6-BA Reduced Yield Loss under Waterlogging Stress by Regulating the Phenylpropanoid Pathway in Wheat
Abstract
:1. Introduction
2. Results
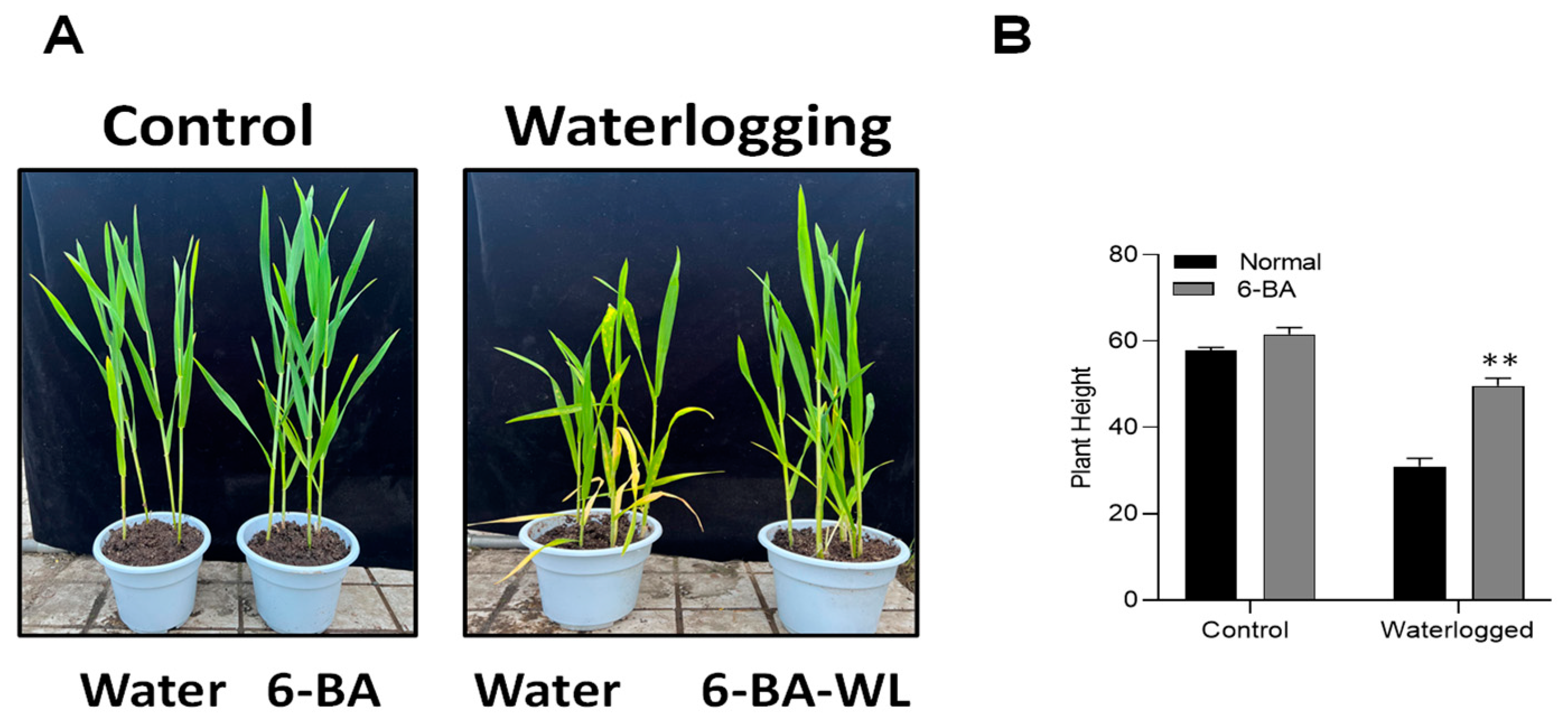
2.1. 6-BA Improved Wheat Morphology under Waterlogging Stress
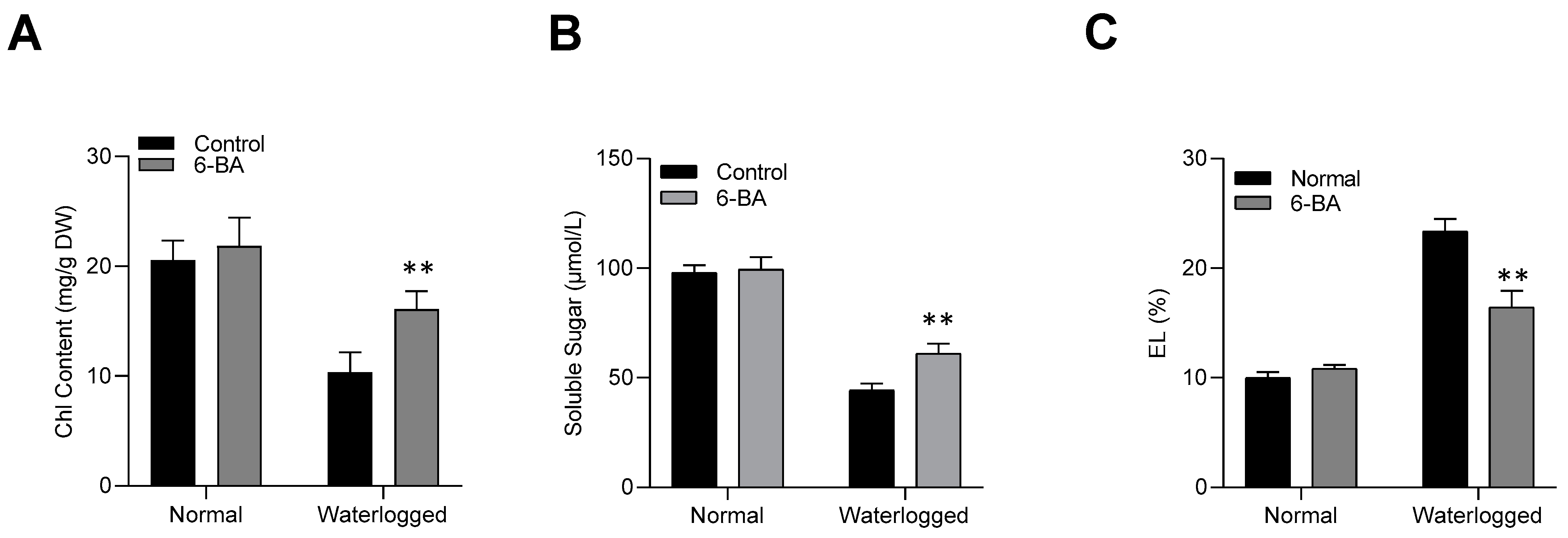
2.2. 6-BA Enhanced the Physiological Response of Wheat under Waterlogging Stress
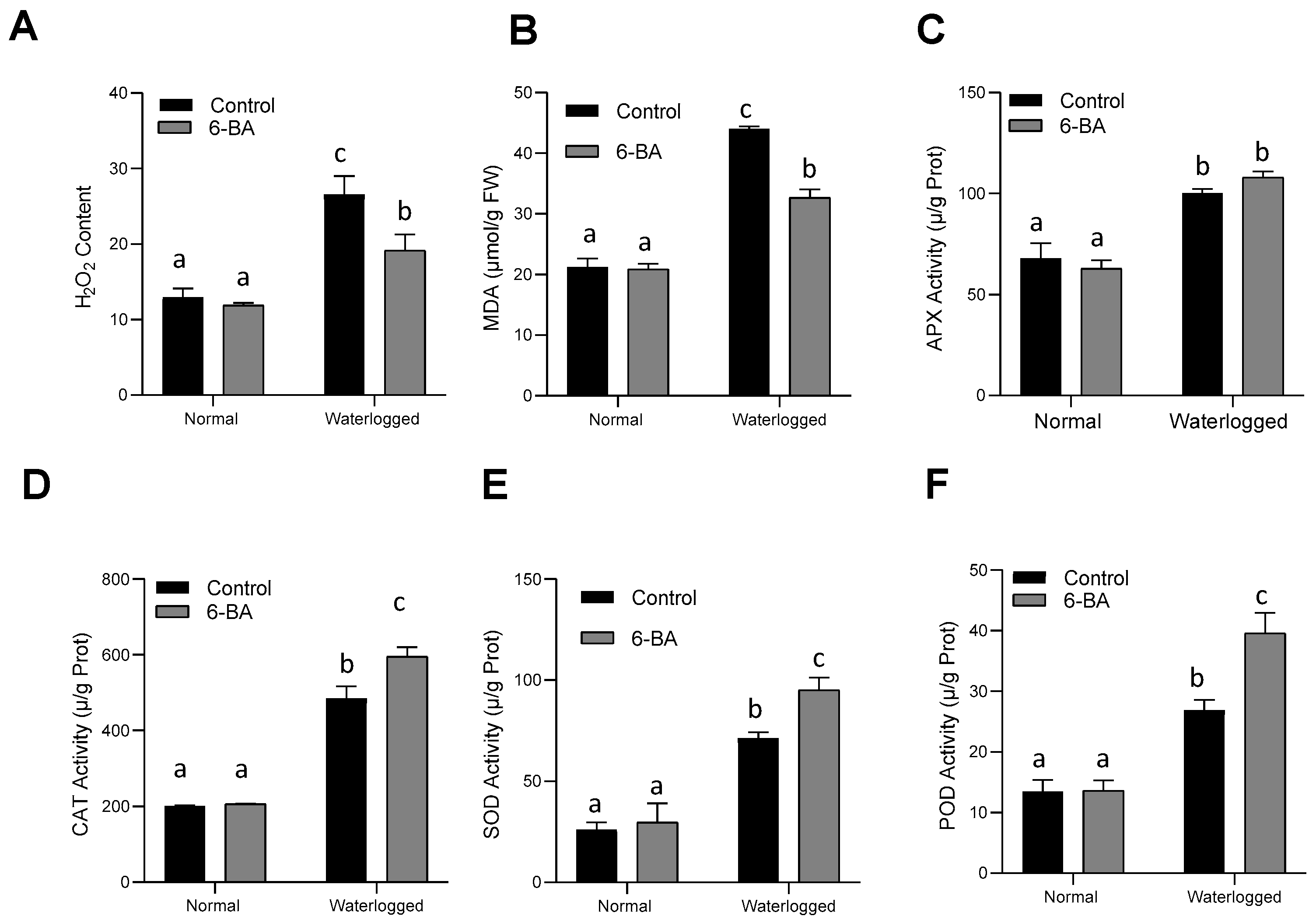
2.3. 6-BA Decreased ROS Accumulation through Increasing Antioxidant Enzyme Activity under Waterlogging Stress
2.4. 6-BA Improved Root Growth System under Waterlogging Stress
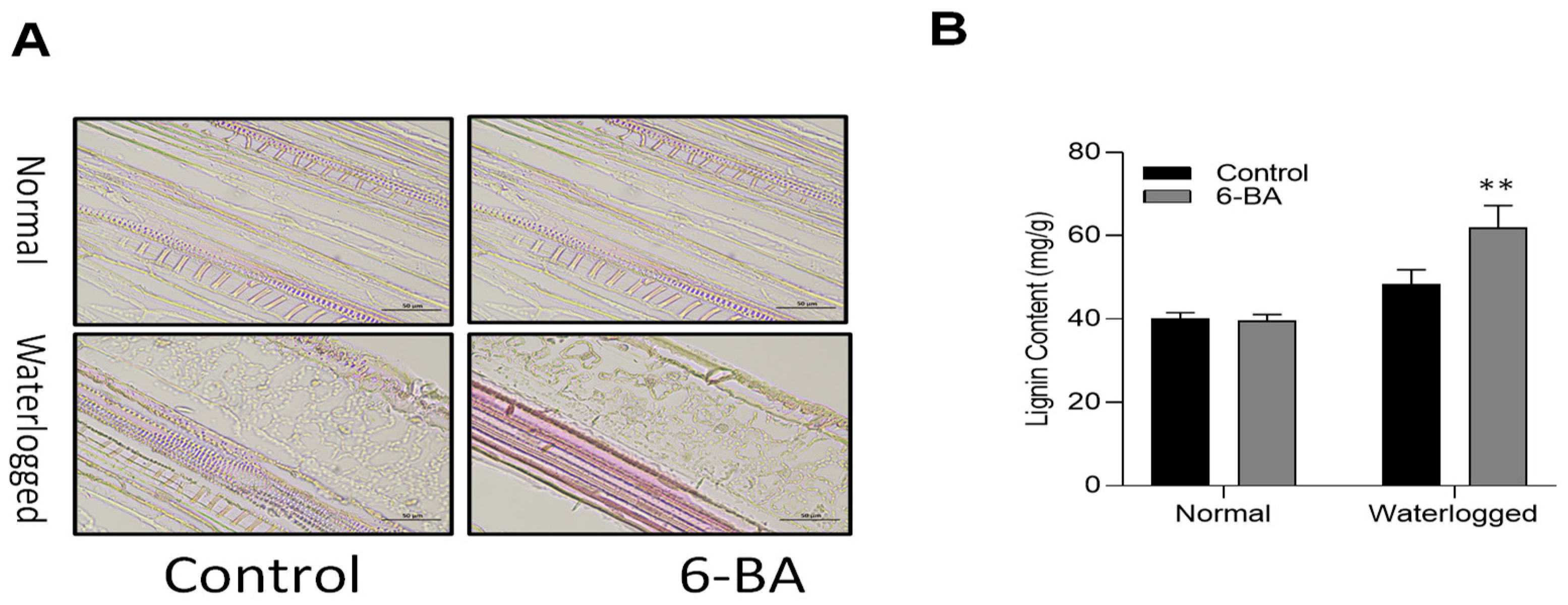
2.5. 6-BA Increased Lignin Content and Deposition in the Wheat under Waterlogging Conditions
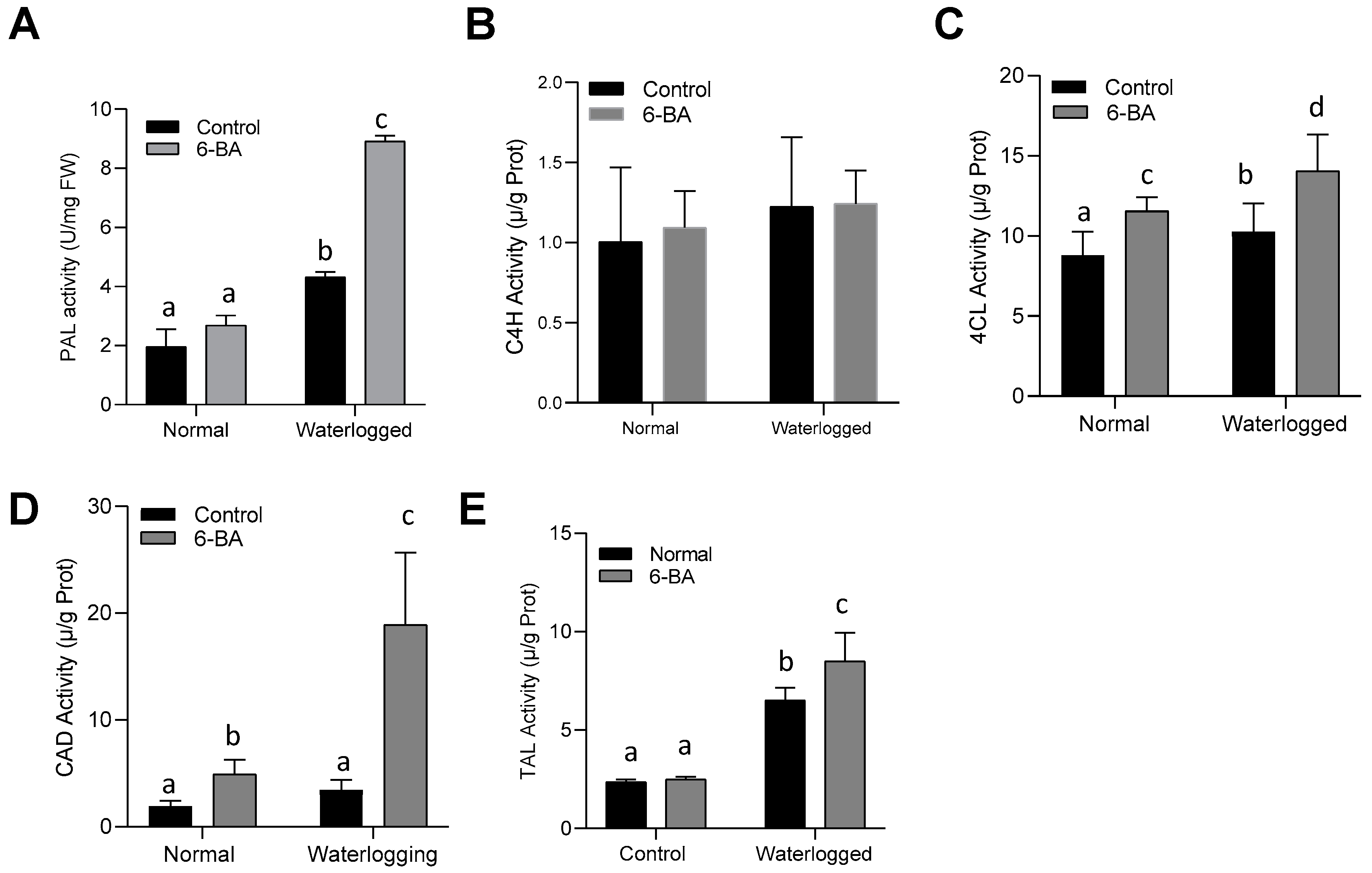
2.6. 6-BA Contributed to the Phenylpropanoid Pathway by Increasing the Related Enzyme Activity under Waterlogging Stress
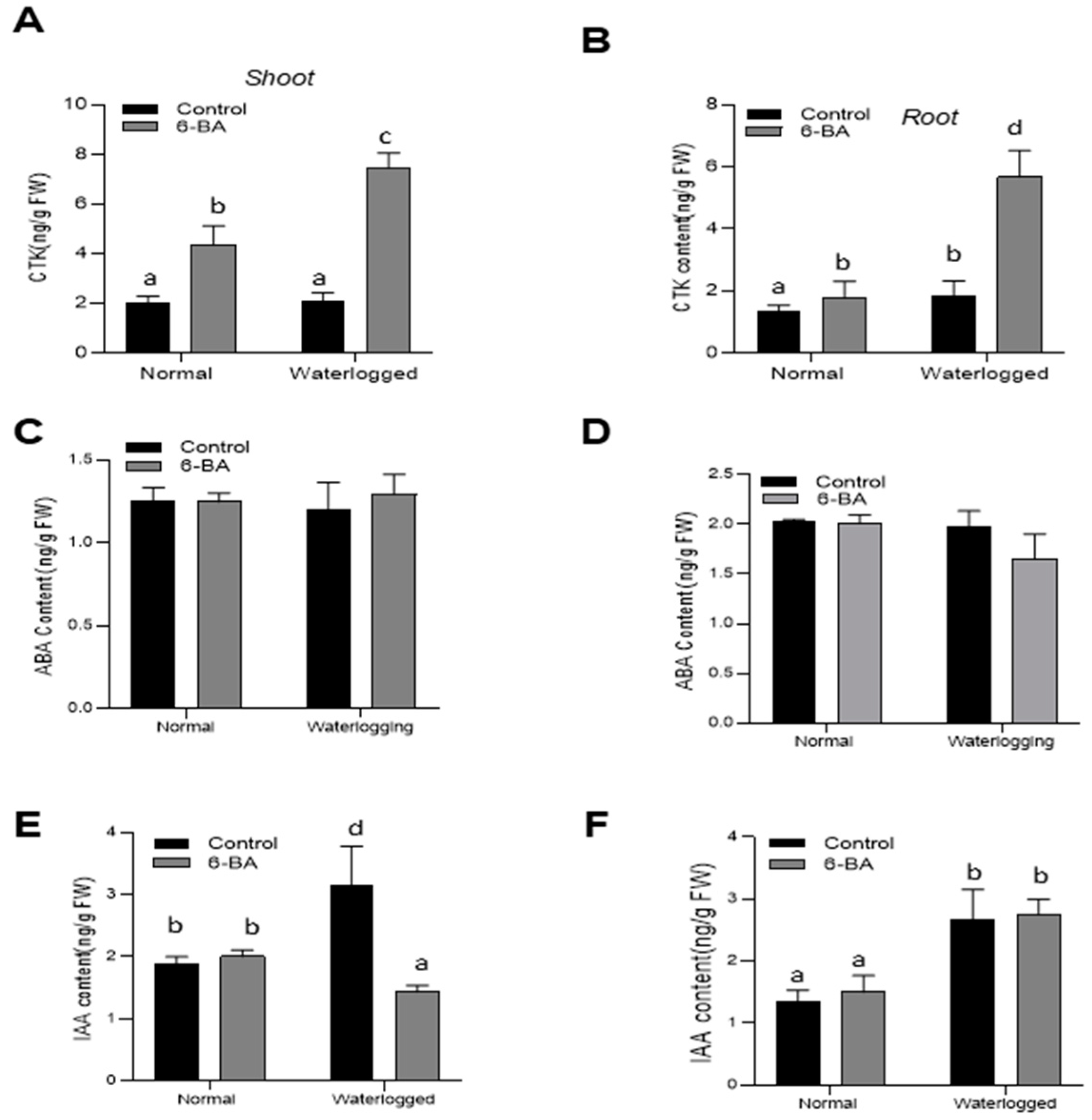
2.7. 6-BA Increased the Level of Endogenous Hormone Content under Waterlogging Stress
2.8. 6-BA Treatment Increased the Expression of Genes Related to IAA Signaling and Phenylpropanoid Biosynthesis
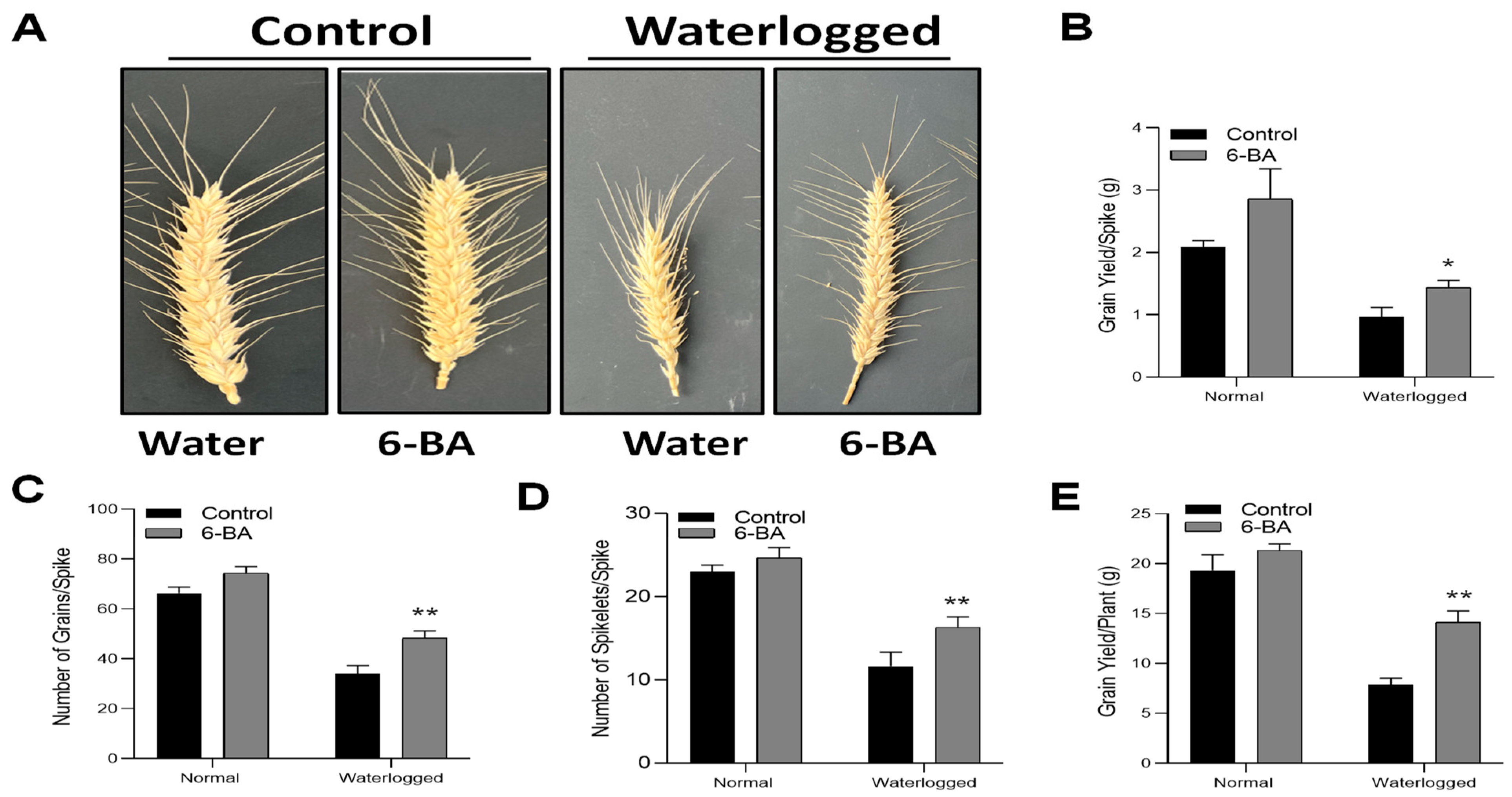
2.9. 6-BA Treatment Decreased the Overall Yield Loss under Waterlogging Stress
3. Discussion
4. Materials and Methods
4.1. Experimental Conditions
4.2. Membrane Lipid Damage (MDA), Antioxidant Enzyme Activity, and Soluble Sugar Content
4.3. Root Growth Measurement
4.4. Total Chlorophyll and Electrolyte Leakage Determination
4.5. Phenylpropanoid-Pathway-Related Enzyme Activities
4.6. Determination of Lignin Content and Lignin Deposition
4.7. Hormone Content Analysis
4.8. RNA Extraction and Gene Expression Analysis (qRT-PCR)
4.9. Grain Yield and Its Related Components
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sendhil, R.; Kumari, B.; Khandoker, S.; Jalali, S.; Acharya, K.K.; Gopalareddy, K.; Singh, G.P.; Joshi, A.K. Wheat in Asia: Trends, challenges and research priorities. In New Horizons in Wheat and Barley Research: Global Trends, Breeding and Quality Enhancement; Springer: Berlin/Heidelberg, Germany, 2022; pp. 33–61. [Google Scholar]
- Mwangi, R.W.; Mustafa, M.; Charles, K.; Wagara, I.W.; Kappel, N. Selected emerging and reemerging plant pathogens affecting the food basket: A threat to food security. J. Agric. Food Res. 2023, 14, 100827. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Pais, I.P.; Moreira, R.; Coelho, A.R.; Semedo, J.N.; Reboredo, F.H.; Coutinho, J.; Lidon, F.C.; Maçãs, B.; Scotti-Campos, P. Unveiling the Impact of Growth Traits on the Yield of Bread Wheat Germplasm Subjected to Waterlogging. Agriculture 2024, 14, 241. [Google Scholar] [CrossRef]
- Sprunger, C.D.; Lindsey, A.; Lightcap, A. Above-and belowground linkages during extreme moisture excess: Leveraging knowledge from natural ecosystems to better understand implications for row-crop agroecosystems. J. Exp. Bot. 2023, 74, 2845–2859. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ogorek, L.L.P.; Pedersen, O.; Sauter, M. Oxygen in the air and oxygen dissolved in the floodwater both sustain growth of aquatic adventitious roots in rice. J. Exp. Bot. 2021, 72, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Kotula, L.; Schreiber, L.; Colmer, T.D.; Nakazono, M. Anatomical and biochemical characterisation of a barrier to radial O2 loss in adventitious roots of two contrasting Hordeum marinum accessions. Funct. Plant Biol. 2017, 44, 845–857. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Hussain, A.; Alam, I.; Khoso, M.A.; Ali, Q.; Liu, F. Waterlogging stress in plants: Unraveling the mechanisms and impacts on growth, development, and productivity. Environ. Exp. Bot. 2024, 244, 105824. [Google Scholar] [CrossRef]
- Pegg, T.; Edelmann, R.R.; Gladish, D.K. Immunoprofiling of cell wall carbohydrate modifications during flooding-induced aerenchyma formation in fabaceae roots. Front. Plant Sci. 2020, 10, 482196. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Son, S.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, L.; Wang, Y.; Li, Y.; Jiang, X.; Liu, Y.; Xie, D.Y.; Gao, L.; Xia, T. Molecular and biochemical characterization of two 4-coumarate: CoA ligase genes in tea plant (Camellia sinensis). Plant Mol. Biol. 2022, 109, 579–593. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, L.; Liu, J.; Zhang, X.; Li, S.; Zhao, K.; Guan, Y.; Song, A.; Wang, H.; Chen, S.; et al. Regulation of lignin biosynthesis by an atypical bHLH protein CmHLB in Chrysanthemum. J. Exp. Bot. 2022, 73, 2403–2419. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Xu, Y.; Li, H.; Chen, X.; Song, Z. Antioxidant activation, cell wall reinforcement, and reactive oxygen species regulation promote resistance to waterlogging stress in hot pepper (Capsicum annuum L.). BMC Plant Biol. 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C. Multiplelevels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Bassham, D.C. Combating stress: The interplay between hormone signaling and autophagy in plants. J. Exp. Bot. 2020, 71, 1723–1733. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Rolletschek, H.; Heinzel, N.; Tikhonov, K.; Dehesh, K. Waterlogging tolerance rendered by oxylipin-mediated metabolic reprogramming in Arabidopsis. J. Exp. Bot. 2019, 70, 2919–2932. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, X.; Ren, H.; Zhao, B.; Zhang, J.; Ren, B.; Gao, H.; Liu, P. Exogenous SA or 6-BA maintains photosynthetic activity in maize leaves under high temperature stress. Crop J. 2023, 11, 605–617. [Google Scholar] [CrossRef]
- Ghaleh, Z.R.; Sarmast, M.K.; Atashi, S. 6-Benzylaminopurine (6-BA) ameliorates drought stress response in tall fescue via the influencing of biochemicals and strigolactone-signaling genes. Plant Physiol. Biochem. 2020, 155, 877–887. [Google Scholar] [CrossRef]
- Hu, J.; Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Phosphoproteomic and physiological analysis revealed 6-benzyladenine improved the operation of photosynthetic apparatus in waterlogged summer maize. Environ. Exp. Bot. 2022, 193, 104679. [Google Scholar] [CrossRef]
- Hu, J.; Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Comparative proteomic analysis reveals that exogenous 6-benzyladenine (6-BA) improves the defense system activity of waterlogged summer maize. BMC Plant Biol. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, F.; Jiang, A.; Hou, X.; Liu, Q.; Fan, H.; Chen, M. Exogenous 6-BA enhances salt tolerance of Limonium bicolor by increasing the number of salt glands. Plant Cell Rep. 2024, 43, 12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhu, Y.; Zhu, H.; Kang, G.; Guo, T.; Wang, C. Floret development and grain setting characteristics in winter wheat in response to pre-anthesis applications of 6-benzylaminopurine and boron. Field Crops Res. 2014, 169, 70–76. [Google Scholar] [CrossRef]
- Liu, C.F.; Yang, N.; Teng, R.M.; Li, J.W.; Chen, Y.; Hu, Z.H.; Li, T.; Zhuang, J. Exogenous methyl jasmonate and cytokinin antagonistically regulate lignin biosynthesis by mediating CsHCT expression in Camellia sinensis. Protoplasma 2023, 260, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Khadr, A.; Wang, Y.H.; Zhang, R.R.; Wang, X.R.; Xu, Z.S.; Xiong, A.S. Cytokinin (6-benzylaminopurine) elevates lignification and the expression of genes involved in lignin biosynthesis of carrot. Protoplasma 2020, 257, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llorca, M.; Pollmann, S.; Müller, M. Ethylene and jasmonates signaling network mediating secondary metabolites under abiotic stress. Int. J. Mol. Sci. 2023, 24, 5990. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, M.; Sánchez-Parra, B.; Pérez Alonso, M.M.; Carrasco Loba, V.; Carrillo, L.; Vicente-Carbajosa, J.; Medina, J.; Pollmann, S. YUCCA8 and YUCCA9 overexpression reveals a link between auxin signaling and lignification through the induction of ethylene biosynthesis. Plant Signal. Behav. 2013, 8, e26363. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, X.; Li, Z.; Song, Q.; Xu, C.; Luo, K. Jasmonic acid regulates lignin deposition in poplar through JAZ5-MYB/NAC interaction. Front. Plant Sci. 2023, 14, 1232880. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Dai, J.; Luo, Z.; Li, Z.; Lu, H.; Xu, S.; Tang, W.; Zhang, D.; Li, W. Global gene expression in cotton (Gossypium hirsutum L.) leaves to waterlogging stress. PLoS ONE 2017, 12, e0185075. [Google Scholar] [CrossRef]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Yu, M.; Demidchik, V.; Shabala, S.; Zhou, M. Identification of QTL related to ROS formation under hypoxia and their association with waterlogging and salt tolerance in barley. Int. J. Mol. Sci. 2019, 20, 699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Dong, H.; Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. Crop J. 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Daniel, K.; Hartman, S. How plant roots respond to waterlogging. J. Exp. Bot. 2024, 75, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Challa, G.S.; Gupta, A.; Gu, L.; Wu, Y.; Li, W. Physiological and transcriptomic characterization of sea-wheatgrass-derived waterlogging tolerance in wheat. Plants 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Shrestha, H.K.; Serrani-Yarce, J.C.; Engle, N.L.; Abraham, P.E.; Tschaplinski, T.J.; Hettich, R.L.; Dixon, R.A. Proteomic and metabolic disturbances in lignin-modified Brachypodium distachyon. Plant Cell 2022, 34, 3339–3363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Zhang, D.; Liu, A.; Zhu, W.; Zhang, J.; Yang, B. Integrative Omic Analysis Reveals the Dynamic Change in Phenylpropanoid Metabolism in Morus alba under Different Stress. Plants 2023, 12, 3265. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.; Fürst-Jansen, J.M.; Irisarri, I.; Dhabalia Ashok, A.; Ischebeck, T.; Feussner, K.; Abreu, I.N.; Petersen, M.; Feussner, I.; de Vries, J. The evolution of the phenylpropanoid pathway entailed pronounced radiations and divergences of enzyme families. Plant J. 2021, 107, 975–1002. [Google Scholar] [CrossRef]
- Alariqi, M.; Ramadan, M.; Wang, Q.; Yang, Z.; Hui, X.; Nie, X.; Ahmed, A.; Chen, Q.; Wang, Y.; Zhu, L. Cotton 4-coumarate-CoA ligase 3 enhanced plant resistance to Verticillium dahliae by promoting jasmonic acid signaling-mediated vascular lignification and metabolic flux. Plant J. 2023, 115, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Özbek, K.; Keskin, C.N.; Zencirci, N. Wheat Genetic Resources. In Advances in Wheat Breeding: Towards Climate Resilience and Nutrient Security; Springer: Berlin/Heidelberg, Germany, 2024; pp. 525–554. [Google Scholar]
- Meng, X.; Lou, H.; Zhai, S.; Zhang, R.; Liu, G.; Xu, W.; Yu, J.; Zhang, Y.; Ni, Z.; Sun, Q. TaNAM-6A is essential for nitrogen remobilisation and regulates grain protein content in wheat (Triticum aestivum L.). Plant Cell Environ. 2024, 47, 2310–2321. [Google Scholar] [CrossRef]
- Xing, F.; Zhang, L.; Ge, W.; Fan, H.; Tian, C.; Meng, F. Comparative transcriptome analysis reveals the importance of phenylpropanoid biosynthesis for the induced resistance of 84K poplar to anthracnose. BMC Genom. 2024, 25, 306. [Google Scholar] [CrossRef]
- Lu, J.; Shi, Y.; Li, W.; Chen, S.; Wang, Y.; He, X.; Yin, X. RcPAL, a key gene in lignin biosynthesis in Ricinus communis L. BMC Plant Biol. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xing, L.L.; Cao, X.H.; Guo, G.Y.; Chai, J.F.; Bei, C.L. Cloning of Ta4CL1 and its function in promoting plant growth and lignin deposition in transgenic Arabidopsis plants. Acta Agron. Sin. 2021, 48, 63–75. [Google Scholar] [CrossRef]
- Gui, J.; Shen, J.; Li, L. Functional characterization of evolutionarily divergent 4-coumarate: Coenzyme a ligases in rice. Plant Physiol. 2011, 157, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Singam, P.; Suravajhala, P. Modulation of Lignin and its Implications in Salt, Drought and Temperature Stress Tolerance. Curr. Chem. Biol. 2023, 17, 2–12. [Google Scholar] [CrossRef]
- Yaqoob, U.; Jan, N.; Raman, P.V.; Siddique, K.H.; John, R. Crosstalk between brassinosteroid signaling, ROS signaling and phenylpropanoid pathway during abiotic stress in plants: Does it exist? Plant Stress 2022, 4, 100075. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Choudhary, K.K. Revisiting the role of phenylpropanoids in plant defense against UV-B stress. Plant Stress 2023, 7, 100143. [Google Scholar] [CrossRef]
- Gala, H.P.; Lanctot, A.; Jean-Baptiste, K.; Guiziou, S.; Chu, J.C.; Zemke, J.E.; George, W.; Queitsch, C.; Cuperus, J.T.; Nemhauser, J.L. A single-cell view of the transcriptome during lateral root initiation in Arabidopsis thaliana. Plant Cell 2021, 33, 2197–2220. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef]
- Shi, M.; Liu, X.; Zhang, H.; He, Z.; Yang, H.; Chen, J.; Feng, J.; Yang, W.; Jiang, Y.; Yao, J.L.; et al. The IAA-and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo. Hortic. Res. 2020, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Koramutla, M.K.; Tuan, P.A.; Ayele, B.T. Salicylic acid enhances adventitious root and aerenchyma formation in wheat under waterlogged conditions. Int. J. Mol. Sci. 2022, 23, 1243. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, M.A.; Song, A.; Faheem, M.; Chen, S.; Jiang, J.; Liu, C.; Fan, Q.; Chen, F. Involvement of CmWRKY10 in drought tolerance of chrysanthemum through the ABA-signaling pathway. Int. J. Mol. Sci. 2016, 17, 693. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY transcription factor ZmWRKY79 positively regulates drought tolerance through elevating ABA biosynthesis. Int. J. Mol. Sci. 2021, 22, 10080. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.J.; Qi, H.; Cheng, B.; Hussain, S.; Peng, Y.; Liu, W.; Feng, G.; Zhao, J.; Li, Z. Enhanced adaptability to limited water supply regulated by diethyl aminoethyl hexanoate (DA-6) associated with lipidomic reprogramming in two white clover genotypes. Front. Plant Sci. 2022, 13, 879331. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 1989, 29, 230–233. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, B.; Ma, L.; Zheng, X.; Gong, D.; Xue, H.; Bi, Y.; Wang, Y.; Zhang, Z.; Prusky, D. Benzo-(1, 2, 3)-thiadiazole-7-carbothioic acid s-methyl ester (BTH) promotes tuber wound healing of potato by elevation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 153, 125–132. [Google Scholar] [CrossRef]
- Sullivan, M.L. Preparation of hydroxycinnamoyl-coenzyme A thioesters using recombinant 4-coumarate: Coenzyme A ligase (4CL) for characterization of BAHD hydroxycinnamoyltransferase enzyme activities. Methods Enzymol. 2023, 683, 3–18. [Google Scholar]
- Tao, S.; Wang, D.; Jin, C.; Sun, W.; Liu, X.; Zhang, S.; Gao, F.; Khanizadeh, S. Cinnamate-4-hydroxylase gene is involved in the step of lignin biosynthesis in Chinese white pear. J. Am. Soc. Hortic. Sci. 2015, 140, 573–579. [Google Scholar] [CrossRef]
- Tao, S.; Khanizadeh, S.; Zhang, H.; Zhang, S. Anatomy, ultrastructure and lignin distribution of stone cells in two Pyrus species. Plant Sci. 2009, 176, 413–419. [Google Scholar] [CrossRef]
- Maslennikova, D.; Ivanov, S.; Petrova, S.; Burkhanova, G.; Maksimov, I.; Lastochkina, O. Components of the phenylpropanoid pathway in the implementation of the protective effect of sodium nitroprusside on wheat under salinity. Plants 2023, 12, 2123. [Google Scholar] [CrossRef] [PubMed]
- Philipp, N.; Weichert, H.; Bohra, U.; Weschke, W.; Schulthess, A.W.; Weber, H. Grain number and grain yield distribution along the spike remain stable despite breeding for high yield in winter wheat. PLoS ONE 2018, 13, e0205452. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulzar, F.; Yang, H.; Chen, J.; Hassan, B.; Huang, X.; Qiong, F. 6-BA Reduced Yield Loss under Waterlogging Stress by Regulating the Phenylpropanoid Pathway in Wheat. Plants 2024, 13, 1991. https://doi.org/10.3390/plants13141991
Gulzar F, Yang H, Chen J, Hassan B, Huang X, Qiong F. 6-BA Reduced Yield Loss under Waterlogging Stress by Regulating the Phenylpropanoid Pathway in Wheat. Plants. 2024; 13(14):1991. https://doi.org/10.3390/plants13141991
Chicago/Turabian StyleGulzar, Faiza, Hongkun Yang, Jiabo Chen, Beenish Hassan, Xiulan Huang, and Fangao Qiong. 2024. "6-BA Reduced Yield Loss under Waterlogging Stress by Regulating the Phenylpropanoid Pathway in Wheat" Plants 13, no. 14: 1991. https://doi.org/10.3390/plants13141991




