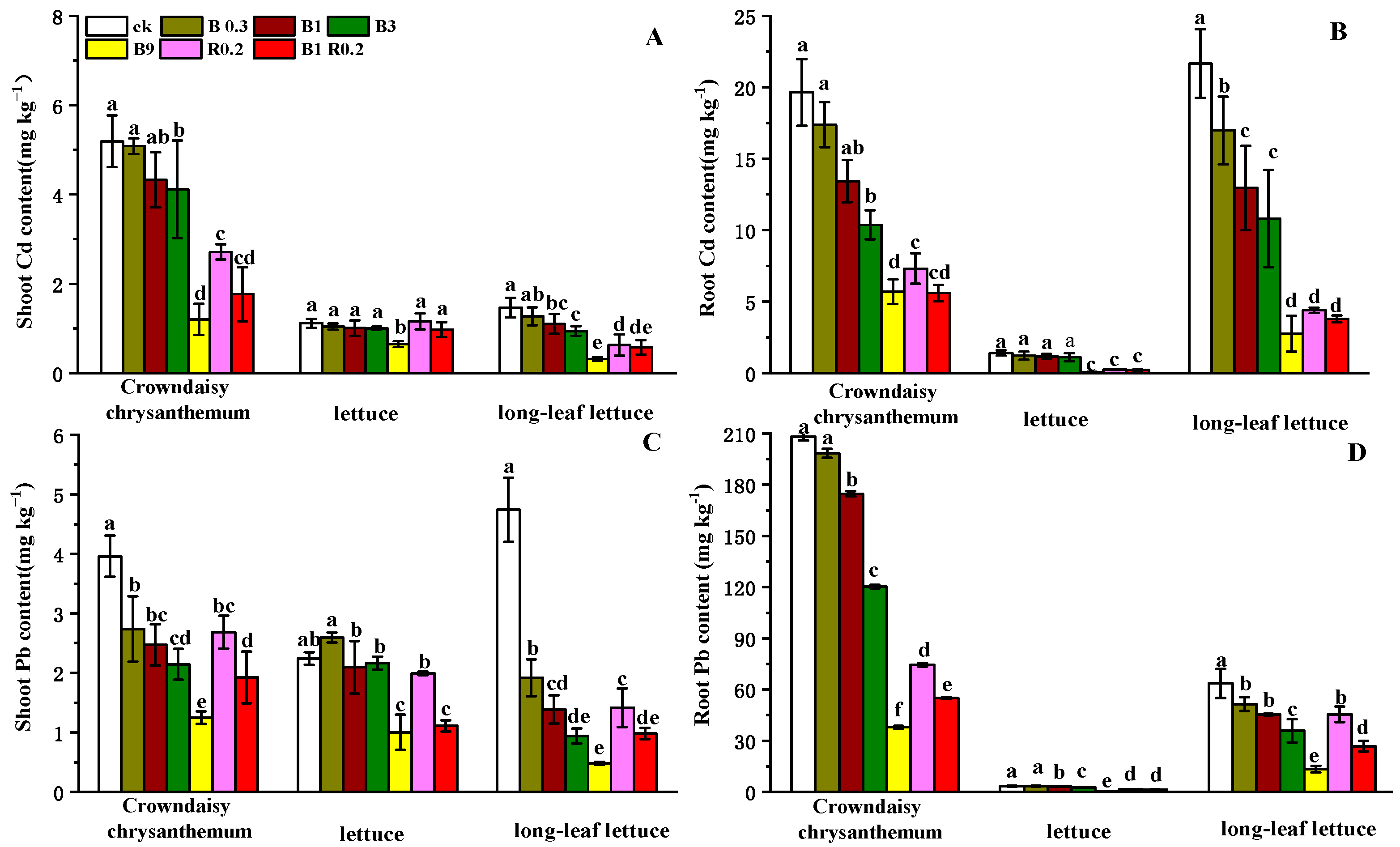
3.2. The Impacts of Amendments with Both Peanut Shell Biochar and Alkaline Mineral on Vegetable Plants
3.2.1. Increase in Vegetable Yields
3.2.2. Reduction in Cd Accumulation in Vegetable Tissues
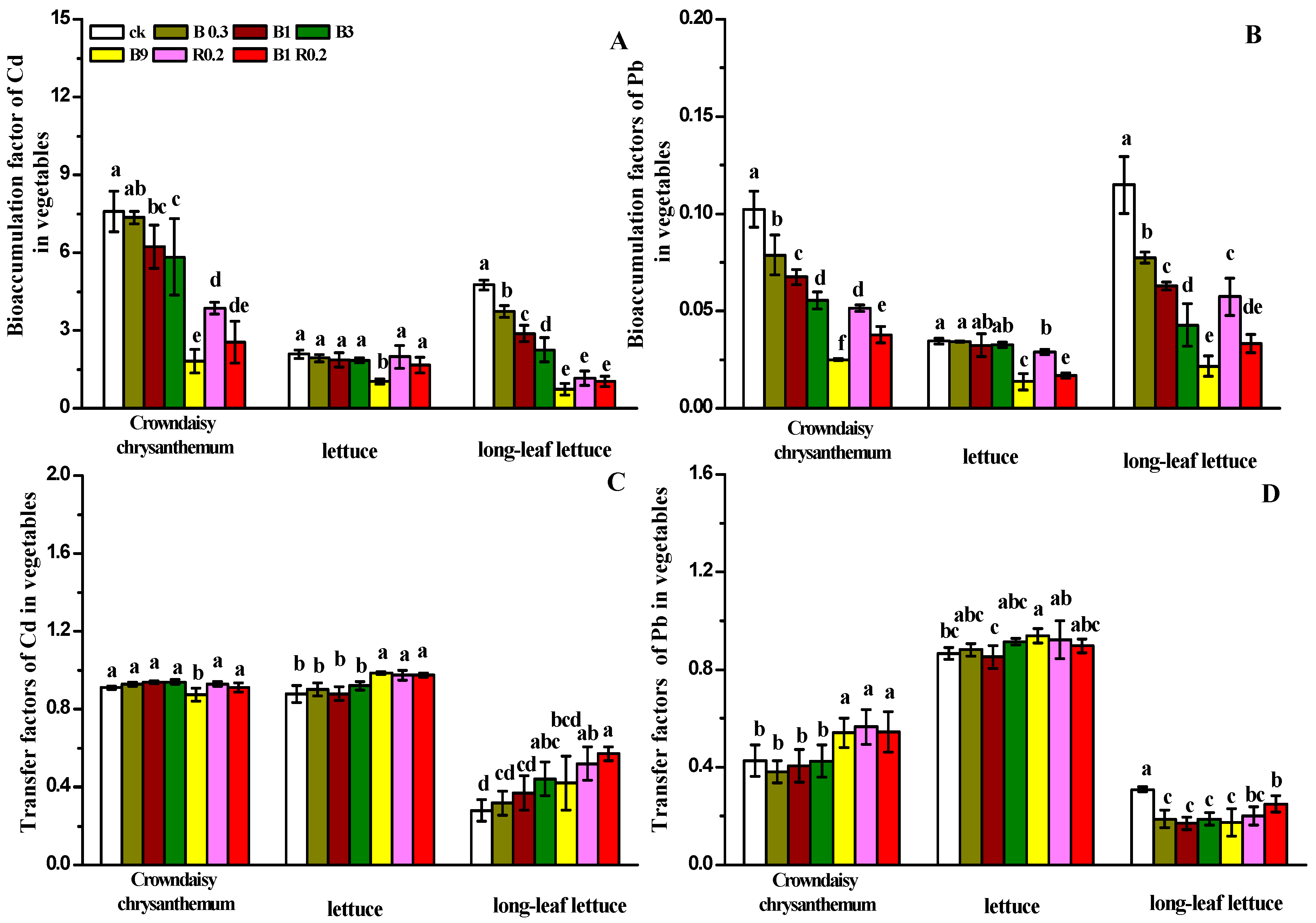
3.3. The Effects of Amendments with Peanut Shell Biochar and Alkaline Mineral on the Absorption and Transport of Heavy Metals in Vegetables
3.4. Analysis of the Main Factors Affecting the Content of Available Cd/Pb in Soil and Total Cd/Pb in Vegetables
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Usman, A.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, P.; Joseph, S.; Munroe, P.; Anawar, H.M.; Storer, P.; Gilkes, R.J.; Solaiman, Z.M. Influences of biochar and biochar-mineral complex on mycorrhizal colonisation and nutrition of wheat and sorghum. Pedosphere 2015, 25, 686–695. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Liang, Y.; Gao, B.; Harris, W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Dai, Y.; Liang, Y.; Xu, X.; Zhao, L.; Cao, X. An integrated approach for simultaneous immobilization of lead in both contaminated soil and groundwater: Laboratory test and numerical modeling. J. Hazard. Mater. 2018, 342, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, S.; Zhong, Q.; Wang, G.; Pan, X.; Xu, X.; Zhou, W.; Li, T.; Luo, L.; Zhang, Y. Soil washing remediation of heavy metal from contaminated soil with EDTMP and PAA: Properties, optimization, and risk assessment. J. Hazard. Mater. 2020, 381, 120997. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Y.; Guo, L.; Wang, Q.; Guan, C.; Yang, F.; Chen, Q. Arsenic adsorption on layered double hydroxides biochars and their amended red and calcareous soils. J. Environ. Manag. 2020, 271, 111045. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Y.; Zhou, Y.; Adeel, M.; Chen, Q. Effects of magnesium ferrite biochar on the cadmium passivation in acidic soil and bioavailability for packoi (Brassica chinensis L.). J. Environ. Manag. 2019, 251, 109610. [Google Scholar] [CrossRef]
- Giao Ngo, D.N.; Chuang, X.Y.; Huang, C.P.; Hua, L.C.; Huang, C. Compositional characterization of nine agricultural waste biochars: The relations between alkaline metals and cation exchange capacity with ammonium adsorption capability. J. Environ. Chem. Eng. 2023, 11, 110003. [Google Scholar] [CrossRef]
- Guan, C.Y.; Tseng, Y.H.; Tsang, D.C.W.; Hu, A.; Yu, C.P. Wetland plant microbial fuel cells for remediation of hexavalent chromium contaminated soils and electricity production. J. Hazard. Mater. 2019, 365, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, J.; Zhang, L.; Wang, Z. Effect of biochar addition on crop yield, water and nitrogen use efficiency: A meta-analysis. J. Cleaner Prod. 2023, 420, 138425. [Google Scholar] [CrossRef]
- Harvey, O.R.; Herbert, B.E.; Rhue, R.D.; Kuo, L.J. Metal interactions at the biochar-water interface: Energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550–5556. [Google Scholar] [CrossRef] [PubMed]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Huang, L.; Wei, L.; Liu, X.; Wu, Y.; Li, X.; Huang, Y.; Huang, Q.; Liu, Z. Effects of biochar on the migration and accumulation of lead and cadmium in soil-plant systems. J. Agro-Environ. Sci. 2020, 39, 2205–2216. [Google Scholar]
- Hussein, S.H.; Qurbani, K.; Ahmed, S.K.; Tawfeeq, W.; Hassan, M. Bioremediation of heavy metals in contaminated environments using Comamonas species: A narrative review. Bioresour. Technol. 2024, 25, 101711. [Google Scholar] [CrossRef]
- Li, J.; Hashimoto, Y.; Riya, S.; Terada, A.; Hou, H.; Shibagaki, Y.; Hosomi, M. Removal and immobilization of heavy metals in contaminated soils by chlorination and thermal treatment on an industrial-scale. Chem. Eng. J. 2019, 359, 385–392. [Google Scholar] [CrossRef]
- Liu, G.; Meng, J.; Huang, Y.; Dai, Z.; Tang, C.; Xu, J. Effects of carbide slag, lodestone and biochar on the immobilization, plant uptake and translocation of As and Cd in a contaminated paddy soil. Environ. Pollut. 2020, 266, 115194. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Fu, S.; Méndez, A.; Gascó, G.; Paz-Ferreiro, J. Can Biochar and Phytoextractors Be Jointly Used for Cadmium Remediation? PLoS ONE 2014, 9, 95218. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul Khalil, H.P.S.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Masto, R.E.; Ansari, M.A.; George, J.; Selvi, V.A.; Ram, L.C. Co-application of biochar and lignite fly ash on soil nutrients and biological parameters at different crop growth stages of Zea mays. Ecol. Eng. 2013, 58, 314–322. [Google Scholar] [CrossRef]
- Meng, J.; Zhong, L.; Wang, L.; Liu, X.; Tang, C.; Chen, H.; Xu, J. Contrasting effects of alkaline amendments on the bioavailability and uptake of Cd in rice plants in a Cd-contaminated acid paddy soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 8827–8835. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Prasher, S.; Elsayed, E.; Dhiman, J.; Mawof, A. Patel R Effect of biochar on heavy metal accumulation in potatoes from wastewater Irrigation. J. Environ. Manag. 2019, 232, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xu, Q.; Lu, Q.; Liu, Y.; Xue, W.; Song, B.; Du, S. Effects of nitrogen forms on the growth and Cd concentration of pakchoi (Brassica chinensis L.) under Cd stress. J. Plant Nutr. Fertil. 2017, 23, 973–982. [Google Scholar]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, J.; Boorboori, M.R.; Li, D.; Chen, S.; Ma, X.; Cheng, P.; Zhang, H. Effect of biochar application rate on changes in soil labile organic carbon fractions and the association between bacterial community assembly and carbon metabolism with time. Sci. Total Environ. 2023, 855, 158876. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhao, Z.; Ali, A.; Guan, W.; Xiao, R.; Wang, J.J.; Ma, S.; Guo, D.; Zhou, B.; Zhang, Z.; et al. Characterization of phosphorus engineered biochar and its impact on immobilization of Cd and Pb from smelting contaminated soils. J. Soils Sediments 2020, 20, 3041–3052. [Google Scholar] [CrossRef]
- Sachdeva, S.; Kumar, R.; Sahoo, P.K.; Nadda, A.K. Recent advances in biochar amendments for immobilization of heavy metals in an agricultural ecosystem: A systematic review. Environ. Pollut. 2023, 319, 120937. [Google Scholar] [CrossRef]
- Schommer, V.A.; Vanin, A.P.; Nazari, M.T.; Ferrari, V.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Biochar-immobilized Bacillus spp. for heavy metals bioremediation:A review on immobilization techniques, bioremediation mechanisms and effects on soil. Sci. Total Environ. 2023, 881, 163385. [Google Scholar] [CrossRef]
- Song, X.; Chen, C.; Arthur, E.; Tuller, M.; Zhou, H.; Shang, J.; Ren, T. Cation exchange capacity and soil pore system play key roles in water vapour sorption. Geoderma 2022, 424, 116017. [Google Scholar] [CrossRef]
- Sun, X.; Yi, Y. Acid washing of incineration bottom ash of municipal solid waste: Effects of pH on removal and leaching of heavy metals. Waste Manag. 2021, 120, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Khan, S.A.; Mahmood, R.; Iqbal, M.; Ramzani, P.M.A.; Fatima, M. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol. Environ. Saf. 2018, 161, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Lima, I.M.; Thomas Klasson, K.; Wartelle, L.H. Contaminant immobilization and nutrient release by biochar soil amendment:Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, N.; Zhao, P.; Chen, M.; Deng, F.; Yu, X.; Zhang, D.; Chen, J.; Sun, J. Effect of a low-cost and highly efficient passivator synthesized by alkali-fused fly ash and swine manure on the leachability of heavy metals in a multi-metal contaminated soil. Chemosphere 2021, 279, 130558. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, Y.; Cheng, L.; Andserson, B.; Zhao, X.; Wang, D.; Ding, A. Review on utilization of biochar for metal-contaminated soil and sediment remediation. J. Environ. Sci. 2018, 63, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Huang, Y.; Huang, L.; Huang, Q.; Li, Y.; Li, X.; Yang, S.; Liu, C.; Liu, Z. Combined biochar and soda residues increases maize yields and decreases grain Cd/Pb in a highly Cd/Pb-polluted acid Udults soil. Agric. Ecosyst. Environ. 2021, 306, 107198. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, H.; Guo, Y.; Liu, Z.; Jiao, W. Immobilization of heavy metals in contaminated soils by modified hydrochar: Efficiency, risk assessment and potential mechanisms. Sci. Total Environ. 2019, 685, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gao, B.; Peng, Y.; Gao, X.; Fan, B.; Chen, Q. Ameta-analysis of heavymetal bioavailability response to biochar aging: Importance of soil and biochar properties. Sci. Total Environ. 2021, 756, 144058. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, Q.; Li, T.; Yu, H.; Zhang, X.; Huang, H. Effects of NTA on Pb phytostabilization efficiency of Athyrium wardii (Hook.) grown in a Pb-contaminated soil. J. Soils Sediments 2019, 19, 3576–3584. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, X.; Gao, Y.; Liu, G.; Liu, Z.; Zhang, Q.; Liu, E.; Sun, S.; Ren, X.; Jia, Z.; et al. Environment and agricultural practices regulate enhanced biochar-induced soil carbon pools and crop yield: A meta-analysis. Sci. Total Environ. 2023, 905, 167290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; Li, Z.G.; Liu, X.D.; Wang, B.; Zhou, G.L.; Huang, X.X.; Lin, C.F.; Wang, A.H.; Brooks, M. Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol. Eng. 2017, 98, 183–188. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Z.; Cai, C.; Tie, B.; Liu, X.; Reid, B.J.; Huang, Q.; Lei, M.; Sun, G.; Baltrėnaitė, E. Mitigating heavy metal accumulation into rice (Oryza sativa L.) using biochar amendment-a field experiment in Hunan, China. Environ. Sci. Pollut. Res. 2015, 22, 11097–11108. [Google Scholar] [CrossRef]
- Zhou, F.; Geng, Z.; Xu, C.; Liu, L.; Zhang, J.; Li, Q.; Chen, S.; Wang, M.; Wang, H. Effect of biochar addition on soil microbial biomass and metabolic activities of carbon sources in Lou soil. J. Plant Nutr. Fertil. 2019, 25, 1277–1289. (In Chinese) [Google Scholar]
- Zhou, R.; Liu, X.; Luo, L.; Zhou, Y.; Wei, J.; Chen, A.; Tang, L.; Wu, H.; Yao, D.; Zhang Feng Wang, Y. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. Int. Biodeterior. Biodegrad. 2017, 118, 73–81. [Google Scholar] [CrossRef]


| Parameters | Peanut Shell Biochar | Alkaline Mineral | Soil |
|---|---|---|---|
| pH | 9.72 ± 0.65 | 9.87 ± 0.68 | 4.73 ± 0.88 |
| Organic Carbon (%) | 42.30 ± 0.32 | 0.01 ± 0.00 | 1.53 ± 0.14 |
| CEC (cmol+·kg−1) | 17.50 ± 0.14 | 20.13 ± 1.23 | 4.53 ± 0.85 |
| Total Cd (mg·kg−1) | 0.16 ± 0.06 | n.d | 5.26 ± 0.69 |
| Total Pb (mg·kg−1) | 5.46 ± 0.24 | n.d | 1110.00 ± 96.32 |
| DTPA Cd (mg·kg−1) | 0.06 ± 0.00 | n.d | 2.45 ± 0.23 |
| DTPA Pb (mg·kg−1) | 2.35 ± 0.05 | n.d | 321.70 ± 22.21 |
| Available N (mg·kg−1) | 211.00 ± 9.87 | 145.66 ± 5.67 | 112.83 ± 3.25 |
| Available P (mg·kg−1) | 317.90 ± 15.63 | 0.94 ± 0.16 | 48.90 ± 1.54 |
| Available K (mg·kg−1) | 33,924.00 ± 368.12 | 269.21 ± 24.13 | 54.67 ± 0.53 |
| Correlation Coefficient (n = 28) | Crown Daisy | Lettuce | Long-Leaf Lettuce | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | CEC | SOC | pH | CEC | SOC | pH | CEC | SOC | |
| Soil-available Cd content | −0.58 ** | −0.50 ** | −0.50 ** | −0.78 ** | −0.69 ** | −0.75 ** | −0.70 ** | −0.33 | −0.52 ** |
| Soil-available Pb content | −0.67 ** | −0.40 * | −0.51 ** | −0.71 ** | −0.65 ** | −0.68 ** | −0.80 ** | −0.57 * | −0.73 ** |
| Shoot Cd content | −0.92 ** | −0.63 ** | −0.55 ** | −0.31 | −0.73 ** | −0.70 ** | −0.85 ** | −0.70 ** | −0.50 ** |
| Root Cd content | −0.89 ** | −0.55 ** | −0.45 * | −0.79 ** | −0.59 ** | −0.59 ** | −0.82 ** | −0.62 ** | −0.50 ** |
| Shoot Pb content | −0.63 ** | −0.69 ** | −0.48 * | −0.64 ** | −0.68 ** | −0.64 ** | −0.55 ** | −0.56 ** | −0.32 |
| Root Pb content | −0.93 ** | −0.62 ** | −0.54 ** | −0.83 ** | −0.77 ** | −0.76 ** | −0.77 ** | −0.86 ** | −0.68 ** |
| Root biomass | 0.08 | −0.35 | −0.33 | 0.77 ** | 0.41 * | 0.41 * | 0.88 ** | 0.31 | 0.45 * |
| Shoot biomass | 0.02 | −0.45 * | −0.42 * | 0.81 ** | 0.27 | 0.34 | 0.82 ** | 0.18 | 0.39 * |
| Correlation Coefficient (n = 28) | Crown Daisy | Lettuce | Long-Leaf Lettuce | |||
|---|---|---|---|---|---|---|
| Soil Available Cd Content | Soil Available Pb Content | Soil Available Cd Content | Soil Available Pb Content | Soil Available Cd Content | Soil Available Pb Content | |
| Shoot Cd/Pb content | 0.52 ** | 0.53 ** | 0.59 ** | 0.84 ** | 0.62 ** | 0.64 ** |
| Root Cd/Pb content | 0.47 * | 0.76 ** | 0.72 ** | 0.79 ** | 0.59 ** | 0.77 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Chen, W.; Wei, L.; Li, X.; Huang, Y.; Huang, Q.; Liu, C.; Liu, Z. Biochar Blended with Alkaline Mineral Can Better Inhibit Lead and Cadmium Uptake and Promote the Growth of Vegetables. Plants 2024, 13, 1934. https://doi.org/10.3390/plants13141934
Huang L, Chen W, Wei L, Li X, Huang Y, Huang Q, Liu C, Liu Z. Biochar Blended with Alkaline Mineral Can Better Inhibit Lead and Cadmium Uptake and Promote the Growth of Vegetables. Plants. 2024; 13(14):1934. https://doi.org/10.3390/plants13141934
Chicago/Turabian StyleHuang, Lianxi, Weisheng Chen, Lan Wei, Xiang Li, Yufen Huang, Qing Huang, Chuanping Liu, and Zhongzhen Liu. 2024. "Biochar Blended with Alkaline Mineral Can Better Inhibit Lead and Cadmium Uptake and Promote the Growth of Vegetables" Plants 13, no. 14: 1934. https://doi.org/10.3390/plants13141934
APA StyleHuang, L., Chen, W., Wei, L., Li, X., Huang, Y., Huang, Q., Liu, C., & Liu, Z. (2024). Biochar Blended with Alkaline Mineral Can Better Inhibit Lead and Cadmium Uptake and Promote the Growth of Vegetables. Plants, 13(14), 1934. https://doi.org/10.3390/plants13141934






