Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens
Abstract
:1. Introduction
2. Results
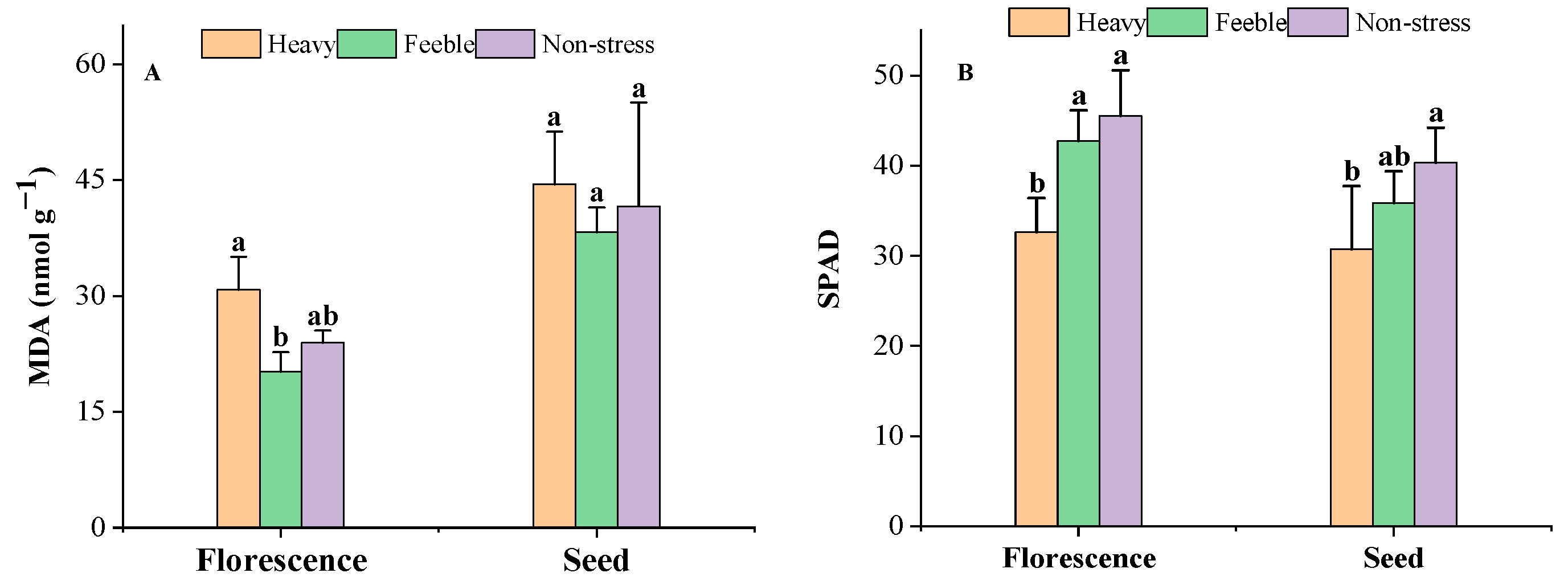
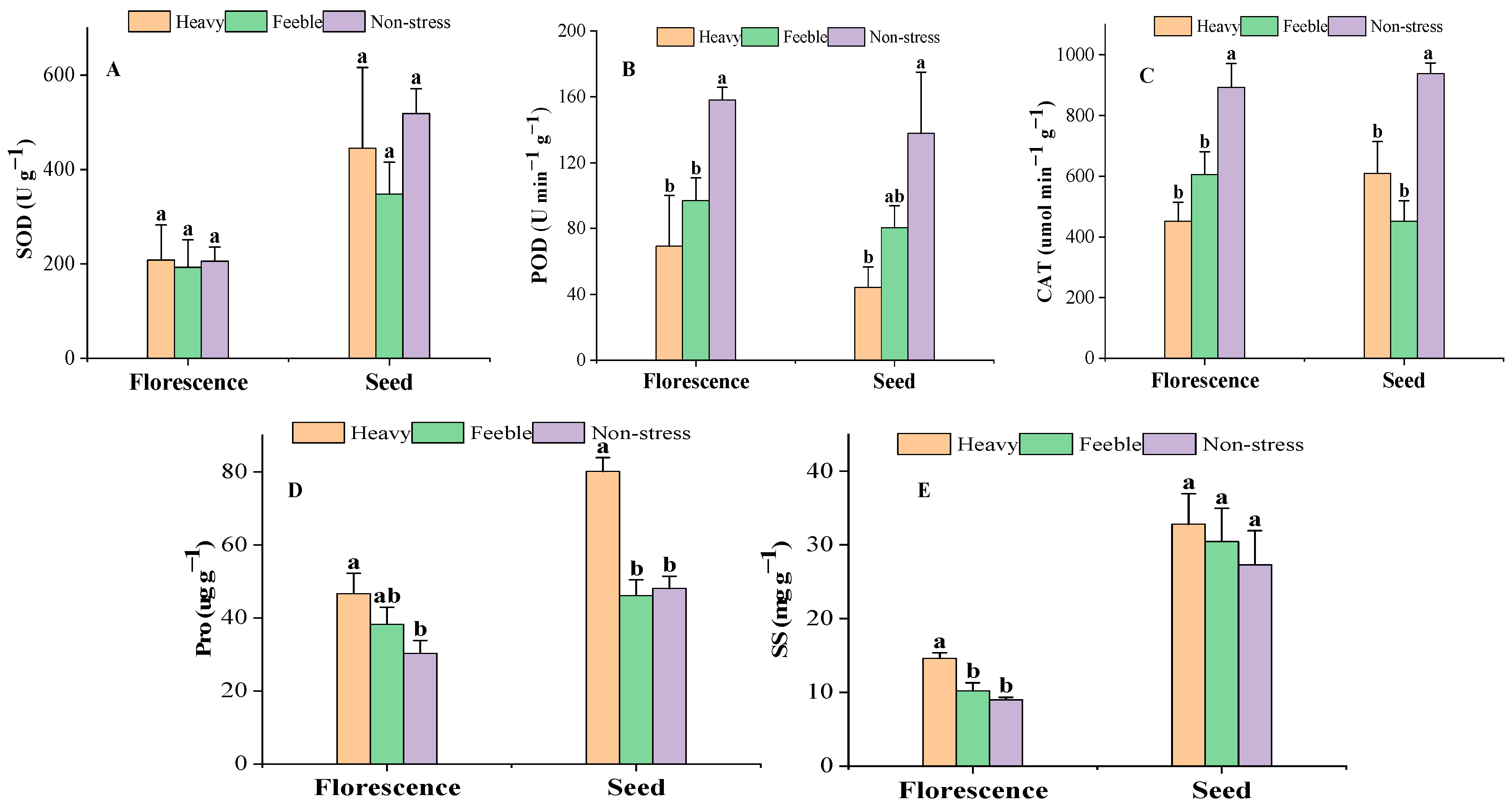
2.1. Leaf Biochemical Traits
2.2. Leaf Morphological Traits
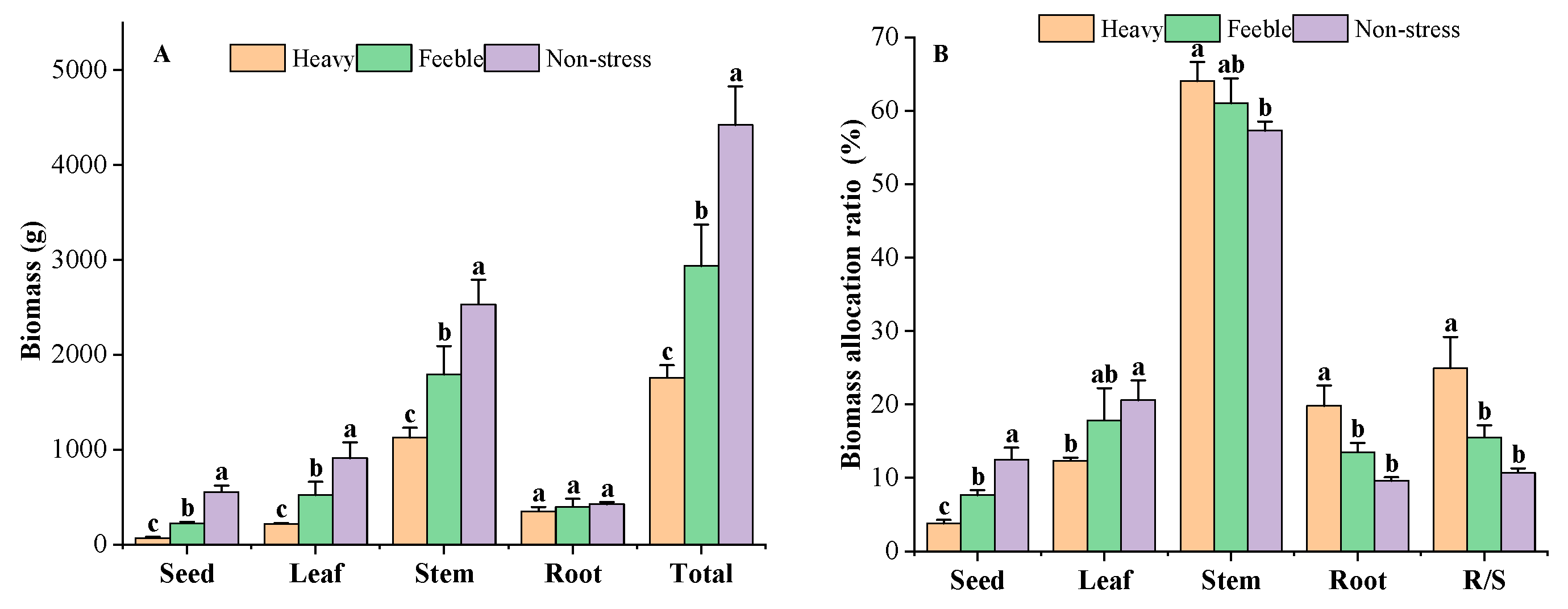
2.3. Biomass Allocation Variables
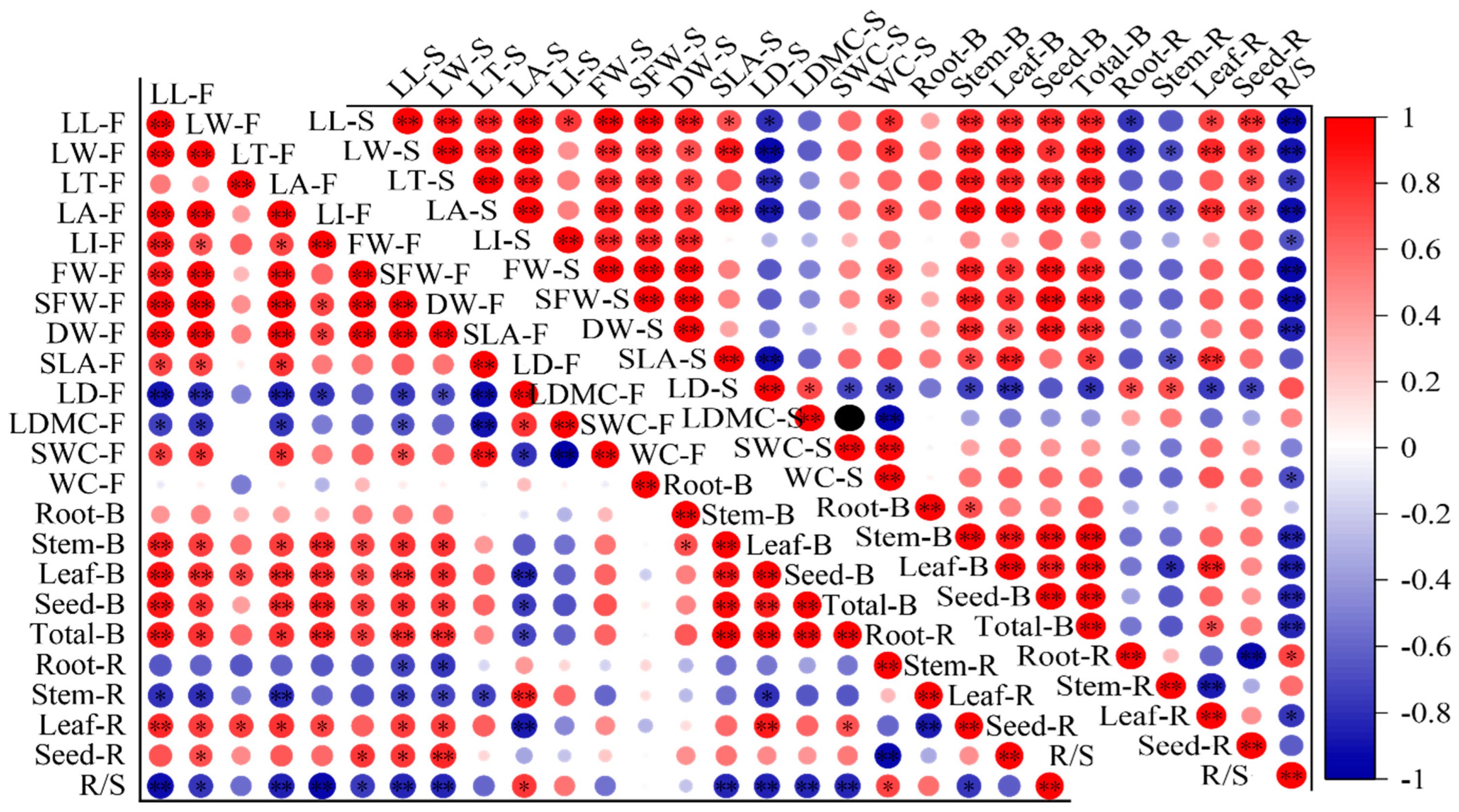
2.4. Correlations between Leaf Functional Traits and Biomass
3. Discussion
3.1. Leaf Biochemical Response to Drought Stress
3.2. Leaf Morphological Response to Drought Stress
3.3. Biomass Allocation Response to Drought Stress
3.4. The Relationship between Leaf Functional Traits and Biomass
4. Materials and Methods
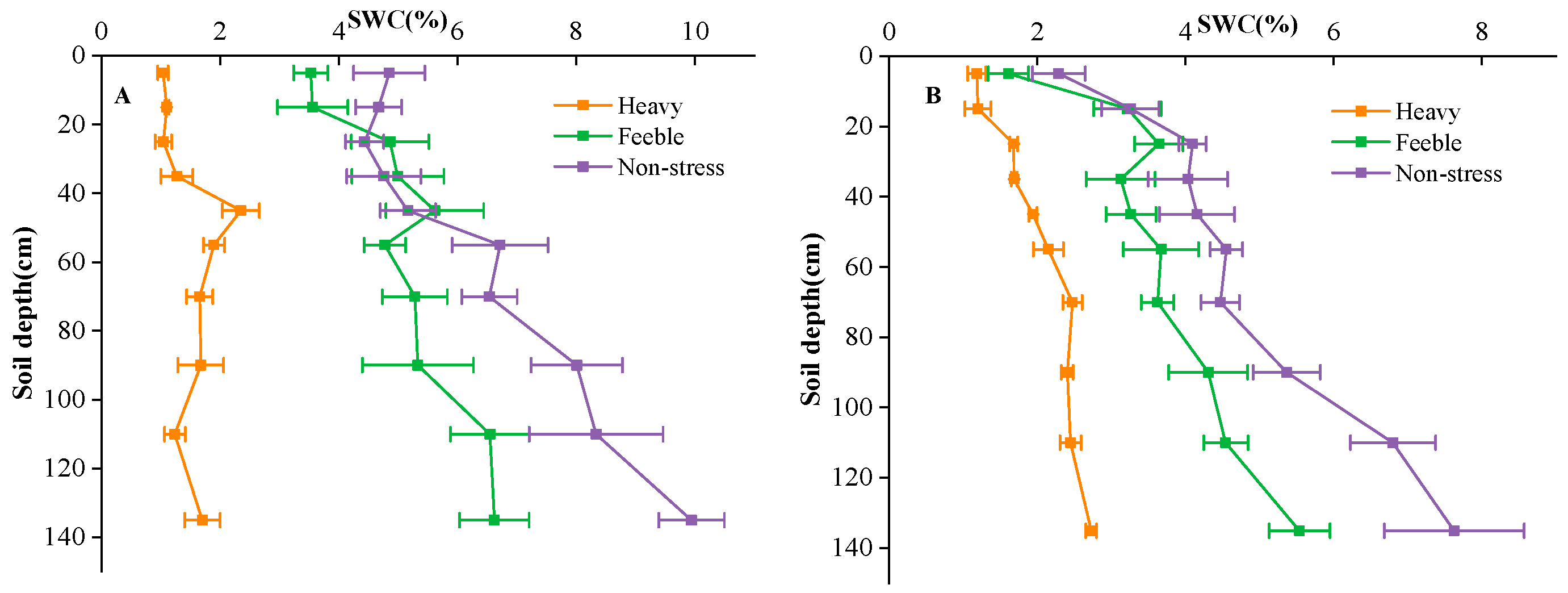
4.1. Experimental Site
4.2. Experimental Design
4.3. Data Collection and Processing
4.3.1. Leaf Functional Traits
4.3.2. Biomass Partitioning Patterns
4.4. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, D.; Li, J.; Luo, J.; Ma, Y.; Wang, Y.; Liu, W.; Rodriguez, L.G.; Yao, Y. Responses to solar UV-B exclusion and drought stress in two cultivars of chestnut rose with different leaf thickness. Forests 2022, 14, 50. [Google Scholar] [CrossRef]
- Ganguly, R.; Sarkar, A.; Dasgupta, D.; Acharya, K.; Keswani, C.; Popova, V.; Minkina, T.; Maksimov, A.Y.; Chakraborty, N. Unravelling the efficient applications of zinc and selenium for mitigation of abiotic stresses in plants. Agriculture 2022, 12, 1551. [Google Scholar] [CrossRef]
- Maseda, P.H.; Fernandez, R.J. Growth potential limits drought morphological plasticity in seedlings from six Eucalyptus provenances. Tree Physiol. 2016, 36, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Q.; Wu, B.; Zhu, Y.; Liu, D.; Zhang, J.; Jin, Z. A review of leaf morphology plasticity linked to plant response and adaptation characteristics in arid ecosystems. Chin. J. Plant Ecol. 2012, 36, 88–98. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.M.; Mood, B.J.; Bonsal, B.; Howat, B.; Laroque, C.P. Comparison of tree-growth drought legacies of three shelterbelt species in the Canadian Prairies. Agric. For. Meteorol. 2023, 330, 109317. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.; Zhou, J.; Zhao, Z.; Zhang, S.; Bu, H.; Qi, W. Geographic variation in the petiole-lamina relationship of 325 Eastern Qinghai-Tibetan woody species: Analysis in three dimensions. Front. Plant Sci. 2021, 12, 748125. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Roa-Fuentes, L.L.; Templer, P.H.; Campo, J. Effects of precipitation regime and soil nitrogen on leaf traits in seasonally dry tropical forests of the Yucatan Peninsula, Mexico. Oecologia 2015, 179, 585–597. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, Z.; Gong, S. Comparisons of relationships between leaf and fine root traits in hilly area of the Loess Plateau, Yanhe River basin, Shaanxi Province, China. Acta Ecol. Sin. 2011, 31, 6805–6814. [Google Scholar]
- Zhang, P.; Li, Y.; Shao, M. Effects of sandy land water habitat and years after rejuvenation pruning on leaf functional traits of Salix psammophila. Chin. J. Appl. Ecol. 2011, 22, 2240–2246. [Google Scholar] [CrossRef]
- Qi, D.; Wen, Z.; Yang, S.; Wang, H.; Guo, R. Trait-based responses and adaptation of Artemisia sacrorum to environmental changes. Chin. J. Appl. Ecol. 2015, 26, 1921–1927. [Google Scholar] [CrossRef]
- Taylor, S.H.; Franks, P.J.; Hulme, S.P.; Spriggs, E.; Christin, P.A.; Edwards, E.J.; Woodward, F.I.; Osborne, C.P. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. New Phytol. 2012, 193, 387–396. [Google Scholar] [CrossRef]
- Collins, C.G.; Wright, S.J.; Wurzburger, N. Root and leaf traits reflect distinct resource acquisition strategies in tropical lianas and trees. Oecologia 2016, 180, 1037–1047. [Google Scholar] [CrossRef]
- Nelson, T.C.; Muir, C.D.; Stathos, A.M.; Vanderpool, D.D.; Anderson, K.; Angert, A.L.; Fishman, L. Quantitative trait locus mapping reveals an independent genetic basis for joint divergence in leaf function, life-history, and floral traits between scarlet monkeyflower (Mimulus cardinalis) populations. Am. J. Bot. 2021, 108, 844–856. [Google Scholar] [CrossRef]
- Jin, N.; Yu, X.; Dong, J.; Duan, M.; Mo, Y.; Feng, L.; Bai, R.; Zhao, J.; Song, J.; Dossa, G.G.O.; et al. Vertical variation in leaf functional traits of Parashorea chinensis with different canopy layers. Front. Plant Sci. 2024, 15, 1335524. [Google Scholar] [CrossRef] [PubMed]
- Avalos, G.; Cambronero, M.; Alvarez-Vergnani, C. Divergence in functional traits in seven species of neotropical palms of different forest strata. Oecologia 2023, 203, 323–333. [Google Scholar] [CrossRef]
- Samanta, S.; Seth, C.S.; Roychoudhury, A. The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol. Biochem. 2024, 206, 108259. [Google Scholar] [CrossRef]
- Yang, J.; Park, S.; Lee, H.; Nam, K.; Lee, K.; Lee, J.; Kim, J.; Kwak, S.; Kim, H.; Kim, Y. Differential responses of antioxidant enzymes and lignin metabolism in susceptible and resistant sweetpotato cultivars during root-knot nematode Infection. Antioxidants 2023, 12, 1164. [Google Scholar] [CrossRef]
- Wu, S.; Tian, J.; Ren, T.; Wang, Y. Osmotic adjustment and antioxidant system regulated by nitrogen deposition improve photosynthetic and growth performance and alleviate oxidative damage in dwarf bamboo under drought stress. Front. Plant Sci. 2022, 13, 819071. [Google Scholar] [CrossRef]
- Kommineni, V.K.; Tautenhahn, S.; Baddam, P.; Gaikwad, J.; Wieczorek, B.; Triki, A.; Kattge, J. Comprehensive leaf size traits dataset for seven plant species from digitised herbarium specimen images covering more than two centuries. Biodivers. Data J. 2021, 9, e69806. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Wilsey, B.J. Exotic species explain plant functional trait differences between seed mixes, restored and reference prairies. Appl. Veg. Sci. 2023, 26, e12709. [Google Scholar] [CrossRef]
- Pritzkow, C.; Szota, C.; Williamson, V.G.; Arndt, S.K. Phenotypic plasticity of drought tolerance traits in a widespread Eucalypt (Eucalyptus obliqua). Forests 2020, 11, 1371. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.; Kang, M.; Li, X. The relationship of the main root-shoot morphological characteristics and biomass allocation of Saussurea salsa under different habitat conditions in Sugan lake wetland on the northern margin of the Qinghai-Tibet Plateau. Ecol. Indic. 2021, 128, 107836. [Google Scholar] [CrossRef]
- Dovrat, G.; Meron, E.; Shachak, M.; Golodets, C.; Osem, Y. Plant size is related to biomass partitioning and stress resistance in water-limited annual plant communities. J. Arid Environ. 2019, 165, 1–9. [Google Scholar] [CrossRef]
- Li, P.; Ma, B.; Guo, S.; Ding, T.; Xiong, Y. Bottom-up redistribution of biomass optimizes energy allocation, water use and yield formation in dryland wheat improvement. J. Sci. Food Agric. 2022, 102, 3336–3349. [Google Scholar] [CrossRef]
- Ma, D.; He, Z.; Bai, X.; Wang, W.; Zhao, P.; Lin, P.; Zhou, H. Atriplex canescens, a valuable plant in soil rehabilitation and forage production. A review. Sci. Total Environ. 2022, 804, 150287. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Lou, J. Research advances on feeding value, processing and utilization of forage shrub Atriplex canescens. Anim. Husb. Feed. Sci. 2021, 42, 28–34. [Google Scholar] [CrossRef]
- Wang, F.; Zhuo, B.; Wang, S.; Lou, J.; Zhang, Y.; Chen, Q.; Shi, Z.; Song, Y.; Tu, P. Atriplex canescens: A new host for Cistanche deserticola. Heliyon 2021, 7, e07368. [Google Scholar] [CrossRef]
- Lei, J.; Nie, X.; Gao, W.; Sailihan, S.; Gao, Y. Study on physiology of drought resistance of Atriplex canescens. Xinjiang Agric. Sci. 2010, 47, 59–66. [Google Scholar]
- Zhang, Z.; Zhang, T.; Yin, B.; Wang, Z.; Li, R.; Li, S. The influence of sodium salt on growth, photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens under drought stress. Agronomy 2023, 13, 2434. [Google Scholar] [CrossRef]
- Hao, G.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Gao, Y.; Yu, Y.; Wang, J.; Hu, s.; Yuan, L.; Wang, S.; Zhang, X. Effect of drought stress on anatomical structure of leave and physiological characteristics in three Atriplex L. seedling. Acta Bot. Boreali-Occident. Sin. 2014, 34, 0976–0987. [Google Scholar] [CrossRef]
- Wang, Q.; Yi, X.; Wang, H.; Han, G.; Zhao, Z. Effects of water stress on seedling biomass of several sand-fixing shrubs. J. Gansu Agric. Univ. 2014, 49, 93–100. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Y.; Yu, X.; Li, G. Activity of protective enzymes of Atriplex canescens and its drought resistance physiological characters under drought stress. J. Arid Land Resour. Environ. 2010, 24, 122–126. [Google Scholar] [CrossRef]
- Huo, J.; Ren, L.; Pan, Y.; Zhao, J.; Xiang, X.; Yu, C.; Meng, D.; Wang, Y.; Lu, R.; Huang, Y. Functional traits of desert plants and their responses to environmental factors in Qaidam Basin, China. Acta Ecol. Sin. 2022, 42, 4494–4503. [Google Scholar] [CrossRef]
- Liu, M.; Ma, J. Responses of plant functional traits and soil factors to slope aspect in alpine meadow of South Gansu, Northwest China. Chin. J. Appl. Ecol. 2012, 23, 3295–3300. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Su, B.; Zhou, Y.; Chang, L.; Ma, X.; Li, X. Ecophysiological responses of five mangrove species (Bruguiera gymnorrhiza, Rhizophora stylosa, Aegiceras corniculatum, Avicennia marina, and Kandelia obovata) to chilling stress. Front. Mar. Sci. 2022, 9, 846566. [Google Scholar] [CrossRef]
- Dudziak, K.; Bulak, P.; Zapalska, M.; Borner, A.; Szczerba, H.; Lesniowska-Nowak, J.; Nowak, M. Using intervarietal substitution lines for the identification of wheat chromosomes involved in early responses to water-deficit stress. PLoS ONE 2019, 14, e0221849. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Kumar, R.; Adhikary, A.; Saini, R.; Khan, S.A.; Yadav, M.; Kumar, S. Drought priming induced thermotolerance in wheat (Triticum aestivum L.) during reproductive stage; a multifaceted tolerance approach against terminal heat stress. Plant Physiol. Biochem. 2023, 201, 107840. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, Y.; Heidari, G.; Weisany, W.; Golezani, K.G.; Mohammadi, K. Changes of antioxidative enzymes, lipid peroxidation and chlorophyll content in chickpea types colonized by different Glomus species under drought stress. Symbiosis 2012, 56, 5–18. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, Y.; Ma, G.; Zhang, X.; Lu, H.; Shi, J.; Jiquan, X. Effects of drought stress on photosynthetic traits and protective enzyme activity in maize seeding. Acta Ecol. Sin. 2011, 31, 1303–1311. [Google Scholar]
- Ali, A.M.; Thind, H.S.; Sharma, S.; Singh, Y. Site-specific nitrogen management in dry direct-seeded rice using chlorophyll meter and leaf colour chart. Pedosphere 2015, 25, 72–81. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef] [PubMed]
- Zareei, E.; Karami, F.; Gholami, M.; Ershadi, A.; Avestan, S.; Aryal, R.; Gohari, G.; Farooq, M. Physiological and biochemical responses of strawberry crown and leaf tissues to freezing stress. BMC Plant Biol. 2021, 21, 532. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zhang, X.; Zhang, C.; Wang, C.; Huang, Y.; Liu, Z. Mitigating the toxicity of reactive oxygen species induced by cadmium via restoring citrate valve and improving the stability of enzyme structure in rice. Chemosphere 2023, 327, 138511. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.J.; William, H.; Outlaw, J. Rapid adjustment of guard-cell abscisic acid levels to current leaf-water status. Plant Physiol. 1991, 95, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, D.; Li, W. Resurrection characteristics, photosynthetic and physiological response to dehydration andrehydration of two species in Gesneriaceae with different habitats. Guihaia 2022, 42, 199–209. [Google Scholar] [CrossRef]
- Zhu, H.; Li, D.; Zhang, T.; Wen, T.; Wen, C.; Wang, X. Effects of saline-alkali resistant agents on osmotic adjustment substances and root activity of double-cropping rice under saline-alkali stress. Chin. J. Soil Sci. 2022, 53, 1098–1105. [Google Scholar] [CrossRef]
- Xu, R.; Liu, J.; Wang, L.; Yan, Y.; Ma, X.; Li, M. Analysis of root and leaf functional traits and C, N, P stoichiometry of Cunninghamia lanceolate from different provenances. Acta Ecol. Sin. 2022, 42, 6298–6310. [Google Scholar] [CrossRef]
- Stahl, U.; Kattge, J.; Reu, B.; Voigt, W.; Ogle, K.; Dickie, J.; Wirth, C. Whole-plant trait spectra of North American woody plant species reflect fundamental ecological strategies. Ecosphere 2013, 4, 1–28. [Google Scholar] [CrossRef]
- Coneva, V.; Chitwood, D.H. Genetic and developmental basis for increased leaf thickness in the Arabidopsis Cviecotype. Front. Plant Sci. 2018, 9, 322. [Google Scholar] [CrossRef]
- Slimani, N.; Arraouadi, S.; Hajlaoui, H.; Borgi, M.A.; Boughattas, N.E.H.; De Feo, V.; Snoussi, M. Theimpact of greenhouse and field growth conditions on Chenopodium quinoa willd accessions’ response to salt stress: A comparative approach. Agronomy 2023, 13, 2303. [Google Scholar] [CrossRef]
- Huo, C.; Zhu, L.; Long, M.; Zhao, L. Functional characters and eco stoichiometric characteristics of leaves of seven landscape shrubs. Chin. J. Trop. Crops 2023, 44, 337–346. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, W.; Wang, J.; Wang, Y.; Li, J.; Yuan, D.; Fan, Y.; Wei, X. Leaf functional traits of oasis plants in extremely arid areas and its response to soil water and salt factors. J. Beijing For. Univ. 2019, 41, 20–29. [Google Scholar] [CrossRef]
- Kleiman, D.; Aarssen, L.W. The leaf size/number trade-off in trees. J. Ecol. 2007, 95, 376–382. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.t.; Morgan, H.D.; Heijden, M.G.A.v.d.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.-h.; Zhang, Z.; Zhang, Q.; Guo, J.I.; Zhou, B.; Deng, Y.; Wang, X.; Wang, F.; She, Y.; et al. Relationship between the characteristics of plant community growth and climate factors in alpine meadow. Acta Ecol. Sin. 2021, 41, 3689–3700. [Google Scholar]
- Revillini, D.; Gehring, C.A.; Johnson, N.C.; Bailey, J. The role of locally adapted mycorrhizas and rhizobacteria in plant–soil feedback systems. Funct. Ecol. 2016, 30, 1086–1098. [Google Scholar] [CrossRef]
- Hu, Q.; Shu, J.; Li, W.; Wang, G. Role of auxin and nitrate signaling in the development of root system architecture. Front. Plant Sci. 2021, 12, 690363. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; Enquist, B.J. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 2007, 21, 713–720. [Google Scholar] [CrossRef]
- Meng, B.; Li, J.; Yao, Y.; Nippert, J.B.; Williams, D.G.; Chai, H.; Collins, S.L.; Sun, W. Soil N enrichment mediates carbon allocation through respiration in a dominant grass during drought. Funct. Ecol. 2022, 36, 1204–1215. [Google Scholar] [CrossRef]
- Zhou, L.; Hong, Y.; Li, C.; Lu, C.; He, Y.; Shao, J.; Sun, X.; Wang, C.; Liu, R.; Liu, H.; et al. Responses of biomass allocation to multi-factor global change: A global synthesis. Agric. Ecosyst. Environ. 2020, 304, 107115. [Google Scholar] [CrossRef]
- Liu, R.; Yang, X.; Gao, R.; Hou, X.; Huo, L.; Huang, Z.; Cornelissen, J.H.C.; Vesk, P. Allometry rather than abiotic drivers explains biomass allocation among leaves, stems and roots of Artemisia across a large environmental gradient in China. J. Ecol. 2020, 109, 1026–1040. [Google Scholar] [CrossRef]
- Schellberg, J.; Pontes, L.d.S. Plant functional traits and nutrient gradients on grassland. Grass Forage Sci. 2012, 67, 305–319. [Google Scholar] [CrossRef]
- Midolo, G. Plant functional traits couple with range size and shape in European trees. Glob. Ecol. Biogeogr. 2024, 00, e13838. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Wen, R.; Zhou, C.; Xu, L.; Xi, X. Mapping spatiotemporal changes in vegetation growth peak and the response to climate and spring phenology over Northeast China. Remote Sens. 2020, 12, 3977. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, H.; Yang, H.; Wei, Z.; Che, J.; Wu, W.; Lyu, L.; Li, W. Physiological and morphological responses of blackberry seedlings to different nitrogen forms. Plants 2023, 12, 1480. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, R.; Liu, X.; Zhou, L.; Dong, M.; Rehman, M.; Fahad, S.; Liu, L.; Deng, G. Effects of light spectra on morphology, gaseous exchange, and antioxidant capacity of industrial hemp. Front. Plant Sci. 2022, 13, 937436. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lai, L.; Zhou, J.; Li, Q.; Yi, S.; Sun, Q.; Zheng, Y. Changes in levels of enzymes and osmotic adjustment compounds in key species and their relevance to vegetation succession in abandoned croplands of a semiarid sandy region. Ecol. Evol. 2020, 10, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; Tian, Y.L.; Ye, D.M.; Qin, J.Q.; Huo, L.S. Model of aboveground biomass of Nitraria tangutorum and relationship between biomass and leaf area. J. Desert Rresearch 2005, 25, 541–546. [Google Scholar]
- Zhang, Y.; Cheng, J.; Su, J.; Cheng, J. Diversity-productivity relationship of plant communities in typical grassland during the long- term grazing exclusion succession. Chin. J. Plant Ecol. 2022, 46, 176–187. [Google Scholar] [CrossRef]
- Pontes, L.D.S.; Soussana, J.F.; Louault, F.; Andueza, D.; Carrere, P. Leaf traits affect the above-ground productivity and quality of pasture grasses. Funct. Ecol. 2007, 21, 844–853. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; Neill, C.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Elger, A.; Willby, N.J. Leaf dry matter content as an integrative expression of plant palatability: The case of freshwater macrophytes. Funct. Ecol. 2003, 17, 58–65. [Google Scholar] [CrossRef]
- Eviner, V.T.; Chapin Iii, F.S. Functional matrix: A conceptual framework for predicting multiple plant effects on ecosystem processes. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 455–485. [Google Scholar] [CrossRef]
- Ma, D.; He, Z.; Wang, L.; Zhao, W.; Chen, L.; Lin, P.; Zhao, P.; Wang, W.; Gao, Y.; Li, J. Soil water and salt migration in oasis farmland during crop growing season. J. Soils Sediments 2022, 23, 355–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W. Vegetation and soil property response of short-time fencing in temperate desert of the Hexi Corridor, northwestern China. Catena 2015, 133, 43–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Huang, P.; Yu, X.; Xiang, Q.; Zhang, L.; Gao, X.; Chen, Q.; Gu, Y. Biochemical and transcriptomic responses of buckwheat to polyethylene microplastics. Sci. Total Environ. 2023, 899, 165587. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Gilgen, A.K.; Wittwer, R.; von Arx, G.; van der Heijden, M.G.A.; Klaus, V.H.; Buchmann, N. Drought effects on trait space of winter wheat are independent of land management. New Phytol. 2024, 243, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, W.; Shen, H.; Niu, X.; Luo, Y.; Liang, C.; Li, Z.; Zheng, C.; Hu, H.; Ma, W. Models for estimating the biomass of 26 temperate shrub species in Inner Mongolia, China. Arid Zone Res. 2021, 36, 1219–1228. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, D.; Zhang, L.; Li, X.; Fu, H. Plant functional traits shape growth rate for xerophytic shrubs. Plant Biol. 2022, 24, 205–214. [Google Scholar] [CrossRef] [PubMed]

| Florescence Stage | Seed Stage | |||||
|---|---|---|---|---|---|---|
| Heavy | Feeble | Non-Stress | Heavy | Feeble | Non-Stress | |
| LL (10−2 m) | 2.65 ± 0.61 c | 4.56 ± 0.52 b | 5.67 ± 0.73 a | 2.87 ± 0.20 c | 4.62 ± 0.35 b | 5.12 ± 0.52 a |
| LW (10−2 m) | 0.50 ± 0.049 b | 0.68 ± 0.068 a | 0.78 ± 0.12 a | 0.48 ± 0.027 b | 0.68 ± 0.069 a | 0.72 ± 0.082 a |
| LT (10−2 m) | 0.044 ± 0.0019 b | 0.049 ± 0.0023 a | 0.047 ± 0.0040 ab | 0.043 ± 0.0033 b | 0.048 ± 0.0042 a | 0.050 ± 0.0022 a |
| LA (10−4 m2) | 0.92 ± 0.22 c | 2.25 ± 0.37 b | 3.06 ± 0.86 a | 0.90 ± 0.073 c | 1.78 ± 0.31 b | 2.13 ± 0.27 a |
| LI | 5.34 ± 1. 02 b | 6.68 ± 0.86 a | 7.31 ± 0.79 a | 5.97 ± 0.49 b | 6.88 ± 0.67 a | 7.18 ± 0.81 a |
| FW (10−3 g) | 51.09 ± 21.33 b | 112.134 ± 22.99 a | 127.42 ± 28.31 a | 40.23 ± 7.74 c | 62.39 ± 4.84 b | 78.75 ± 17.68 a |
| SFW (10−3 g) | 66.43 ± 16.77 b | 159.36 ± 28.25 a | 188.60 ± 55.01 a | 49.94 ± 11.59 c | 73.26 ± 5.21 b | 91.91 ± 20.91 a |
| DW (10−3 g) | 12.93 ± 4.25 b | 30.10 ± 5.18 a | 32.36 ± 8.95 a | 13.48 ± 4.40 b | 18.75 ± 2.33 ab | 22.91 ± 7.06 a |
| SLA (10−4 m2 g−1) | 73.68 ± 10.59 b | 76.36 ± 14.89 b | 94.86 ± 9.52 a | 73.00 ± 20.31 b | 97.16 ± 25.95 a | 101.31 ± 27.36 a |
| LD (106 g m−3) | 0.31 ± 0.038 a | 0.28 ± 0.043 a | 0.23 ± 0.0243 b | 0.35 ± 0.13 a | 0.22 ± 0.040 b | 0.22 ± 0.082b |
| LDMC (%) | 19.20 ± 2.46 a | 18.91 ± 1.27 a | 17.21 ± 0.82 a | 26.57 ± 3.43 a | 25.61 ± 2.69 a | 24.56 ± 2.15 a |
| SWC (%) | 80.80 ± 2.46 a | 81.09 ± 1.27 a | 82.79 ± 0.82 a | 73.43 ± 3.42 a | 74.39 ± 2.69 a | 75.44 ± 2.15 a |
| WC (%) | 73.50 ± 3.59 a | 72.84 ± 2.65 a | 74.13 ± 5.30 a | 67.17 ± 5.03 a | 69.90 ± 3.24 a | 71.30 ± 2.95 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhou, H.; He, Z.; Ma, D.; Sun, W.; Xu, X.; Tian, Q. Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens. Plants 2024, 13, 2006. https://doi.org/10.3390/plants13142006
Wang S, Zhou H, He Z, Ma D, Sun W, Xu X, Tian Q. Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens. Plants. 2024; 13(14):2006. https://doi.org/10.3390/plants13142006
Chicago/Turabian StyleWang, Shuai, Hai Zhou, Zhibin He, Dengke Ma, Weihao Sun, Xingzhi Xu, and Quanyan Tian. 2024. "Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens" Plants 13, no. 14: 2006. https://doi.org/10.3390/plants13142006
APA StyleWang, S., Zhou, H., He, Z., Ma, D., Sun, W., Xu, X., & Tian, Q. (2024). Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens. Plants, 13(14), 2006. https://doi.org/10.3390/plants13142006


.png)




