Heat Stress and Plant–Biotic Interactions: Advances and Perspectives
Abstract
:1. Introduction
2. Heat Stress and Plant–Biotic Interactions
3. Plant Responses to Heat Stress
4. Plant Responses to Biotic Stressors
5. Plant Responses to Concurrent Heat Stress and Biotic Interactions
5.1. Plant–Heat Stress–Bacterial Interactions
5.2. Plant–Heat Stress–Fungal Interactions
5.3. Plant–Heat Stress–Viral Interactions
5.4. Plant–Heat Stress–Nematode Interactions
5.5. Plant–Heat Stress–Insect Interactions
5.6. Plant–Heat Stress–Weed/Parasitic Plant Interactions
6. Perspectives on Dealing with Combined Heat and Biotic Stresses in Agriculture
6.1. Potential Use of Plant Microbiome
| Plant | Plant-Beneficial Microbes | Pathogen (Disease) | Pathogen/Lifestyle | Details | Ref. |
|---|---|---|---|---|---|
| Arabidopsis | Bacillus subtilis PTA-CT2, Pseudomonas fluorescens PTA-271 | Pseudomonas syringae pv. tomato DC3000 (Psto) | Bacterial hemibiotroph | Root-drench, priming effect on plant immunity, ISR | [259,260] |
| Botrytis cinerea (Grey mold) | Fungal necrotroph | ||||
| Bacillus proteolyticus OSUB18 | Psto | Bacterial hemibiotroph | Root-drench, priming effect on plant immunity, ISR | [261] | |
| Botrytis cinerea | Fungal necrotroph | ||||
| Soybean | Trichoderma viride GT-8, T. reesei GT-31, T. longibrachiatum GT-32 | Macrophomina phaseolina (Charcoal rot), Sclerotinia sclerotiorum (White mold), Fusarium sp. (Seedling damping-off) | Fungal necrotroph | Seed treatment, plant growth promotion (PGP), competition | [262] |
| Trichoderma harzianum, T. asperellum T00 | Pratylenchus brachyurus (Root lesion) | Nematode | Seed treatment, production of SM, lytic enzymes | [263,264] | |
| Dragon fruit | T. viride, T. asperellum, T. harzianum | Neoscytalidium dimidiate (Brown spot) | Fungal | Added to base soil of tree, competition, ISR, antibiosis synthesis | [265] |
| Kale | Pseudomonas fluorescens SP007S | Pectobacterium carotovorum (Soft rot disease) | Bacterial necrotroph | Soil treatment, SAR, phenol compounds, PGP | [266] |
| Olive | Bacillus sp., Pseudomonas fluorescens (alone or combined) | Pseudomonas savastanoi (Knot disease) | Bacterial necrotroph | Foliar spray, SM, PGP | [267] |
| Chili pepper | Bacillus thuringiensis MW740161.1, Pseudomonas fluorescens, Bacillus subtilis | Leveillula Taurica (Powdery mildew) | Fungal | Foliar spray, production of SM, peroxidases, ISR | [268] |
| Sorghum | Trichoderma viride | Dickeyadadantii (Stalk rot) | Bacterial | Seed treatment, production of defense enzymes, PGP | [269] |
| Potato | Trichoderma harzianum, Pseudomonas fluorescens | Alternaria solani (Early Blight) | Fungal | Tuber treatment, PGP | [270] |
| Cucumber | Trichoderma harzianum | Fusarium oxysporum | Fungal | Seed treatment, production of defense enzymes, PGP | [271] |
| Tomato | Trichoderma asperellum | Agroathelia rolfsii (Collar rot) | Fungal necrotroph | Root-drench, production of hydrolytic enzymes, SM | [272] |
| Trichoderma longibrachiatum, T. atroviride, T. harzianum | Alternaria solani (Early Blight) | Fungal | Foliar spray, production of SM, antioxidant enzymes, PGP | [273] | |
| Trichoderma asperellum, T. harzianum, Bacillus subtilis Verticillium lecanii, Metarhizium anisopliae, | Meloidogyne spp. (Root-knot nematode) | Nematode | Cavity chamber method, production of lytic enzymes | [274] | |
| Cucumber, tomato | Pseudomonas protegens 1B1, P. clororaphis 48G9, P. brassicacearum 93G8 | Agrobacterium rhizogenes (Hairy root disease) | Bacterial | Root dip method | [275] |
| Wheat | Beauveria bassiana, Metarhizium anisopliae | Rhopalosiphum rufiabdominalis (Aphid) | Insect | Foliar spray, penetration of hyphae through the insect cuticle | [276] |
| Beauveria bassiana (Balsamo) Vuillemin | Tenebrio molitor (Mealworm), Monochamus alternatus (Japanese pine sawyer), Allomyrina dichotoma (Japanese rhinoceros beetle) | Insects | Syringe application, production of toxins | [277] | |
| Sugarcane | Metarhizium anisopliae | Termite (Odontotermes obesus (Ramb.), Microtermes obesi (Holgren) | Insect | Soil drenching, muscle contraction, flaccid paralysis | [278] |
| M. anisopliae | Holotrichia serrata (White grub) | Insect | Soil application, protease production, flaccid paralysis | [279] | |
| Walnut | Lecanicillium lecanii, Metarhizium anisopliae sensu lato, Beauveria bassiana | Myllocerus fotedari Ahmad (Weevil) | Insect | Foliar spray, production of toxin and flaccid paralysis | [280] |
| Orange and mandarin | Bacillus thuringiensis, Metarhizium anisopliae, Trichoderma harzianum | Monacha obstructa (Land snail) | Animal pest | Leaf dipping, poisonous baiting, and foliar spray, hyper parasitism | [281] |
6.2. Building Precise Regulatory Networks of Plant Responses to Combined Heat and Biotic Stresses
6.3. Developing Reliable Stress Prediction Models and Priming Plants against Combined HS–Biotic Stresses
6.4. Developing Stress-Resilient Crop Varieties along with Novel Agricultural Practices
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate Change and Ecosystems: Threats, Opportunities and Solutions. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Climate Change and Future of Agri-Food Production. In Future Foods: Global Trends, Opportunities, and Sustainability Challenges; Elsevier: Amsterdam, The Netherlands, 2021; pp. 49–79. ISBN 9780323910019. [Google Scholar]
- Shelake, R.M.; Kadam, U.S.; Kumar, R.; Pramanik, D.; Singh, A.K.; Kim, J.Y. Engineering Drought and Salinity Tolerance Traits in Crops through CRISPR-Mediated Genome Editing: Targets, Tools, Challenges, and Perspectives. Plant Commun. 2022, 3, 100417. [Google Scholar] [CrossRef] [PubMed]
- Sundström, J.F.; Albihn, A.; Boqvist, S.; Ljungvall, K.; Marstorp, H.; Martiin, C.; Nyberg, K.; Vågsholm, I.; Yuen, J.; Magnusson, U. Future Threats to Agricultural Food Production Posed by Environmental Degradation, Climate Change, and Animal and Plant Diseases—A Risk Analysis in Three Economic and Climate Settings. Food Secur. 2014, 6, 201–215. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y. The Molecular Basis of Heat Stress Responses in Plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Y.; Mahmood, K.; Ashraf, M.; Akhter, J.; Hussain, F. Optimal Supply of Micronutrients Improves Drought Tolerance in Legumes. In Crop Production for Agricultural Improvement; Springer: Dordrecht, The Netherlands, 2012; pp. 637–657. ISBN 9789400741164. [Google Scholar]
- Desaint, H.; Aoun, N.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Fight Hard or Die Trying: When Plants Face Pathogens under Heat Stress. New Phytol. 2021, 229, 712–734. [Google Scholar] [CrossRef] [PubMed]
- IPCC 2021. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021; Volume 2391, ISBN 9789291691586. [Google Scholar]
- Nejat, N.; Mantri, N. Plant Immune System: Crosstalk between Responses to Biotic and Abiotic Stresses the Missing Link in Understanding Plant Defence. Curr. Issues Mol. Biol. 2017, 23, 1–16. [Google Scholar] [CrossRef]
- Kwon, T.; Shibata, H.; Kepfer-Rojas, S.; Schmidt, I.K.; Larsen, K.S.; Beier, C.; Berg, B.; Verheyen, K.; Lamarque, J.F.; Hagedorn, F.; et al. Effects of Climate and Atmospheric Nitrogen Deposition on Early to Mid-Term Stage Litter Decomposition Across Biomes. Front. For. Glob. Chang. 2021, 4, 678480. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sengupta, S.; Fritschi, F.B.; Azad, R.K.; Nechushtai, R.; Mittler, R. The Impact of Multifactorial Stress Combination on Plant Growth and Survival. New Phytol. 2021, 230, 1034–1048. [Google Scholar] [CrossRef]
- Nissan, H.; Goddard, L.; de Perez, E.C.; Furlow, J.; Baethgen, W.; Thomson, M.C.; Mason, S.J. On the Use and Misuse of Climate Change Projections in International Development. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, e579. [Google Scholar] [CrossRef]
- Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life 2022, 12, 1634. [Google Scholar] [CrossRef]
- Leisner, C.P.; Potnis, N.; Sanz-Saez, A. Crosstalk and Trade-Offs: Plant Responses to Climate Change-Associated Abiotic and Biotic Stresses. Plant Cell Environ. 2023, 46, 2946–2963. [Google Scholar] [CrossRef] [PubMed]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A.; Ainsworth, E.A.; Bahuguna, R.N.; Broadley, M.R.; Busch, W.; Carpita, N.C.; Castrillo, G.; Chory, J.; Dehaan, L.R.; Duarte, C.M.; et al. Climate Change Challenges, Plant Science Solutions. Plant Cell 2023, 35, 24–66. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.T.; Humphreys, A.M. Global Variation in the Thermal Tolerances of Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13580–13587. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Raza, A.; Iqbal, J.; Shaukat, M.; Mahmood, T. Analyzing the Regulatory Role of Heat Shock Transcription Factors in Plant Heat Stress Tolerance: A Brief Appraisal. Mol. Biol. Rep. 2022, 49, 5771–5785. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Leach, J.E. High Temperature-Induced Plant Disease Susceptibility: More than the Sum of Its Parts. Curr. Opin. Plant Biol. 2020, 56, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef] [PubMed]
- Sewelam, N.; El-Shetehy, M.; Mauch, F.; Maurino, V.G. Combined Abiotic Stresses Repress Defense and Cell Wall Metabolic Genes and Render Plants More Susceptible to Pathogen Infection. Plants 2021, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate Change-Mediated Temperature Extremes and Insects: From Outbreaks to Breakdowns. Glob. Chang. Biol. 2020, 26, 6685–6701. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil, A.; Senthil-Kumar, M. Stress Combinations and Their Interactions in Crop Plants. Plant Physiol. Rep. 2024, 29, 1–5. [Google Scholar] [CrossRef]
- Pandey, P.; Patil, M.; Priya, P.; Senthil-Kumar, M. When Two Negatives Make a Positive: The Favorable Impact of the Combination of Abiotic Stress and Pathogen Infection on Plants. J. Exp. Bot. 2024, 75, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Roussin-Léveillée, C.; Rossi, C.A.M.; Castroverde, C.D.M.; Moffett, P. The Plant Disease Triangle Facing Climate Change: A Molecular Perspective. Trends Plant Sci. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Mahalingam, R.; Pandey, P.; Senthil-Kumar, M. Progress and Prospects of Concurrent or Combined Stress Studies in Plants. Ann. Plant Rev. 2021, 4, 813–868. [Google Scholar] [CrossRef]
- Son, S.; Park, S.R. Climate Change Impedes Plant Immunity Mechanisms. Front. Plant Sci. 2022, 13, 1032820. [Google Scholar] [CrossRef]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Habib-ur-Rahman, M.; Noor, M.A.; Sher, A.; Fahad, S. Linking Plants Functioning to Adaptive Responses Under Heat Stress Conditions: A Mechanistic Review. J. Plant Growth Regul. 2022, 41, 2596–2613. [Google Scholar] [CrossRef]
- Manghwar, H.; Zaman, W. Plant Biotic and Abiotic Stresses. Life 2024, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Balasubramanian, S. Thermomorphogenesis. Annu. Rev. Plant Biol. 2019, 70, 321–346. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; Van Zanten, M. Molecular and Genetic Control of Plant Thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Al Zeidi, M.; Siddique, K.H.M.; Farooq, M. Plant Photosynthesis under Heat Stress: Effects and Management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Wang, M.; Shah, L.; Lu, S.; Wang, X.; Ma, C. Unraveling Field Crops Sensitivity to Heat Stress: Mechanisms, Approaches, and Future Prospects. Agronomy 2018, 8, 128. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive Oxygen Species, Heat Stress and Oxidative-Induced Mitochondrial Damage. A Review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Kim, S.H.; Yun, M.; Choi, W.G. Recapitulation of the Function and Role of Ros Generated in Response to Heat Stress in Plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex Plant Responses to Drought and Heat Stress under Climate Change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V. (Biotechnologist) Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 9781119432364. [Google Scholar]
- Li, Z.-G.; Fang, J.-R.; Bai, S.-J. Hydrogen Sulfide Signaling in Plant Response to Temperature Stress. Front. Plant Sci. 2024, 15, 1337250. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Lee, K.; Hoshikawa, K.; Kang, M.; Jang, S. Molecular Bases of Heat Stress Responses in Vegetable Crops With Focusing on Heat Shock Factors and Heat Shock Proteins. Front. Plant Sci. 2022, 13, 837152. [Google Scholar] [CrossRef] [PubMed]
- Jhu, M.-Y.; Sinha, N.R. Parasitic Plants: An Overview of Mechanisms by Which Plants Perceive and Respond to Parasites. Annu. Rev. Plant Biol. 2022, 73, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Mandadi, K.K.; Scholthof, K.B.G. Plant Immune Responses against Viruses: How Does a Virus Cause Disease? Plant Cell 2013, 25, 1489–1505. [Google Scholar] [CrossRef]
- Calil, I.P.; Fontes, E.P.B. Plant Immunity against Viruses: Antiviral Immune Receptors in Focus. Ann. Bot. 2017, 119, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kadota, Y.; Shirasu, K. Plant Immune Responses to Parasitic Nematodes. Front. Plant Sci. 2019, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of Infection by Parasitic Weeds: A Review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty Years of Resistance: Zig-Zag through the Plant Immune System. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Gong, D.; Wang, Z.; Liu, L.; He, J.; Han, X.; Tsuda, K. How Plants Manage Pathogen Infection. EMBO Rep. 2024, 25, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in Plant Innate Immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef]
- Ferrusquía-Jiménez, N.I.; Chandrakasan, G.; Torres-Pacheco, I.; Rico-Garcia, E.; Feregrino-Perez, A.A.; Guevara-González, R.G. Extracellular DNA: A Relevant Plant Damage-Associated Molecular Pattern (DAMP) for Crop Protection Against Pests—A Review. J. Plant Growth Regul. 2021, 40, 451–463. [Google Scholar] [CrossRef]
- Islam, M.M.; El-Sappah, A.H.; Ali, H.M.; Zandi, P.; Huang, Q.; Soaud, S.A.; Alazizi, E.M.Y.; Wafa, H.A.; Hossain, M.A.; Liang, Y. Pathogenesis-related proteins (PRs) countering environmental stress in plants: A review. S. Afr. J. Bot. 2023, 160, 414–427. [Google Scholar] [CrossRef]
- dos Santos, C.; Franco, O.L. Pathogenesis-Related Proteins (PRs) with Enzyme Activity Activating Plant Defense Responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Ge, D.; Yeo, I.-C.; Shan, L. Knowing Me, Knowing You: Self and Non-Self Recognition in Plant Immunity. Essays Biochem. 2022, 66, 447–458. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant-Pathogen Interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the Plant Immune System from Dissection to Deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Feng, Y.; Xue, J.; Chen, P.; Zhang, A.; Yu, Y. Advances in Receptor-like Protein Kinases in Balancing Plant Growth and Stress Responses. Plants 2023, 12, 427. [Google Scholar] [CrossRef]
- Lin, P.-A.; Chen, Y.; Ponce, G.; Acevedo, F.E.; Lynch, J.P.; Anderson, C.T.; Ali, J.G.; Felton, G.W. Stomata-Mediated Interactions between Plants, Herbivores, and the Environment. Trends Plant Sci. 2022, 27, 287–300. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Gou, M.; Balint-Kurti, P.; Xu, M.; Yang, Q. Quantitative Disease Resistance: Multifaceted Players in Plant Defense. J. Integr. Plant Biol. 2023, 65, 594–610. [Google Scholar] [CrossRef]
- Silvestri, A.; Bansal, C.; Rubio-Somoza, I. After Silencing Suppression: MiRNA Targets Strike Back. Trends Plant Sci. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate Change Impacts on Plant Pathogens, Food Security and Paths Forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of Temperature Modulation of Plant Defense against Biotrophic Microbes. Mol. Plant Microbe Interact. 2009, 22, 498–506. [Google Scholar] [CrossRef]
- Janda, M.; Lamparová, L.; Zubíková, A.; Burketová, L.; Martinec, J.; Krčková, Z. Temporary Heat Stress Suppresses PAMP-Triggered Immunity and Resistance to Bacteria in Arabidopsis thaliana. Mol. Plant Pathol. 2019, 20, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Menna, A.; Nguyen, D.; Guttman, D.S.; Desveaux, D. Elevated Temperature Differentially Influences Effector-Triggered Immunity Outputs in Arabidopsis. Front. Plant Sci. 2015, 6, 995. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Poovaiah, B.W. Interplay between Ca2+/Calmodulin-Mediated Signaling and AtSR1/CAMTA3 during Increased Temperature Resulting in Compromised Immune Response in Plants. Int. J. Mol. Sci. 2022, 23, 2175. [Google Scholar] [CrossRef] [PubMed]
- Aoun, N.; Tauleigne, L.; Lonjon, F.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Quantitative Disease Resistance under Elevated Temperature: Genetic Basis of New Resistance Mechanisms to Ralstonia solanacearum. Front. Plant Sci. 2017, 8, 1387. [Google Scholar] [CrossRef]
- Webb, K.M.; Oña, I.; Bai, J.; Garrett, K.A.; Mew, T.; Vera Cruz, C.M.; Leach, J.E. A Benefit of High Temperature: Increased Effectiveness of a Rice Bacterial Blight Disease Resistance Gene. New Phytol. 2010, 185, 568–576. [Google Scholar] [CrossRef]
- Cohen, S.P.; Liu, H.; Argueso, C.T.; Pereira, A.; Cruz, C.V.; Verdier, V.; Leach, J.E. RNA-Seq Analysis Reveals Insight into Enhanced Rice Xa7-Mediated Bacterial Blight Resistance at High Temperature. PLoS ONE 2017, 12, e0187625. [Google Scholar] [CrossRef] [PubMed]
- Dossa, G.S.; Quibod, I.; Atienza-Grande, G.; Oliva, R.; Maiss, E.; Vera Cruz, C.; Wydra, K. Rice Pyramided Line IRBB67 (Xa4/Xa7) Homeostasis under Combined Stress of High Temperature and Bacterial Blight. Sci. Rep. 2020, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Si, F.; Feng, Z.; Li, S.; Liang, D.; Yang, P.; Yang, C.; Yan, B.; Tang, J.; Yang, Y.; et al. The OsSGS3-TasiRNA-OsARF3 Module Orchestrates Abiotic-Biotic Stress Response Trade-off in Rice. Nat. Commun. 2023, 14, 4441. [Google Scholar] [CrossRef] [PubMed]
- Krausz, J.P.; Thurston, D. Breakdown of Resistance to Pseudomonas solanacearum in Tomato. Phytopathology 1975, 65, 1272. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Shen, L.; Wu, R.; Cao, J.; Tang, W.; Lu, Q.; Huang, Y.; Guan, D.; He, S. Solanaceous Plants Switch to Cytokinin-Mediated Immunity against Ralstonia solanacearum under High Temperature and High Humidity. Plant Cell Environ. 2022, 45, 459–478. [Google Scholar] [CrossRef]
- Scalschi, L.; Fernández-Crespo, E.; Pitarch-Marin, M.; Llorens, E.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; García-Agustín, P. Response of Tomato-Pseudomonas Pathosystem to Mild Heat Stress. Horticulturae 2022, 8, 174. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Wang, A.; Huang, X.; Zheng, X.; Liu, Q.; Cheng, X.; Wan, M.; Lv, J.; Guan, D.; et al. MADS-Box Protein AGL8 Interacts with Chromatin-Remodelling Component SWC4 to Activate Thermotolerance and Environment-Dependent Immunity in Pepper. J. Exp. Bot. 2023, 74, 3667–3683. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Wu, R.; Huang, Y.; Lu, Q.; Wang, H.; Huang, X.; Zhang, Y.; Wu, Q.; Cheng, X.; et al. Differential CaKAN3-CaHSF8 Associations Underlie Distinct Immune and Heat Responses under High Temperature and High Humidity Conditions. Nat. Commun. 2023, 14, 4477. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, S.; Zhang, Y.; Shi, Y.; Shen, L.; Zhang, Q.; Qiu, A.; Guan, D.; He, S. Temperature-Dependent Action of Pepper Mildew Resistance Locus O 1 in Inducing Pathogen Immunity and Thermotolerance. J. Exp. Bot. 2024, 75, 2064–2083. [Google Scholar] [CrossRef]
- Onaga, G.; Wydra, K.D.; Koopmann, B.; Séré, Y.; Von Tiedemann, A. Elevated Temperature Increases in Planta Expression Levels of Virulence Related Genes in Magnaporthe oryzae and Compromises Resistance in Oryza Sativa Cv. Nipponbare. Funct. Plant Biol. 2017, 44, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Onaga, G.; Wydra, K.; Koopmann, B.; Chebotarov, D.; Séré, Y.; Von Tiedemann, A. High Temperature Effects on Pi54 Conferred Resistance to Magnaporthe oryzae in Two Genetic Backgrounds of Oryza sativa. J. Plant Physiol. 2017, 212, 80–93. [Google Scholar] [CrossRef]
- Qiu, J.; Xie, J.; Chen, Y.; Shen, Z.; Shi, H.; Naqvi, N.I.; Qian, Q.; Liang, Y.; Kou, Y. Warm Temperature Compromises JA-Regulated Basal Resistance to Enhance Magnaporthe oryzae Infection in Rice. Mol. Plant 2022, 15, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cai, C.; Song, L.; Qiu, J.; Ma, C.; Wang, D.; Gu, X.; Yang, X.; Wei, W.; Tao, Y.; et al. Elevated CO2 and Temperature under Future Climate Change Increase Severity of Rice Sheath Blight. Front. Plant Sci. 2023, 14, 1115614. [Google Scholar] [CrossRef]
- Matić, S.; Garibaldi, A.; Gullino, M.L. Combined and Single Effects of Elevated CO2 and Temperatures on Rice Bakanae Disease under Controlled Conditions in Phytotrons. Plant Pathol. 2021, 70, 815–826. [Google Scholar] [CrossRef]
- Chilakala, A.R.; Mali, K.V.; Irulappan, V.; Patil, B.S.; Pandey, P.; Rangappa, K.; Ramegowda, V.; Kumar, M.N.; Puli, C.O.R.; Mohan-Raju, B.; et al. Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes. Front. Plant Sci. 2022, 13, 890551. [Google Scholar] [CrossRef]
- Sharath Chandran, U.S.; Tarafdar, A.; Mahesha, H.S.; Sharma, M. Temperature and Soil Moisture Stress Modulate the Host Defense Response in Chickpea During Dry Root Rot Incidence. Front. Plant Sci. 2021, 12, 653265. [Google Scholar] [CrossRef] [PubMed]
- Toniutti, L.; Breitler, J.C.; Etienne, H.; Campa, C.; Doulbeau, S.; Urban, L.; Lambot, C.; Pinilla, J.C.H.; Bertrand, B. Influence of Environmental Conditions and Genetic Background of Arabica Coffee (C. arabica L.) on Leaf Rust (Hemileia vastatrix) Pathogenesis. Front. Plant Sci. 2017, 8, 2025. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, E.J.; Miranda, B.R.; Dreaden, T.J.; Pinchot, C.C.; Jacobs, D.F. Beyond Blight: Phytophthora Root Rot under Climate Change Limits Populations of Reintroduced American Chestnut. Ecosphere 2022, 13, e3917. [Google Scholar] [CrossRef]
- Dorado, F.J.; Alías, J.C.; Chaves, N.; Solla, A. Warming Scenarios and Phytophthora cinnamomi Infection in Chestnut (Castanea sativa Mill.). Plants 2023, 12, 556. [Google Scholar] [CrossRef]
- Mayama, S.; Daly, J.M.; Rehfeld, D.W.; Daly, C.R. Hypersensitive Response of Near-Isogenic Wheat Carrying the Temperature-Sensitive Sr6 Allele for Resistance to Stem Rust. Physiol. Plant Pathol. 1975, 7, 35–47. [Google Scholar] [CrossRef]
- Harder, D.E.; Samborski, D.J.; Rohringer, R.; Rimmer, S.R.; Kim, W.K.; Chong, J. Electron Microscopy of Susceptible and Resistant Near-Isogenic (Sr6/Sr6) Lines of Wheat Infected by Puccinia graminis tritici. III. Ultrastructure of Incompatible Interact. Can. J. Bot. 1979, 57, 2626–2634. [Google Scholar] [CrossRef]
- Ge, Y.-F.; Johnson, J.W.; Roberts, J.J.; Rajaram, S. Temperature and Resistance Gene Interactions in the Expression of Resistance to Blumeria graminis f. sp. tritici. Euphytica 1998, 99, 103–109. [Google Scholar] [CrossRef]
- Gousseau, H.D.M.; Deverall, B.J.; McIntosh, R.A. Temperature-Sensitivity of the Expression of Resistance to Puccinia graminis Conferred by the Sr15, Sr9b and Sr14 Genes in Wheat. Physiol. Plant Pathol. 1985, 27, 335–343. [Google Scholar] [CrossRef]
- Dyck, P.L.; Johnson, R. Temperature Sensitivity of Genes for Resistance in Wheat to Puccinia recondita. Can. J. Plant Pathol. 1983, 5, 229–234. [Google Scholar] [CrossRef]
- Wang, C.; Yin, G.; Xia, X.; He, Z.; Zhang, P.; Yao, Z.; Qin, J.; Li, Z.; Liu, D. Molecular Mapping of a New Temperature-Sensitive Gene LrZH22 for Leaf Rust Resistance in Chinese Wheat Cultivar Zhoumai 22. Mol. Breed. 2016, 36, 18. [Google Scholar] [CrossRef]
- Chen, X.; Coram, T.; Huang, X.; Wang, M.; Dolezal, A. Understanding Molecular Mechanisms of Durable and Non-Durable Resistance to Stripe Rust in Wheat Using a Transcriptomics Approach. Curr. Genom. 2013, 14, 111–126. [Google Scholar] [CrossRef]
- Tao, F.; Wang, J.; Guo, Z.; Hu, J.; Xu, X.; Yang, J.; Chen, X.; Hu, X. Transcriptomic Analysis Reveal the Molecular Mechanisms of Wheat Higher-Temperature Seedling-Plant Resistance to Puccinia striiformis f. sp. tritici. Front. Plant Sci. 2018, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Shang, H.; Chen, X.; Xu, X.; Hu, X. TaXa21, a Leucine-Rich Repeat Receptor-like Kinase Gene Associated with TaWRKY76 and TaWRKY62, Plays Positive Roles in Wheat High-Temperature Seedling Plant Resistance to Puccinia striiformis f. sp. tritici. Mol. Plant Microbe Interact. 2019, 32, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, M.; See, D.R.; Chao, S.; Zheng, Y.; Chen, X. Characterization of Novel Gene Yr79 and Four Additional Quantitative Trait Loci for All-Stage and High-Temperature Adult-Plant Resistance to Stripe Rust in Spring Wheat PI 182103. Phytopathology 2018, 108, 737–747. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, M.; Chen, X.; See, D.; Chao, S.; Jing, J. Mapping of Yr62 and a Small-Effect QTL for High-Temperature Adult-Plant Resistance to Stripe Rust in Spring Wheat PI 192252. Theor. Appl. Genet. 2014, 127, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Wang, M.N.; Chen, X.M.; Lu, Y.; Kang, Z.S.; Jing, J.X. Identification of Yr59 Conferring High-Temperature Adult-Plant Resistance to Stripe Rust in Wheat Germplasm PI 178759. Theor. Appl. Genet. 2014, 127, 935–945. [Google Scholar] [CrossRef]
- Ren, R.S.; Wang, M.N.; Chen, X.M.; Zhang, Z.J. Characterization and Molecular Mapping of Yr52 for High-Temperature Adult-Plant Resistance to Stripe Rust in Spring Wheat Germplasm PI 183527. Theor. Appl. Genet. 2012, 125, 847–857. [Google Scholar] [CrossRef]
- Uauy, C.; Brevis, J.C.; Chen, X.; Khan, I.; Jackson, L.; Chicaiza, O.; Distelfeld, A.; Fahima, T.; Dubcovsky, J. High-Temperature Adult-Plant (HTAP) Stripe Rust Resistance Gene Yr36 from Triticum turgidum spp. Dicoccoides Is Closely Linked to the Grain Protein Content Locus Gpc-B1. Theor. Appl. Genet. 2005, 112, 97–105. [Google Scholar] [CrossRef]
- Fu, D.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.; Sela, H.; Fahima, T.; Dubcovsky, J. A Kinase-START Gene Confers Temperature-Dependent Resistance to Wheat Stripe Rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef]
- Martens, J.W.; McKenzie, R.I.H.; Green, G.J. Thermal Stability of Stem Rust Resistance in Oat Seedlings. Can. J. Bot. 1967, 45, 451–458. [Google Scholar] [CrossRef]
- Künstler, A.; Füzék, K.; Schwarczinger, I.; Nagy, J.K.; Bakonyi, J.; Fodor, J.; Hafez, Y.M.; Király, L. Heat Shock-Induced Enhanced Susceptibility of Barley to Bipolaris sorokiniana Is Associated with Elevated ROS Production and Plant Defence-Related Gene Expression. Plant Biol. 2023, 25, 803–812. [Google Scholar] [CrossRef]
- Schwarczinger, I.; Nagy, J.K.; Király, L.; Mészáros, K.; Bányai, J.; Kunos, V.; Fodor, J.; Künstler, A. Heat Stress Pre-Exposure May Differentially Modulate Plant Defense to Powdery Mildew in a Resistant and Susceptible Barley Genotype. Genes 2021, 12, 776. [Google Scholar] [CrossRef]
- Barna, B.; Harrach, B.D.; Viczián, O.; Fodor, J. Heat Induced Susceptibility of Barley Lines with Various Types of Resistance Genes to Powdery Mildew. Acta Phytopathol. Entomol. Hung. 2014, 49, 177–188. [Google Scholar] [CrossRef]
- Kolozsváriné Nagy, J.; Schwarczinger, I.; Király, L.; Bacsó, R.; Ádám, A.L.; Künstler, A. Near-Isogenic Barley Lines Show Enhanced Susceptibility to Powdery Mildew Infection Following High-Temperature Stress. Plants 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Fodor, J.; Nagy, J.K.; Király, L.; Mészáros, K.; Bányai, J.; Cséplő, M.K.; Schwarczinger, I.; Künstler, A. Heat Treatments at Varying Ambient Temperatures and Durations Differentially Affect Plant Defense to Blumeria Hordei in a Resistant and a Susceptible Hordeum Vulgare Line. Phytopathology 2024, 114, 418–426. [Google Scholar] [CrossRef]
- Yang, S.; Hua, J. A Haplotype-Specific Resistance Gene Regulated by BONZAI1 Mediates Temperature-Dependent Growth Control in Arabidopsis. Plant Cell 2004, 16, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Gijzen, M.; MacGregor, T.; Bhattacharyya, M.; Buzzell, R. Temperature Induced Susceptibility to Phytophthora sojaein Soybean Isolines Carrying Different Rps Genes. Physiol. Mol. Plant Pathol. 1996, 48, 209–215. [Google Scholar] [CrossRef]
- Lu, J.; Guo, M.; Zhai, Y.; Gong, Z.; Lu, M. Differential Responses to the Combined Stress of Heat and Phytophthora capsici Infection Between Resistant and Susceptible Germplasms of Pepper (Capsicum annuum L.). J. Plant Growth Regul. 2017, 36, 161–173. [Google Scholar] [CrossRef]
- Elad, Y.; Omer, C.; Nisan, Z.; Harari, D.; Goren, H.; Adler, U.; Silverman, D.; Biton, S. Passive Heat Treatment of Sweet Basil Crops Suppresses Peronospora Belbahrii Downy Mildew. Ann. Appl. Biol. 2016, 168, 373–389. [Google Scholar] [CrossRef]
- Whitham, S.; Dinesh-Kumar, S.P.; Choi, D.; Hehl, R.; Corr, C.; Baker, B. The Product of the Tobacco Mosaic Virus Resistance Gene N: Similarity to Toll and the Interleukin-1 Receptor. Cell 1994, 78, 1101–1115. [Google Scholar] [CrossRef]
- Valkonen, J.P.T. Novel Resistances to Four Potyviruses in Tuber-Bearing Potato Species, and Temperature-Sensitive Expression of Hypersensitive Resistance to Potato Virus Y. Ann. Appl. Biol. 1997, 130, 91–104. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection With Potato Virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef] [PubMed]
- Hayano-Saito, Y.; Hayashi, K. Stvb-i, a Rice Gene Conferring Durable Resistance to Rice Stripe Virus, Protects Plant Growth From Heat Stress. Front. Plant Sci. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, A.; Adi, M.; Lilia, F.; Linoy, A.; Or, R.; Mikhail, K.; Mouhammad, Z.; Henryk, C.; Rena, G. Tomato Yellow Leaf Curl Virus Infection Mitigates the Heat Stress Response of Plants Grown at High Temperatures. Sci. Rep. 2016, 6, 19715. [Google Scholar] [CrossRef]
- Tsai, W.A.; Shafiei-Peters, J.R.; Mitter, N.; Dietzgen, R.G. Effects of Elevated Temperature on the Susceptibility of Capsicum Plants to Capsicum Chlorosis Virus Infection. Pathogens 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Seifers, D.L.; Qi, L.L.; Friebe, B.; Gill, B.S. A Compensating Wheat-Thinopyrum intermedium Robertsonian Translocation Conferring Resistance to Wheat Streak Mosaic Virus and Triticum Mosaic Virus. Crop Sci. 2011, 51, 2382–2390. [Google Scholar] [CrossRef]
- Farahbakhsh, F.; Massah, A.; Hamzehzarghani, H.; Yassaie, M.; Amjadi, Z.; El-Zaeddi, H.; Carbonell-Barrachina, A.A. Comparative Profiling of Volatile Organic Compounds Associated to Temperature Sensitive Resistance to Wheat Streak Mosaic Virus (WSMV) in Resistant and Susceptible Wheat Cultivars at Normal and Elevated Temperatures. J. Plant Physiol. 2023, 281, 153903. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhsh, F.; Hamzehzarghani, H.; Massah, A.; Tortosa, M.; Yasayee, M.; Rodriguez, V.M. Comparative Metabolomics of Temperature Sensitive Resistance to Wheat Streak Mosaic Virus (WSMV) in Resistant and Susceptible Wheat Cultivars. J. Plant Physiol. 2019, 237, 30–42. [Google Scholar] [CrossRef]
- Sawada, H.; Takeuchi, S.; Hamada, H.; Kiba, A.; Matsumoto, M.; Hikichi, Y. A New Tobamovirus-Resistance Gene, L1a, of Sweet Pepper (Capsicum annuum L.). Engei Gakkai Zasshi 2004, 73, 552–557. [Google Scholar] [CrossRef]
- Meziadi, C.; Lintz, J.; Naderpour, M.; Gautier, C.; Blanchet, S.; Noly, A.; Gratias-Weill, A.; Geffroy, V.; Pflieger, S. R-BPMV-Mediated Resistance to Bean Pod Mottle Virus in Phaseolus vulgaris L. Is Heat-Stable but Elevated Temperatures Boost Viral Infection in Susceptible Genotypes. Viruses 2021, 13, 1239. [Google Scholar] [CrossRef]
- Jablonska, B.; Ammiraju, J.S.S.; Bhattarai, K.K.; Mantelin, S.; De Ilarduya, O.M.; Roberts, P.A.; Kaloshian, I. The Mi-9 Gene from Solanum arcanum Conferring Heat-Stable Resistance to Root-Knot Nematodes Is a Homolog of Mi-1. Plant Physiol. 2007, 143, 1044–1054. [Google Scholar] [CrossRef]
- Marques de Carvalho, L.; Benda, N.D.; Vaughan, M.M.; Cabrera, A.R.; Hung, K.; Cox, T.; Abdo, Z.; Allen, L.H.; Teal, P.E.A. Mi-1-Mediated Nematode Resistance in Tomatoes Is Broken by Short-Term Heat Stress but Recovers Over Time. J. Nematol. 2015, 47, 133–140. [Google Scholar] [PubMed]
- Veremis, J.C.; Roberts, P.A. Relationships between Meloidogyne incognita Resistance Genes in Lycopersicon peruvianum Differentiated by Heat Sensitivity and Nematode Virulence. Theor. Appl. Genet. 1996, 93, 950–959. [Google Scholar] [CrossRef]
- Hwang, C.-F.; Bhakta, A.V.; Truesdell, G.M.; Pudlo, W.M.; Williamson, V.M. Evidence for a Role of the N Terminus and Leucine-Rich Repeat Region of the Mi Gene Product in Regulation of Localized Cell Death. Plant Cell 2000, 12, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Djian-Caporalino, C.; Pijarowski, L.; Januel, A.; Lefebvre, V.; Daubèze, A.; Palloix, A.; Dalmasso, A.; Abad, P. Spectrum of Resistance to Root-Knot Nematodes and Inheritance of Heat-Stable Resistance in in Pepper (Capsicum annuum L.). Theor. Appl. Genet. 1999, 99, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Lin, P.A.; Hoover, K.; Felton, G.W.; Rajotte, E.G. Asymmetric Responses to Climate Change: Temperature Differentially Alters Herbivore Salivary Elicitor and Host Plant Responses to Herbivory. J. Chem. Ecol. 2020, 46, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Havko, N.E.; Das, M.R.; McClain, A.M.; Kapali, G.; Sharkey, T.D.; Howe, G.A. Insect Herbivory Antagonizes Leaf Cooling Responses to Elevated Temperature in Tomato. Proc. Natl. Acad. Sci. USA 2020, 117, 2211–2217. [Google Scholar] [CrossRef]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.; Digilio, M.C. Temperature Differentially Influences the Capacity of Trichoderma Species to Induce Plant Defense Responses in Tomato Against Insect Pests. Front. Plant Sci. 2021, 12, 678830. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.Y.; Liu, B.; Kaurilind, E.; Niinemets, Ü. Phloem-Feeding Insect Infestation Antagonizes Volatile Organic Compound Emissions and Enhances Heat Stress Recovery of Photosynthesis in Origanum vulgare. Environ. Exp. Bot. 2021, 189, 104551. [Google Scholar] [CrossRef]
- Guyer, A.; van Doan, C.; Maurer, C.; Machado, R.A.R.; Mateo, P.; Steinauer, K.; Kesner, L.; Hoch, G.; Kahmen, A.; Erb, M.; et al. Climate Change Modulates Multitrophic Interactions Between Maize, A Root Herbivore, and Its Enemies. J. Chem. Ecol. 2021, 47, 889–906. [Google Scholar] [CrossRef]
- Beetge, L.; Krüger, K. Drought and Heat Waves Associated with Climate Change Affect Performance of the Potato Aphid Macrosiphum euphorbiae. Sci. Rep. 2019, 9, 3645. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Wang, Q.; Liu, D.; Tian, Z.; Liu, J. Effects of Heat Wave on Development, Reproduction, and Morph Differentiation of Aphis glycines (Hemiptera: Aphididae). Environ. Entomol. 2023, 52, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ngoc Ha, V.; Shen, Z.; Dang, P.; Zhu, H.; Zhao, F.; Zhao, Z. Response of the Rhizosphere Microbial Community to Fine Root and Soil Parameters Following Robinia pseudoacacia L. Afforestation. Appl. Soil. Ecol. 2018, 132, 11–19. [Google Scholar] [CrossRef]
- Wang, R.; Bai, B.; Li, D.; Wang, J.; Huang, W.; Wu, Y.; Zhao, L. Phytoplasma: A Plant Pathogen That Cannot Be Ignored in Agricultural Production-Research Progress and Outlook. Mol. Plant Pathol. 2024, 25, e13437. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Nagendran, K.; Rai, A.B.; Singh, B.; Rao, G.P.; Bertaccini, A. Global Status of Phytoplasma Diseases in Vegetable Crops. Front. Microbiol. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Reddy, M.G.; Baranwal, V.K.; Sagar, D.; Rao, G.P. Molecular Characterization of Chickpea Chlorotic Dwarf Virus and Peanut Witches’ Broom Phytoplasma Associated with Chickpea Stunt Disease and Identification of New Host Crops and Leafhopper Vectors in India. 3 Biotech 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.A.; Farrag, A.A.; Kheder, A.A.; Shaaban, A. Effect of Phytoplasma Associated with Sesame Phyllody on Ultrastructural Modification, Physio-Biochemical Traits, Productivity and Oil Quality. Plants 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Pecher, P.; Moro, G.; Canale, M.C.; Capdevielle, S.; Singh, A.; MacLean, A.; Sugio, A.; Kuo, C.-H.; Lopes, J.R.S.; Hogenhout, S.A. Phytoplasma SAP11 Effector Destabilization of TCP Transcription Factors Differentially Impact Development and Defence of Arabidopsis versus Maize. PLoS Pathog. 2019, 15, e1008035. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.J.; Kazemi, N.; Marzban, A. Life Cycle Environmental Impacts of Cucumber and Tomato Production in Open-Field and Greenhouse. J. Saudi Soc. Agric. Sci. 2019, 18, 249–255. [Google Scholar] [CrossRef]
- Bianco, P.A.; Romanazzi, G.; Mori, N.; Myrie, W.; Bertaccini, A. Integrated Management of Phytoplasma Diseases. In Phytoplasmas: Plant Pathogenic Bacteria—II; Springer: Singapore, 2019; pp. 237–258. [Google Scholar]
- Saracco, P.; Bosco, D.; Veratti, F.; Marzachì, C. Quantification over Time of Chrysanthemum Yellows Phytoplasma (16Sr-I) in Leaves and Roots of the Host Plant Chrysanthemum carinatum (Schousboe) Following Inoculation with Its Insect Vector. Physiol. Mol. Plant Pathol. 2005, 67, 212–219. [Google Scholar] [CrossRef]
- Brochu, A.-S.; Dionne, A.; Fall, M.L.; Pérez-López, E. A Decade of Hidden Phytoplasmas Unveiled Through Citizen Science. Plant Dis. 2023, 107, 3389–3393. [Google Scholar] [CrossRef] [PubMed]
- Sabato, E.O.; Landau, E.C.; Barros, B.A.; Oliveira, C.M. Differential Transmission of Phytoplasma and Spiroplasma to Maize Caused by Variation in the Environmental Temperature in Brazil. Eur. J. Plant Pathol. 2020, 157, 163–171. [Google Scholar] [CrossRef]
- Bahar, M.H.; Wist, T.J.; Bekkaoui, D.R.; Hegedus, D.D.; Olivier, C.Y. Aster Leafhopper Survival and Reproduction, and Aster Yellows Transmission under Static and Fluctuating Temperatures, Using DdPCR for Phytoplasma Quantification. Sci. Rep. 2018, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Plante, N.; Durivage, J.; Brochu, A.-S.; Dumonceaux, T.; Almeida Santos, A.; Torres, D.; Bahder, B.; Kits, J.; Dionne, A.; Légaré, J.-P.; et al. Leafhoppers as Markers of the Impact of Climate Change on Agriculture. Cell Rep. Sustain. 2024, 1, 100029. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Chofong, G.N.; Minarovits, J.; Richert-Pöggeler, K.R. Chapter 4—Virus Latency: Heterogeneity of Host-Virus Interaction in Shaping the Virosphere. In Plant Virus-Host Interaction, 2nd ed.; Gaur, R.K., Khurana, S.M.P., Sharma, P., Hohn, T., Eds.; Academic Press: Boston, MA, USA, 2021; pp. 111–137. ISBN 978-0-12-821629-3. [Google Scholar]
- Sett, S.; Prasad, A.; Prasad, M. Resistance Genes on the Verge of Plant–Virus Interaction. Trends Plant Sci. 2022, 27, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Jeger, M.; Bragard, C. The Epidemiology of Xylella fastidiosa; A Perspective on Current Knowledge and Framework to Investigate Plant Host–Vector–Pathogen Interactions. Phytopathology 2019, 109, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, H.; Carhuapoma, P.; Cumapa, L.; González, G.; Muñoz, J.; Sporleder, M.; Kreuze, J. A Temperature-Driven Model for Potato Yellow Vein Virus Transmission Efficacy by Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Virus Res. 2020, 289, 198109. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Sett, S.; Prasad, M. Plant-Virus-Abiotic Stress Interactions: A Complex Interplay. Environ. Exp. Bot. 2022, 199, 104869. [Google Scholar] [CrossRef]
- Islam, W.; Naveed, H.; Zaynab, M.; Huang, Z.; Chen, H.Y.H. Plant Defense against Virus Diseases; Growth Hormones in Highlights. Plant Signal. Behav. 2019, 14, 1596719. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N. Roles of Plant Hormones in the Regulation of Host–Virus Interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, B.; Jiang, L.; Zhang, M.; Lu, Y.; Wang, S.; Yan, F.; Xin, X. Triticum Aestivum Heat Shock Protein 23.6 Interacts with the Coat Protein of Wheat Yellow Mosaic Virus. Virus Genes. 2019, 55, 209–217. [Google Scholar] [CrossRef]
- Kim, M.Y.; Oglesbee, M. Virus-Heat Shock Protein Interaction and a Novel Axis for Innate Antiviral Immunity. Cells 2012, 1, 646–666. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Wang, D.; Chen, Z. Mode of Action of Heat Shock Protein (HSP) Inhibitors against Viruses through Host HSP and Virus Interactions. Genes 2023, 14, 792. [Google Scholar] [CrossRef]
- Du, Z.; Xiao, D.; Wu, J.; Jia, D.; Yuan, Z.; Liu, Y.; Hu, L.; Han, Z.; Wei, T.; Lin, Q.; et al. P2 of Rice Stripe Virus (RSV) Interacts with OsSGS3 and Is a Silencing Suppressor. Mol. Plant Pathol. 2011, 12, 808–814. [Google Scholar] [CrossRef]
- Tian, B.; Li, J.; Vodkin, L.O.; Todd, T.C.; Finer, J.J.; Trick, H.N. Host-Derived Gene Silencing of Parasite Fitness Genes Improves Resistance to Soybean Cyst Nematodes in Stable Transgenic Soybean. Theor. Appl. Genet. 2019, 132, 2651–2662. [Google Scholar] [CrossRef]
- Mitchum, M.G. Soybean Resistance to the Soybean Cyst Nematode Heterodera Glycines: An Update. Phytopathology 2016, 106, 1444–1450. [Google Scholar] [CrossRef]
- Guang, Y.; Luo, S.; Ahammed, G.J.; Xiao, X.; Li, J.; Zhou, Y.; Yang, Y. The OPR Gene Family in Watermelon: Genome-Wide Identification and Expression Profiling under Hormone Treatments and Root-Knot Nematode Infection. Plant Biol. 2021, 23, 80–88. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Liu, Y.; Morelli, M.; Koskimäki, J.J.; Qin, S.; Zhu, Y.H.; Zhang, X.X. Editorial: Role of Endophytic Bacteria in Improving Plant Stress Resistance. Front. Plant Sci. 2022, 13, 1106701. [Google Scholar] [CrossRef]
- Li, Z.; Howell, S.H. Heat Stress Responses and Thermotolerance in Maize. Int. J. Mol. Sci. 2021, 22, 948. [Google Scholar] [CrossRef]
- Thurau, T.; Kifle, S.; Jung, C.; Cai, D. The Promoter of the Nematode Resistance Gene Hs1 Pro-1 Activates a Nematode-Responsive and Feeding Site-Specific Gene Expression in Sugar Beet (Beta vulgaris L.) and Arabidopsis thaliana. Plant Mol. Biol. 2003, 52, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.A.; Wu, D.Q.; Huang, W.K.; Peng, H.; Wang, G.F.; Cui, J.K.; Liu, S.M.; Li, Z.G.; Yang, J.; Peng, D.L. Large-Scale Identification of Wheat Genes Resistant to Cereal Cyst Nematode Heterodera avenae Using Comparative Transcriptomic Analysis. BMC Genom. 2015, 16, 801. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cui, L.; Chen, Y.; Zhang, H.; Liu, P.; Wu, P.; Qiu, D.; Zou, J.; Yang, D.; Yang, L.; et al. Transcriptional Responses of Wheat and the Cereal Cyst Nematode Heterodera avenae during Their Early Contact Stage. Sci. Rep. 2017, 7, 14471. [Google Scholar] [CrossRef]
- Bodlah, M.A.; Iqbal, J.; Ashiq, A.; Bodlah, I.; Jiang, S.; Mudassir, M.A.; Rasheed, M.T.; Fareen, A.G.E. Insect Behavioral Restraint and Adaptation Strategies under Heat Stress: An Inclusive Review. J. Saudi Soc. Agric. Sci. 2023. [CrossRef]
- Neven, L.G. Physiological Responses of Insects to Heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Hou, L.; Wu, Y.; Kou, X.; Li, R.; Wang, S. Developing High-Temperature-Short-Time Radio Frequency Disinfestation Treatments in Coix Seeds: Insect Mortality, Product Quality and Energy Consumption. Biosyst. Eng. 2022, 215, 262–270. [Google Scholar] [CrossRef]
- Sakka, M.K.; Jagadeesan, R.; Nayak, M.K.; Athanassiou, C.G. Insecticidal Effect of Heat Treatment in Commercial Flour and Rice Mills for the Control of Phosphine-Resistant Insect Pests. J. Stored Prod. Res. 2022, 99, 102023. [Google Scholar] [CrossRef]
- Iltis, C.; Tougeron, K.; Hance, T.; Louâpre, P.; Foray, V. A Perspective on Insect–Microbe Holobionts Facing Thermal Fluctuations in a Climate-Change Context. Environ. Microbiol. 2022, 24, 18–29. [Google Scholar] [CrossRef]
- Tougeron, K.; Iltis, C. Impact of Heat Stress on the Fitness Outcomes of Symbiotic Infection in Aphids: A Meta-Analysis. Proc. R. Soc. B Biol. Sci. 2022, 289, 20212660. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Zhang, Y.; Zhong, J.; Ge, Y.; Abou El-Ela, A.S.; Mao, Z.; Ntiri, E.S.; Mao, L.; Zhu, Z.; Zhou, W. Heat Stress Affects Potato’s Volatile Emissions That Mediate Agronomically Important Trophic Interactions. Plant Cell Environ. 2022, 45, 3036–3051. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.A.; Michaud, D.; Cloutier, C. A Proteomic Analysis of the Aphid Macrosiphum euphorbiae under Heat and Radiation Stress. Insect Biochem. Mol. Biol. 2009, 39, 20–30. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, C.; Liu, R. Indirect Interactions between Arbuscular Mycorrhizal Fungi and Spodoptera Exigua Alter Photosynthesis and Plant Endogenous Hormones. Mycorrhiza 2017, 27, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.; Zavalnitskaya, J.; Isaacs, R.; Szendrei, Z. Heat of the Moment: Extreme Heat Poses a Risk to Bee–Plant Interactions and Crop Yields. Curr. Opin. Insect Sci. 2022, 52, 100927. [Google Scholar] [CrossRef] [PubMed]
- Kask, K.; Kännaste, A.; Talts, E.; Copolovici, L.; Niinemets, Ü. How Specialized Volatiles Respond to Chronic and Short-Term Physiological and Shock Heat Stress in Brassica nigra. Plant Cell Environ. 2016, 39, 2027–2042. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Guo, P.; Zhang, Y.; Tan, X.; Chen, J.; Li, Y.; Wu, Y. Effect of High Temperature and Natural Enemies on the Interspecies Competition Between Two Wheat Aphid Species, Rhopalosiphum Padi and Sitobion miscanthi. J. Econ. Entomol. 2022, 115, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lwalaba, D.; Hoffmann, K.H.; Woodring, J. Control of the release of digestive enzymes in the larvae of the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2010, 73, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, T.J.; Donze-Reiner, T.; Marchi-Werle, L.; Palmer, N.A.; Hunt, T.E.; Sarath, G.; Heng-Moss, T. Transcriptional Responses of Tolerant and Susceptible Soybeans to Soybean Aphid (Aphis glycines Matsumura) Herbivory. Arthropod Plant Interact. 2015, 9, 347–359. [Google Scholar] [CrossRef]
- Liu, F.H.; Kang, Z.; Tan, X.; Fan, Y.; Tian, H.; Liu, T. Physiology and Defense Responses of Wheat to the Infestation of Different Cereal Aphids. J. Integr. Agric. 2020, 19, 1464–1474. [Google Scholar] [CrossRef]
- Lee, S.; Cassone, B.J.; Wijeratne, A.; Jun, T.H.; Michel, A.P.; Mian, M.A.R. Transcriptomic Dynamics in Soybean Near-Isogenic Lines Differing in Alleles for an Aphid Resistance Gene, Following Infestation by Soybean Aphid Biotype 2. BMC Genom. 2017, 18, 472. [Google Scholar] [CrossRef] [PubMed]
- Gyan, N.M.; Yaakov, B.; Weinblum, N.; Singh, A.; Cna’ani, A.; Ben-Zeev, S.; Saranga, Y.; Tzin, V. Variation Between Three Eragrostis Tef Accessions in Defense Responses to Rhopalosiphum padi Aphid Infestation. Front. Plant Sci. 2020, 11, 598483. [Google Scholar] [CrossRef]
- Jasrotia, P.; Sharma, S.; Nagpal, M.; Kamboj, D.; Kashyap, P.L.; Kumar, S.; Mishra, C.N.; Kumar, S.; Singh, G.P. Comparative Transcriptome Analysis of Wheat in Response to Corn Leaf Aphid, Rhopalosiphum maidis F. Infestation. Front. Plant Sci. 2022, 13, 989365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX Makes Rice More Susceptible to Chewing Herbivores, but Enhances Resistance to a Phloem Feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Xue, R.; Li, Q.; Guo, R.; Yan, H.; Ju, X.; Liao, L.; Zeng, R.; Song, Y.; Wang, J. Rice Defense Responses Orchestrated by Oral Bacteria of the Rice Striped Stem Borer, Chilo suppressalis. Rice 2023, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.; CaraDonna, P.J.; Rakosy, D.; Fründ, J.; Pascual Tudanca, M.P.; Dormann, C.F.; Burkle, L.A.; Kaiser-Bunbury, C.N.; Knight, T.M.; Resasco, J.; et al. Predicting Plant–Pollinator Interactions: Concepts, Methods, and Challenges. Trends Ecol. Evol. 2024, 39, 494–505. [Google Scholar] [CrossRef]
- Bishop, J.; Jones, H.E.; Lukac, M.; Potts, S.G. Insect Pollination Reduces Yield Loss Following Heat Stress in Faba Bean (Vicia faba L.). Agric. Ecosyst. Environ. 2016, 220, 89–96. [Google Scholar] [CrossRef]
- Hemberger, J.A.; Rosenberger, N.M.; Williams, N.M. Experimental Heatwaves Disrupt Bumblebee Foraging through Direct Heat Effects and Reduced Nectar Production. Funct. Ecol. 2023, 37, 591–601. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- O’Connell, D.P.; Baker, B.M.; Atauri, D.; Jones, J.C. Increasing Temperature and Time in Glasshouses Increases Honey Bee Activity and Affects Internal Brood Conditions. J. Insect Physiol. 2024, 155, 104635. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Vangnai, A.S.; Kamaraj, B.; Cornejo, P. Plant Growth-Promoting Actinobacterial Inoculant Assisted Phytoremediation Increases Cadmium Uptake in Sorghum bicolor under Drought and Heat Stresses. Environ. Pollut. 2022, 307, 119489. [Google Scholar] [CrossRef] [PubMed]
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable Crop and Weed Management in the Era of the EU Green Deal: A Survival Guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Dubey, A.; Kumar, K.; Srinivasan, T.; Kondreddy, A.; Kumar, K.R.R. An Invasive Weed-Associated Bacteria Confers Enhanced Heat Stress Tolerance in Wheat. Heliyon 2022, 8, e09893. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Chu, S.; You, Y.; Chi, Y.; Wang, R.; Yang, X.; Hayat, K.; Zhang, D.; Zhou, P. Comparative Cytology Combined with Transcriptomic and Metabolomic Analyses of Solanum nigrum L. in Response to Cd Toxicity. J. Hazard. Mater. 2022, 423, 127168. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive Effects of Drought and Heat Stresses on Morpho-Physiological Attributes, Yield, Nutrient Uptake and Oxidative Status in Maize Hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ayyub, C.M.; Hussain, Z.; Hussain, R.; Rashid, S. Optimization of Chitosan Level to Alleviate the Drastic Effects of Heat Stress in Cucumber (Cucumis sativus L.). J. Pure Appl. Agric. 2020, 5, 30–38. [Google Scholar]
- Matloob, A.; Khaliq, A.; Chauhan, B.S. Chapter Five—Weeds of Direct-Seeded Rice in Asia: Problems and Opportunities. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 291–336. ISBN 0065-2113. [Google Scholar]
- Touzy, G.; Lafarge, S.; Redondo, E.; Lievin, V.; Decoopman, X.; Le Gouis, J.; Praud, S. Identification of QTLs Affecting Post-Anthesis Heat Stress Responses in European Bread Wheat. Theor. Appl. Genet. 2022, 135, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, H. Ecological Management of the Nitrogen Cycle in Organic Farms. Nitrogen 2023, 4, 58–84. [Google Scholar] [CrossRef]
- Wen, B. Effects of High Temperature and Water Stress on Seed Germination of the Invasive Species Mexican Sunflower. PLoS ONE 2015, 10, e0141567. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Abugho, S.B. Effect of Water Stress on the Growth and Development of Amaranthus spinosus, Leptochloa Chinensis, and Rice. Am. J. Plant Sci. 2013, 4, 989–998. [Google Scholar] [CrossRef]
- Admassie, M.; Woldehawariat, Y.; Alemu, T.; Gonzalez, E.; Jimenez, J.F. The Role of Plant Growth-Promoting Bacteria in Alleviating Drought Stress on Pepper Plants. Agric. Water Manag. 2022, 272, 107831. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F. Induction of Abiotic Stress Tolerance in Plants by Endophytic Microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Afkhami, M.E.; Bell-Dereske, L.; Chung, Y.A.; Crawford, K.M.; Kivlin, S.N.; Mann, M.A.; Nuñez, M.A. Climate Disruption of Plant-Microbe Interactions. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 561–586. [Google Scholar] [CrossRef]
- Teiba, I.I.; El-Bilawy, E.H.; Elsheery, N.I.; Rastogi, A. Microbial Allies in Agriculture: Harnessing Plant Growth-Promoting Microorganisms as Guardians against Biotic and Abiotic Stresses. Horticulturae 2024, 10, 12. [Google Scholar] [CrossRef]
- Singh, B.K.; Trivedi, P.; Egidi, E.; Macdonald, C.A.; Delgado-Baquerizo, M. Crop Microbiome and Sustainable Agriculture. Nat. Rev. Microbiol. 2020, 18, 601–602. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S. Bin From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Shahzad, R.; Ullah, I.; Khan, A.R.; Lee, I.J. Mutualistic Fungal Endophytes Produce Phytohormones and Organic Acids That Promote Japonica Rice Plant Growth under Prolonged Heat Stress. J. Zhejiang Univ. Sci. B 2015, 16, 1011–1018. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Bacillus Velezensis 5113 Induced Metabolic and Molecular Reprogramming during Abiotic Stress Tolerance in Wheat. Sci. Rep. 2019, 9, 16282. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. Mitigation of Salt Stress in Wheat Plant (Triticum aestivum) by ACC Deaminase Bacterium Enterobacter sp. SBP-6 Isolated from Sorghum bicolor. Acta Physiol. Plant 2016, 38, 110. [Google Scholar] [CrossRef]
- Zulfikar Ali, S.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of Inoculation with a Thermotolerant Plant Growth Promoting Pseudomonas putida Strain AKMP7 on Growth of Wheat (Triticum spp.) under Heat Stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef]
- Ashraf, A.; Bano, A.; Ali, S.A. Characterisation of Plant Growth-Promoting Rhizobacteria from Rhizosphere Soil of Heat-Stressed and Unstressed Wheat and Their Use as Bio-Inoculant. Plant Biol. 2019, 21, 762–769. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Fridborg, I.; Meijer, J. Identifying Potential Molecular Factors Involved in Bacillus amyloliquefaciens 5113 Mediated Abiotic Stress Tolerance in Wheat. Plant Biol. 2018, 20, 271–279. [Google Scholar] [CrossRef]
- Khan, A.N.; Hassan, M.N.; Keyani, R.; Amir, Z.; Raish, M.; Singh, R.; Yasmin, H. Potential of Lactobacillus agilis, Lactobacillus plantarum, and Lactobacillus acidophilus to Enhance Wheat Growth under Drought and Heat Stress. J. King Saud Univ. Sci. 2024, 103334, online ahead of print. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Mishra, S.; Kumar, S.; Saxena, A.K.; Suman, A. Appraisal of Diversity and Functional Attributes of Thermotolerant Wheat Associated Bacteria from the Peninsular Zone of India. Saudi J. Biol. Sci. 2019, 26, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; ur Rehman, S.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-Deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Issa, A.; Esmaeel, Q.; Sanchez, L.; Courteaux, B.; Guise, J.-F.; Gibon, Y.; Ballias, P.; Clément, C.; Jacquard, C.; Vaillant-Gaveau, N.; et al. Impacts of Paraburkholderia phytofirmans Strain PsJN on Tomato (Lycopersicon esculentum L.) Under High Temperature. Front. Plant Sci. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Mukhtar, T.; Ali, F.; Rafique, M.; Ali, J.; Afridi, M.S.; Smith, D.; Mehmood, S.; Amna; Souleimanov, A.; Jellani, G.; et al. Biochemical Characterization and Potential of Bacillus safensis Strain SCAL1 to Mitigate Heat Stress in Solanum lycopersicum L. J. Plant Growth Regul. 2023, 42, 523–538. [Google Scholar] [CrossRef]
- Duarte, B.; Carreiras, J.A.; Cruz-Silva, A.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Redondo-Gómez, S.; Mesa-Marín, J.; Figueiredo, A. Marine Plant Growth Promoting Bacteria (PGPB) Inoculation Technology: Testing the Effectiveness of Different Application Methods to Improve Tomato Plants Tolerance against Acute Heat Wave Stress. Plant Stress. 2024, 11, 100434. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Extending Thermotolerance to Tomato Seedlings by Inoculation with SA1 Isolate of Bacillus cereus and Comparison with Exogenous Humic Acid Application. PLoS ONE 2020, 15, e0232228. [Google Scholar] [CrossRef]
- Bensalim, S.; Nowak, J.; Asiedu, S.I. A Plant Growth Promoting Rhizobacterium and Temperature Effects on Performance of 18 Clones of Potato. Am. J. Potato Res. 1998, 75, 145–152. [Google Scholar] [CrossRef]
- Ali, S.; Khan, N. Delineation of Mechanistic Approaches Employed by Plant Growth Promoting Microorganisms for Improving Drought Stress Tolerance in Plants. Microbiol. Res. 2021, 249, 126771. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.M.; Hasnain, A. Functional and Morphological Characterization of Low-Substituted Acetylated White Sorghum (Sorghum bicolor) Starch. Int. J. Polym. Anal. Charact. 2011, 16, 187–198. [Google Scholar] [CrossRef]
- Bruno, L.B.; Karthik, C.; Ma, Y.; Kadirvelu, K.; Freitas, H.; Rajkumar, M. Amelioration of Chromium and Heat Stresses in Sorghum Bicolor by Cr6+ Reducing-Thermotolerant Plant Growth Promoting Bacteria. Chemosphere 2020, 244, 125521. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. Strain AKM-P6 Enhances Tolerance of Sorghum Seedlings to Elevated Temperatures. Biol. Fertil. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Shin, D.J.; Yoo, S.J.; Hong, J.K.; Weon, H.Y.; Song, J.; Sang, M.K. Effect of Bacillus aryabhattai H26-2 and b. Siamensis H30-3 on Growth Promotion and Alleviation of Heat and Drought Stresses in Chinese Cabbage. Plant Pathol J (Faisalabad) 2019, 35, 178–187. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.-Y.; Gu, H.; Du, Y.; Zuo, J.-F.; Zhang, Z.; Zhang, M.; Li, P.; Dunwell, J.M.; Cao, Y.; et al. Bradyrhizobium diazoefficiens USDA 110–Glycine max Interactome Provides Candidate Proteins Associated with Symbiosis. J. Proteome Res. 2018, 17, 3061–3074. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Vessey, J.K.; Smith, D.L. Response of Field-Grown Soybean to Co-Inoculation with the Plant Growth Promoting Rhizobacteria Serratia proteamaculans or Serratia liquefaciens, and Bradyrhizobium japonicum Pre-Incubated with Genistein. Eur. J. Agron. 2002, 17, 143–153. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance Effect of Plant Growth-Promoting Bacillus cereus SA1 on Soybean during Heat Stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Handa, N.; Arora, U.; Arora, N.; Kaur, P.; Kapoor, D.; Bhardwaj, R. Role of Metabolites in Abiotic Stress Tolerance in Legumes. In Abiotic Stress and Legumes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–276. [Google Scholar]
- Liu, Y.S.; Geng, J.C.; Sha, X.Y.; Zhao, Y.X.; Hu, T.M.; Yang, P.Z. Effect of Rhizobium Symbiosis on Low-Temperature Tolerance and Antioxidant Response in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2019, 10, 538. [Google Scholar] [CrossRef]
- Carreiras, J.; Cruz-Silva, A.; Fonseca, B.; Carvalho, R.C.; Cunha, J.P.; Proença Pereira, J.; Paiva-Silva, C.; Santos, S.A.; Janeiro Sequeira, R.; Mateos-Naranjo, E.; et al. Improving Grapevine Heat Stress Resilience with Marine Plant Growth-Promoting Rhizobacteria Consortia. Microorganisms 2023, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Tienda, S.; Vida, C.; Villar-Moreno, R.; de Vicente, A.; Cazorla, F.M. Development of a Pseudomonas-Based Biocontrol Consortium with Effective Root Colonization and Extended Beneficial Side Effects for Plants under High-Temperature Stress. Microbiol. Res. 2024, 285, 127761. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Yin, Y.-Q.; Zhu, X.-M.; Xia, X.; Han, J.-J. High Ambient Temperature Regulated the Plant Systemic Response to the Beneficial Endophytic Fungus Serendipita indica. Front. Plant Sci. 2022, 13, 844572. [Google Scholar] [CrossRef] [PubMed]
- Macabuhay, A.; Arsova, B.; Watt, M.; Nagel, K.A.; Lenz, H.; Putz, A.; Adels, S.; Müller-Linow, M.; Kelm, J.; Johnson, A.A.T.; et al. Plant Growth Promotion and Heat Stress Amelioration in Arabidopsis Inoculated with Paraburkholderia phytofirmans PsJN Rhizobacteria Quantified with the GrowScreen-Agar II Phenotyping Platform. Plants 2022, 11, 2927. [Google Scholar] [CrossRef] [PubMed]
- Delamare, J.; Brunel-Muguet, S.; Boukerb, A.M.; Bressan, M.; Dumas, L.; Firmin, S.; Leroy, F.; Morvan-Bertrand, A.; Prigent-Combaret, C.; Personeni, E. Impact of PGPR Inoculation on Root Morphological Traits and Root Exudation in Rapeseed and Camelina: Interactions with Heat Stress. Physiol. Plant 2023, 175, e14058. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Imtiaz, M.; Nawaz, M.S.; Mubeen, F.; Sarwar, Y.; Hayat, M.; Asif, M.; Naqvi, R.Z.; Ahmad, M.; Imran, A. Thermotolerant PGPR Consortium B3P Modulates Physio-Biochemical and Molecular Machinery for Enhanced Heat Tolerance in Maize during Early Vegetative Growth. Ann. Microbiol. 2023, 73, 34. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The Role of Plant Growth Promoting Rhizobacteria in Plant Drought Stress Responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Doornbos, R.F.; Zamioudis, C.; Berendsen, R.L.; Pieterse, C.M.J. Induced Systemic Resistance and the Rhizosphere Microbiome. Plant Pathol. J. 2013, 29, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, J.; Lu, X.; Zhou, C. Development of Plant Systemic Resistance by Beneficial Rhizobacteria: Recognition, Initiation, Elicitation and Regulation. Front. Plant Sci. 2022, 13, 952397. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced Systemic Resistance (ISR) in Plants: Mechanism of Action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Tyagi, A.; Bae, H. Plant Microbiome: An Ocean of Possibilities for Improving Disease Resistance in Plants. Microorganisms 2023, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S.; et al. New Opportunities in Plant Microbiome Engineering for Increasing Agricultural Sustainability under Stressful Conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef] [PubMed]
- Arif, I.; Batool, M.; Schenk, P.M. Plant Microbiome Engineering: Expected Benefits for Improved Crop Growth and Resilience. Trends Biotechnol. 2020, 38, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Kashyap, S.; Agarwala, N. Biotic Stress-Induced Changes in Root Exudation Confer Plant Stress Tolerance by Altering Rhizospheric Microbial Community. Front. Plant Sci. 2023, 14, 1132824. [Google Scholar] [CrossRef] [PubMed]
- Shinwari, Z.K.; Tanveer, F.; Iqrar, I. Role of Microbes in Plant Health, Disease Management. In Microbiome in Plant Health and Disease: Challenges and Opportunities; Springer: Singapore, 2019; pp. 231–250. ISBN 9789811384950. [Google Scholar]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.H.; Baki, M.Z.I.; Ahmed, B.; Mostofa, M.G. Current, Faltering, and Future Strategies for Advancing Microbiome-Assisted Sustainable Agriculture and Environmental Resilience. New Crops 2024, 1, 100013. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Schwarzenberg, A.; Nguema-Ona, E.; Clément, C.; Baillieul, F.; Aziz, A. Bacillus subtilis and Pseudomonas fluorescens Trigger Common and Distinct Systemic Immune Responses in Arabidopsis thaliana Depending on the Pathogen Lifestyle. Vaccines 2020, 8, 503. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; ClCrossed D Sign©ment, C.; Baillieul, F.; Aziz, A. Priming of Camalexin Accumulation in Induced Systemic Resistance by Beneficial Bacteria against Botrytis cinerea and Pseudomonas syringae Pv. Tomato DC3000. J. Exp. Bot. 2022, 73, 3743–3757. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.O.; Xia, Y. Bacillus proteolyticus OSUB18 Triggers Induced Systemic Resistance against Bacterial and Fungal Pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef] [PubMed]
- Mattos, R.; Galeano, S.; Souza, J.V.; Samanta, R.; Silva, M.; Lorena, A.; Simas, O.; Cavalieri De Alencar, N.; Douglas, G.; Masui, C.; et al. New Strains of Trichoderma with Potential for Biocontrol and Plant Growth Promotion Improve Early Soybean Growth and Development. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Oshiquiri, L.H.; Almeida, N.O.; Steindorf, A.S.; da Rocha, M.R.; Georg, R.C.; Ulhoa, C.J. Trichoderma harzianum Transcriptome in Response to the Nematode Pratylenchus Brachyurus. Biol. Control 2023, 183, 105245. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Almeida, N.O.; Côrtes, M.V.d.C.B.; Júnior, M.L.; da Rocha, M.R.; Ulhoa, C.J. Biological Control of Pratylenchus brachyurus with Isolates of Trichoderma spp. on Soybean. Biol. Cont. 2021, 152, 104425. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Phan, Q.K.; Do, A.D. Antagonistic Activities of Trichoderma spp. Isolates against Neoscytalidium dimidiatum Causing Brown Spot Disease on Dragon Fruit Hylocereus undatus. J. Appl. Biol. Biotechnol. 2024, 12, 265–272. [Google Scholar] [CrossRef]
- Nilmat, A.; Thepbandit, W.; Chuaboon, W.; Athinuwat, D. Pseudomonas fluorescens SP007S Formulations in Controlling Soft Rot Disease and Promoting Growth in Kale. Agronomy 2023, 13, 1856. [Google Scholar] [CrossRef]
- Ali, A.O.; Awla, H.K.; Rashid, T.S. Investigating the in Vivo Biocontrol and Growth-Promoting Efficacy of Bacillus sp. and Pseudomonas fluorescens against Olive Knot Disease. Microb. Pathog. 2024, 191, 106645. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Abdel-Aal, A.M.K.; Rawa, M.J.; Mousa, M.A.A.; Moustafa, Y.M.M.; Abo-Elyousr, K.A.M. Enhancing Chili Pepper (Capsicum annuum L.) Resistance and Yield against Powdery Mildew (Leveillula taurica) with Beneficial Bacteria. Egypt. J. Biol. Pest. Control 2023, 33, 114. [Google Scholar] [CrossRef]
- Yadav, S.S.; Arya, A.; Singh, V.; Singh, Y. Elicitation of Native Bio Protective Microbial Agents Associated Systemic Defense Responses and Plant Growth Promotion against Bacterial Stalk Rot Pathogen in Sorghum (Sorghum bicolor). Phytopathol. Res. 2023, 5, 47. [Google Scholar] [CrossRef]
- Shahni, Y.S.; Banik, S.; Pongener, N.; Neog, P.; Singh, A.P. Effects of Biocontrol Agents on Early Blight Disease of Potato in Field. J. Mycopathol. Res. 2023, 61, 2583–6315. [Google Scholar] [CrossRef]
- Lian, H.; Li, R.; Ma, G.; Zhao, Z.; Zhang, T.; Li, M. The Effect of Trichoderma harzianum Agents on Physiological-Biochemical Characteristics of Cucumber and the Control Effect against Fusarium Wilt. Sci. Rep. 2023, 13, 17606. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, C.; Kamil, D.; Kundu, A.; Singh, P.K.; Das, A.; Hussain, Z.; Gogoi, R.; Shashank, P.R.; Gangaraj, R.; Chaithra, M. Exploring the Potential Biocontrol Isolates of Trichoderma asperellum for Management of Collar Rot Disease in Tomato. Horticulturae 2023, 9, 1116. [Google Scholar] [CrossRef]
- Imran, M.; Abo-Elyousr, K.A.M.; Mousa, M.A.A.; Saad, M.M. Use of Trichoderma Culture Filtrates as a Sustainable Approach to Mitigate Early Blight Disease of Tomato and Their Influence on Plant Biomarkers and Antioxidants Production. Front. Plant Sci. 2023, 14, 1192818. [Google Scholar] [CrossRef] [PubMed]
- Dhayal, R.; Chandrawat, B.S.; Choudhary, K.; Gurjar, V.; Bishnoi, S.P.; Gurjar, H.; Singh, H. In Vitro Evaluation of Bio-Agents on Hatching and Mortality of Root-Knot Nematode, Meloidogyne javanica. Biol. Forum 2023, 15, 357–360. [Google Scholar]
- de Freitas, C.C.; Taylor, C.G. Biological Control of Hairy Root Disease Using Beneficial Pseudomonas Strains. Biol. Control 2023, 177, 105098. [Google Scholar] [CrossRef]
- Saif, I.; Sufyan, M.; Baboo, I.; Jabbar, M.; Shafiq, A.; Saif, R.N.; Liaqat, U.; Lackner, M. Efficacy of Beauveria bassiana and Metarhizium anisopliae against Wheat Aphid. Eurobiotech J. 2024, 8, 23–31. [Google Scholar] [CrossRef]
- Sarker, S.; Choi, H.W.; Lim, U.T. Evaluation of New Strain (AAD16) of Beauveria bassiana Recovered from Japanese Rhinoceros Beetle: Effects on Three Coleopteran Insects. PLoS ONE 2024, 19, e0296094. [Google Scholar] [CrossRef]
- Rani, D.S.; Krishnamma, K.; Rani, J.S. Entomopathogenic Fungi, Metarhizium Anisopliae as a Chemical Substitute for Termite Pest Management in Sugarcane. J. Environ. Biol. 2024, 45, 235–242. [Google Scholar] [CrossRef]
- Kammar ICAR-Krishi Vigyan Kendra, M.R.; Kammar, M.R.; Sulagitti, A.R. Performance of Biopesticides for Management of White Grub Holotrichia serrata in Sugarcane. Pharma Innov. J. 2022, 11, 2152–2154. [Google Scholar]
- Gull, S.; Ahmad, T.; Khanday, A.L.; Sureshan, P.M.; Rashid, G. Pathogenicity of the Entomopathogenic Fungi against Myllocerus Fotedari Ahmad, 1974 (Coleoptera: Curculionidae) under Laboratory Conditions in India. J. For. Sci. 2023, 69, 277–286. [Google Scholar] [CrossRef]
- Abo-Elwfa, M.M.; Omar, M.M.A.; El-Shamy, E.A.; Ibrahim, H.A.M. Biocontrol Potential of Some Bacterial and Fungal Isolates against the Terrestrial Snail, Monacha Obstructa, Evaluating Their Laboratory and Field Efficiency. Egypt. J. Biol. Pest. Control 2024, 34, 25. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Ijaz, U.; Shahid, M.; Azizullah; Li, D.; Manzoor, I.; Song, F. Plant–Microbiome Crosstalk: Dawning from Composition and Assembly of Microbial Community to Improvement of Disease Resilience in Plants. Int. J. Mol. Sci. 2021, 22, 6852. [Google Scholar] [CrossRef]
- Singh, D.P.; Gupta, V.K.; Prabha, R. Microbial Interventions in Agriculture and Environment: Volume 2: Rhizosphere, Microbiome and Agro-Ecology; Springer: Singapore, 2019; ISBN 9789811383830. [Google Scholar]
- Saleem, M.A.; Malik, W.; Qayyum, A.; Ul-Allah, S.; Ahmad, M.Q.; Afzal, H.; Amjid, M.W.; Ateeq, M.F.; Zia, Z.U. Impact of Heat Stress Responsive Factors on Growth and Physiology of Cotton (Gossypium hirsutum L.). Mol. Biol. Rep. 2021, 48, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mazahar, S.; Chapadgaonkar, S.S.; Giri, P.; Shourie, A. Phyto-Microbiome to Mitigate Abiotic Stress in Crop Plants. Front. Microbiol. 2023, 14, 1210890. [Google Scholar] [CrossRef] [PubMed]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Solomon, W.; Janda, T.; Molnár, Z. Unveiling the Significance of Rhizosphere: Implications for Plant Growth, Stress Response, and Sustainable Agriculture. Plant Physiol Biochem 2024, 206, 108290. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Yadav, A.N.; Santoyo, G.; Babalola, O.O. Understanding the Plant-Microbe Interactions in Environments Exposed to Abiotic Stresses: An Overview. Microbiol. Res. 2023, 271, 127368. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and Their Potential in Biotic Stress Management and Crop Production. Front. Microbiol. 2022, 13, 933017. [Google Scholar] [CrossRef]
- José Pereira Lima Teixeira, P.; Colaianni, N.R.; Fitzpatrick, C.R. Beyond Pathogens: Microbiota Interactions with the Plant Immune System. Curr. Opin. Microbiol. 2019, 49, 7–17. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.S.N.A.; Hamid, N.W.A.; Nadarajah, K. Effects of Abiotic Stress on Soil Microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant Growth Promoting Rhizobacterial Mitigation of Drought Stress in Crop Plants: Implications for Sustainable Agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Di Lelio, I.; Forni, G.; Magoga, G.; Brunetti, M.; Bruno, D.; Becchimanzi, A.; De Luca, M.G.; Sinno, M.; Barra, E.; Bonelli, M.; et al. A Soil Fungus Confers Plant Resistance against a Phytophagous Insect by Disrupting the Symbiotic Role of Its Gut Microbiota. Proc. Natl. Acad. Sci. USA 2023, 120, e2216922120. [Google Scholar] [CrossRef] [PubMed]
- Waghunde, R.R.; Shelake, R.M.; Sabalpara, A.N. Trichoderma: A Significant Fungus for Agriculture and Environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Huo, X.; Lai, X.; Zhu, W.; Hao, Y.; Zheng, Z.; Guo, K. Enhanced Resistance to Heat and Fungal Infection in Transgenic Trichoderma via Over-Expressing the HSP70 Gene. AMB Express 2024, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Tamosiune, I.; Baniulis, D.; Stanys, V. Role of Endophytic Bacteria in Stress Tolerance of Agricultural Plants: Diversity of Microorganisms and Molecular Mechanisms. In Probiotics in Agroecosystem; Springer: Singapore, 2017; pp. 1–29. [Google Scholar]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular Mechanisms Associated with Microbial Biostimulant-Mediated Growth Enhancement, Priming and Drought Stress Tolerance in Maize Plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Peláez-Vico, M.Á.; Sinha, R.; Pascual, L.S.; Mittler, R. The Impact of Multifactorial Stress Combination on Plants, Crops, and Ecosystems: How Should We Prepare for What Comes Next? Plant J. 2024, 117, 1800–1814. [Google Scholar] [CrossRef]
- Joshi, S.; Patil, S.; Shaikh, A.; Jamla, M.; Kumar, V. Modern Omics Toolbox for Producing Combined and Multifactorial Abiotic Stress Tolerant Plants. Plant Stress 2024, 11, 100301. [Google Scholar] [CrossRef]
- Murmu, S.; Sinha, D.; Chaurasia, H.; Sharma, S.; Das, R.; Jha, G.K.; Archak, S. A Review of Artificial Intelligence-Assisted Omics Techniques in Plant Defense: Current Trends and Future Directions. Front. Plant Sci. 2024, 15, 1292054. [Google Scholar] [CrossRef]
- Li, P.; Jiang, J.; Zhang, G.; Miao, S.; Lu, J.; Qian, Y.; Zhao, X.; Wang, W.; Qiu, X.; Zhang, F.; et al. Integrating GWAS and Transcriptomics to Identify Candidate Genes Conferring Heat Tolerance in Rice. Front. Plant Sci. 2023, 13, 1102938. [Google Scholar] [CrossRef]
- Camp, E.F.; Kahlke, T.; Signal, B.; Oakley, C.A.; Lutz, A.; Davy, S.K.; Suggett, D.J.; Leggat, W.P. Proteome Metabolome and Transcriptome Data for Three Symbiodiniaceae under Ambient and Heat Stress Conditions. Sci. Data 2022, 9, 153. [Google Scholar] [CrossRef]
- Naik, B.; Kumar, V.; Rizwanuddin, S.; Chauhan, M.; Choudhary, M.; Gupta, A.K.; Kumar, P.; Kumar, V.; Saris, P.E.J.; Rather, M.A.; et al. Genomics, Proteomics, and Metabolomics Approaches to Improve Abiotic Stress Tolerance in Tomato Plant. Int. J. Mol. Sci. 2023, 24, 3025. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Das, S.P.; Gupta, A.; Parihar, P.; Chandrasekhar, K.; Sarker, U.; Kumar, A.; Ramrao, D.P.; Sudhakar, C. Multi-Omics Pipeline and Omics-Integration Approach to Decipher Plant’s Abiotic Stress Tolerance Responses. Genes 2023, 14, 1281. [Google Scholar] [CrossRef]
- Raza, A.; Bashir, S.; Khare, T.; Karikari, B.; Copeland, R.G.R.; Jamla, M.; Abbas, S.; Charagh, S.; Nayak, S.N.; Djalovic, I.; et al. Temperature-smart Plants: A New Horizon with Omics-driven Plant Breeding. Physiol. Plant 2024, 176, e14188. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant Heat Stress: Concepts Directing Future Research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Esgario, J.G.M.; Krohling, R.A.; Ventura, J.A. Deep Learning for Classification and Severity Estimation of Coffee Leaf Biotic Stress. Comput. Electron. Agric. 2020, 169, 105162. [Google Scholar] [CrossRef]
- Singh, A.K.; Ganapathysubramanian, B.; Sarkar, S.; Singh, A. Deep Learning for Plant Stress Phenotyping: Trends and Future Perspectives. Trends Plant Sci. 2018, 23, 883–898. [Google Scholar] [CrossRef]
- Rico-Chávez, A.K.; Franco, J.A.; Fernandez-Jaramillo, A.A.; Contreras-Medina, L.M.; Guevara-González, R.G.; Hernandez-Escobedo, Q. Machine Learning for Plant Stress Modeling: A Perspective towards Hormesis Management. Plants 2022, 11, 970. [Google Scholar] [CrossRef]
- Webber, H.; Rezaei, E.E.; Ryo, M.; Ewert, F. Framework to Guide Modeling Single and Multiple Abiotic Stresses in Arable Crops. Agric. Ecosyst. Environ. 2022, 340, 108179. [Google Scholar] [CrossRef]
- Dangi, A.K.; Sharma, B.; Khangwal, I.; Shukla, P. Combinatorial Interactions of Biotic and Abiotic Stresses in Plants and Their Molecular Mechanisms: Systems Biology Approach. Mol. Biotechnol. 2018, 60, 636–650. [Google Scholar] [CrossRef]
- Mohan, N.; Jhandai, S.; Bhadu, S.; Sharma, L.; Kaur, T.; Saharan, V.; Pal, A. Acclimation Response and Management Strategies to Combat Heat Stress in Wheat for Sustainable Agriculture: A State-of-the-Art Review. Plant Sci. 2023, 336, 111834. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Jiang, D. Priming: A Promising Strategy for Crop Production in Response to Future Climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Schwachtje, J.; Whitcomb, S.J.; Firmino, A.A.P.; Zuther, E.; Hincha, D.K.; Kopka, J. Induced, Imprinted, and Primed Responses to Changing Environments: Does Metabolism Store and Process Information? Front. Plant Sci. 2019, 10, 106. [Google Scholar] [CrossRef]
- Charng, Y.; Mitra, S.; Yu, S.-J. Maintenance of Abiotic Stress Memory in Plants: Lessons Learned from Heat Acclimation. Plant Cell 2023, 35, 187–200. [Google Scholar] [CrossRef]
- Wang, X.; Xin, C.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Heat Priming Induces Trans-Generational Tolerance to High Temperature Stress in Wheat. Front. Plant Sci. 2016, 7, 501. [Google Scholar] [CrossRef]
- Pazzaglia, J.; Badalamenti, F.; Bernardeau-Esteller, J.; Ruiz, J.M.; Giacalone, V.M.; Procaccini, G.; Marín-Guirao, L. Thermo-Priming Increases Heat-Stress Tolerance in Seedlings of the Mediterranean Seagrass P. Oceanica. Mar. Pollut. Bull. 2022, 174, 113164. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, V.; Pandey, K.; Sopory, S.K.; Sanan-Mishra, N. Thermo-Priming Mediated Cellular Networks for Abiotic Stress Management in Plants. Front. Plant Sci. 2022, 13, 866409. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Leibman-Markus, M.; Marash, I.; Kovetz, N.; Rav-David, D.; Elad, Y.; Bar, M. Root Zone Warming Represses Foliar Diseases in Tomato by Inducing Systemic Immunity. Plant Cell Environ. 2021, 44, 2277–2289. [Google Scholar] [CrossRef]
- Shelake, R.M.; Pramanik, D.; Kim, J.-Y. Evolution of Plant Mutagenesis Tools: A Shifting Paradigm from Random to Targeted Genome Editing. Plant Biotechnol. Rep. 2019, 13, 423–445. [Google Scholar] [CrossRef]
- Magdy, M.; Mostofa, M.G.; Rahimi, M.; Abd El Moneim, D. Editorial: Abiotic Stress Alleviation in Plants: Morpho-Physiological and Molecular Aspects. Front. Plant Sci. 2023, 14, 1295638. [Google Scholar] [CrossRef]
- Rauf, S.; Al-Khayri, J.M.; Zaharieva, M.; Monneveux, P.; Khalil, F. Breeding Strategies to Enhance Drought Tolerance in Crops. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; Volume 2, pp. 397–445. ISBN 9783319225180. [Google Scholar]
- Selosse, M.-A.; Baudoin, E.; Vandenkoornhuyse, P. Symbiotic Microorganisms, a Key for Ecological Success and Protection of Plants. Comptes Rendus Biol. 2004, 327, 639–648. [Google Scholar] [CrossRef]
- Carter, E.K.; Riha, S.J.; Melkonian, J.; Steinschneider, S. Yield Response to Climate, Management, and Genotype: A Large-Scale Observational Analysis to Identify Climate-Adaptive Crop Management Practices in High-Input Maize Systems. Environ. Res. Lett. 2018, 13, 114006. [Google Scholar] [CrossRef]
- Williams, P.A.; Crespo, O.; Abu, M. Adapting to Changing Climate through Improving Adaptive Capacity at the Local Level—The Case of Smallholder Horticultural Producers in Ghana. Clim. Risk Manag. 2019, 23, 124–135. [Google Scholar] [CrossRef]
- Landaverde, R.; Rodriguez, M.T.; Niewoehner-Green, J.; Kitchel, T.; Chuquillanqui, J. Climate Change Perceptions and Adaptation Strategies: A Mixed Methods Study with Subsistence Farmers in Rural Peru. Sustainability 2022, 14, 16015. [Google Scholar] [CrossRef]
- Murrell, E.G. Can Agricultural Practices That Mitigate or Improve Crop Resilience to Climate Change Also Manage Crop Pests? Curr. Opin. Insect Sci. 2017, 23, 81–88. [Google Scholar] [CrossRef]
- Zuma, M.; Arthur, G.; Coopoosamy, R.; Naidoo, K. Incorporating Cropping Systems with Eco-Friendly Strategies and Solutions to Mitigate the Effects of Climate Change on Crop Production. J. Agric. Food Res. 2023, 14, 100722. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Bouri, M.; Arslan, K.S.; Şahin, F. Climate-Smart Pest Management in Sustainable Agriculture: Promises and Challenges. Sustainability 2023, 15, 4592. [Google Scholar] [CrossRef]
- Al-Zahrani, W.; Bafeel, S.O.; El-Zohri, M. Jasmonates Mediate Plant Defense Responses to Spodoptera Exigua Herbivory in Tomato and Maize Foliage. Plant Signal. Behav. 2020, 15, 1746898. [Google Scholar] [CrossRef]
- Erdoğan, İ.; Cevher-Keskin, B.; Bilir, Ö.; Hong, Y.; Tör, M. Recent Developments in CRISPR/Cas9 Genome-Editing Technology Related to Plant Disease Resistance and Abiotic Stress Tolerance. Biology 2023, 12, 1037. [Google Scholar] [CrossRef]
- Debbarma, J.; Sarki, Y.N.; Saikia, B.; Boruah, H.P.D.; Singha, D.L.; Chikkaputtaiah, C. Ethylene Response Factor (ERF) Family Proteins in Abiotic Stresses and CRISPR–Cas9 Genome Editing of ERFs for Multiple Abiotic Stress Tolerance in Crop Plants: A Review. Mol. Biotechnol. 2019, 61, 153–172. [Google Scholar] [CrossRef]
- Farhat, S.; Jain, N.; Singh, N.; Sreevathsa, R.; Dash, P.K.; Rai, R.; Yadav, S.; Kumar, P.; Sarkar, A.K.; Jain, A.; et al. CRISPR-Cas9 Directed Genome Engineering for Enhancing Salt Stress Tolerance in Rice. Semin. Cell Dev. Biol. 2019, 96, 91–99. [Google Scholar] [CrossRef]
- Luo, T.; Ma, C.; Fan, Y.; Qiu, Z.; Li, M.; Tian, Y.; Shang, Y.; Liu, C.; Cao, Q.; Peng, Y.; et al. CRISPR-Cas9-mediated Editing of GmARM Improves Resistance to Multiple Stresses in Soybean. Plant Sci. 2024, 346, 112147. [Google Scholar] [CrossRef]
- Shelake, R.M.; Pramanik, D.; Kim, J.Y. CRISPR Base Editor-Based Targeted Random Mutagenesis (BE-TRM) Toolbox for Directed Evolution. BMB Rep. 2024, 57, 30–39. [Google Scholar] [CrossRef]
- Hu, Y.; Patra, P.; Pisanty, O.; Shafir, A.; Belew, Z.M.; Binenbaum, J.; Ben Yaakov, S.; Shi, B.; Charrier, L.; Hyams, G.; et al. Multi-Knock—A Multi-Targeted Genome-scale CRISPR Toolbox to Overcome Functional Redundancy in Plants. Nat. Plants 2023, 9, 572–587. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; Mcroberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.; Cammarano, D.; Kimball, B.; Ottman, M.; Wall, G.; White, J.W. Rising Temperatures Reduce Global Wheat Production. Nat. Clim. Chang. 2015, 5, 143. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centenso, G.S.; Khush, G.S.; Cassman, K.G. Rice Yields Decline with Higher Night Temperature from Global Warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production Since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops that feed the World 2. Soybean—Worldwide Production, Use, and Constraints Caused by Pathogens and Pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Battisti, D.S.; Naylor, R.L. Historical Warnings of Future Food Insecurity with Unprecedented Seasonal Heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef]
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Hareau, G.; Wolf, J. Climate Change Impact on Global Potato Production. Eur. J. Agron 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hodges, H.F.; McKinion, J.M.; Wall, G.W. Temperature Effects on Pima Cotton Growth and Development. Agron. J. 1992, 84, 237–243. [Google Scholar] [CrossRef]
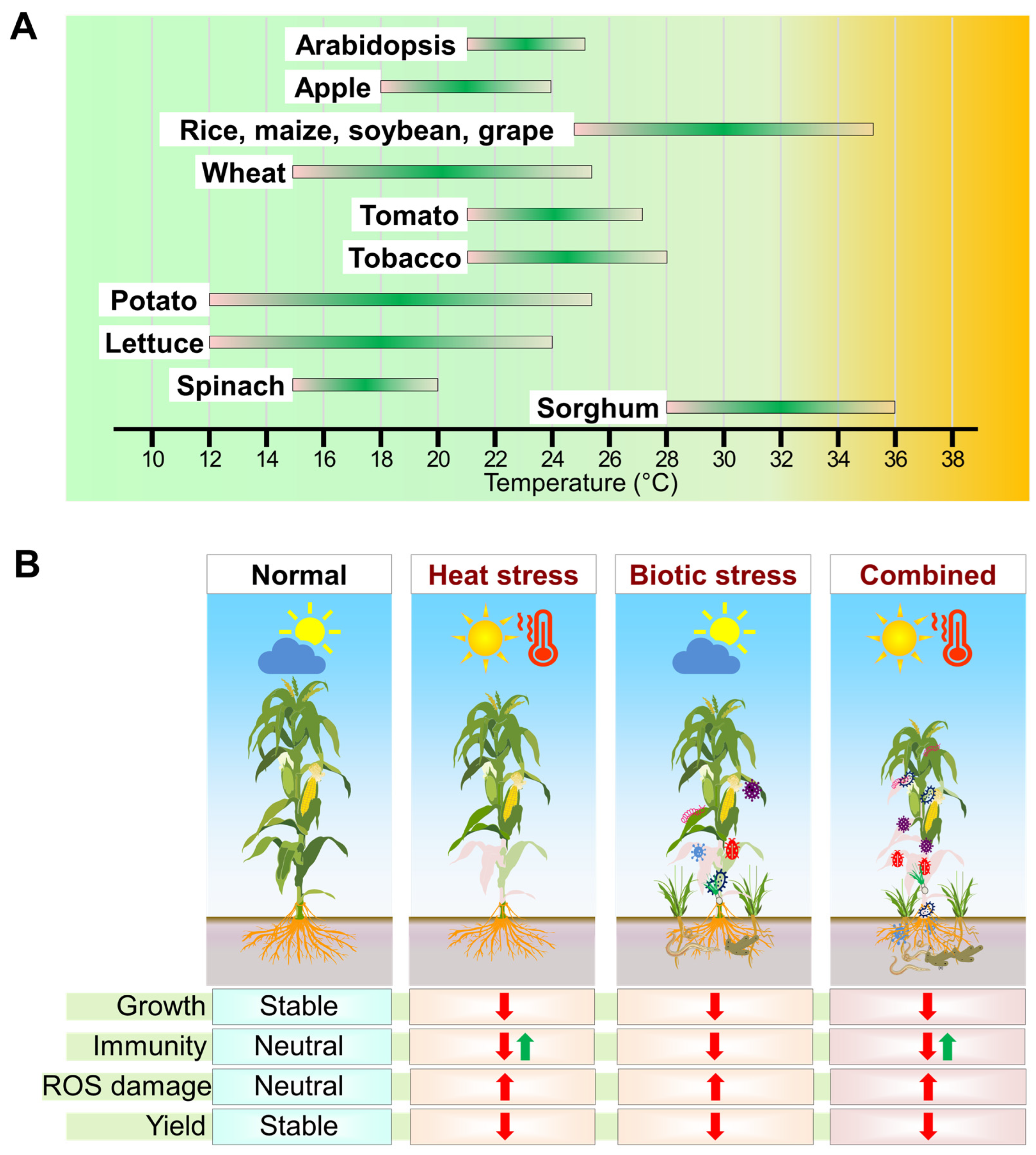
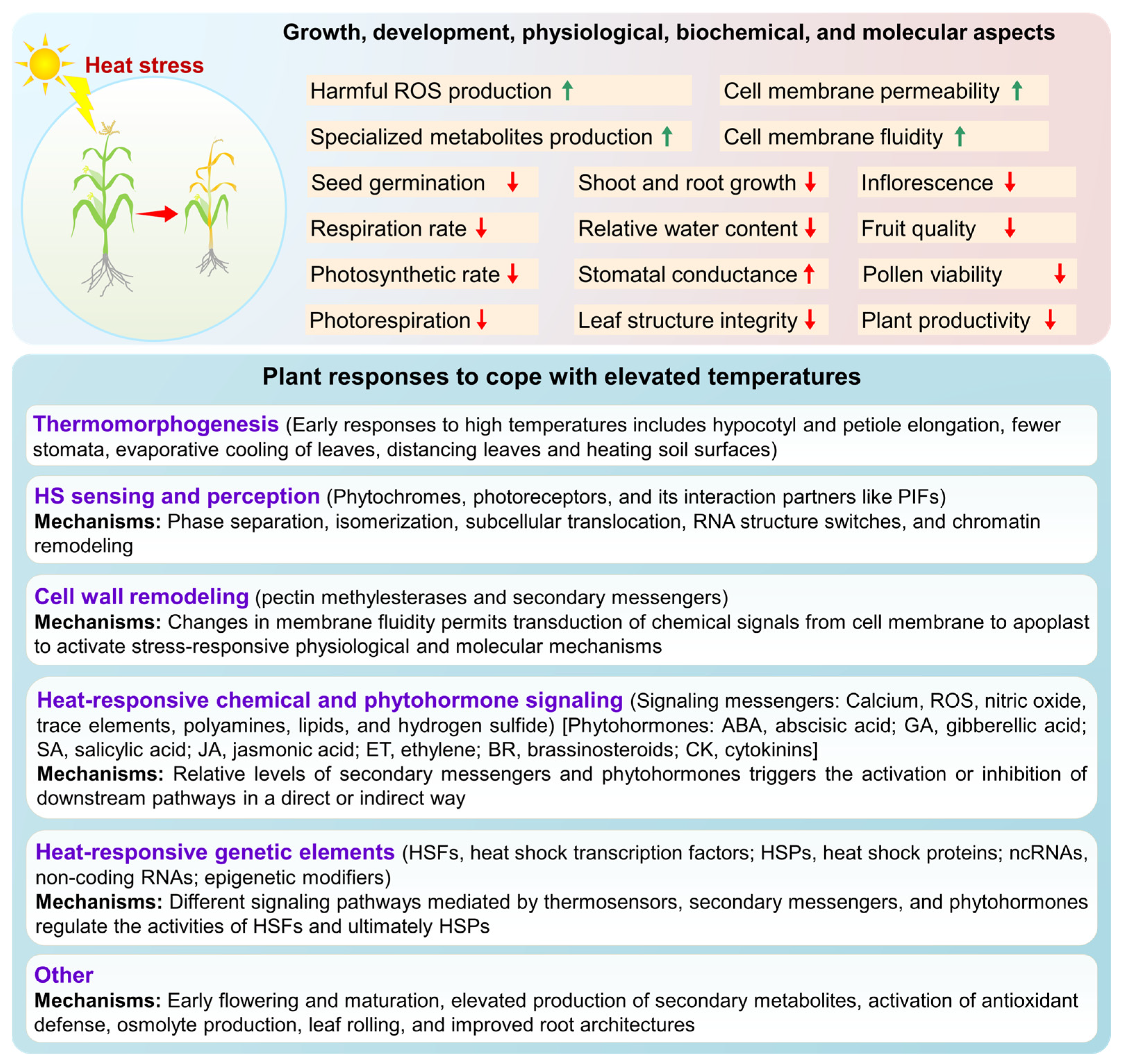

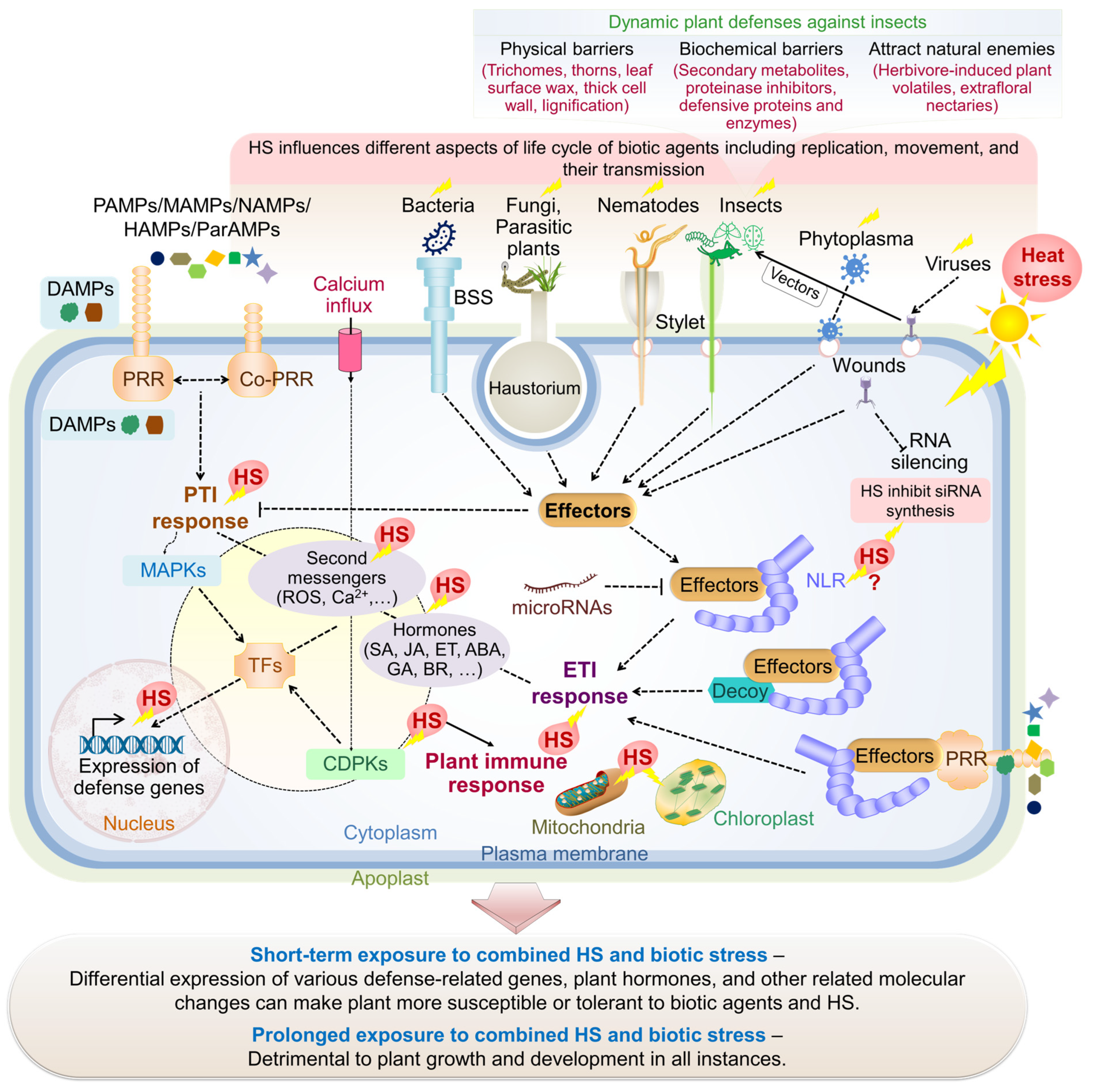
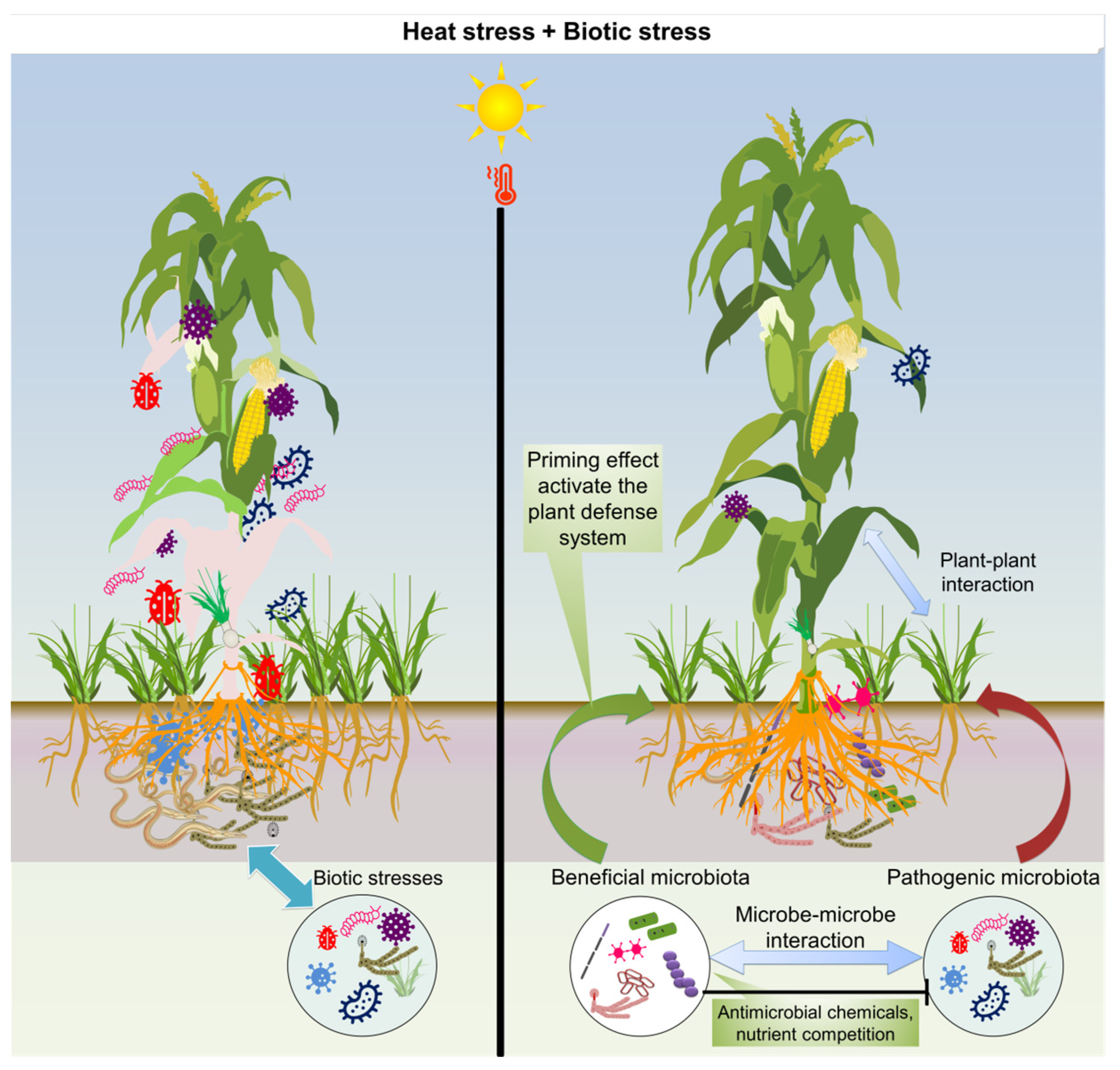
| Host Plant (Average Temp.) | Biotic Factor | Disease/Nature of Interaction | Set Up | Temp. (°C) | Genes or Other Explored Factors | Effect | Details | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bacteria | ||||||||
| Arabidopsis (22 °C) | Pseudomonas syringae pv. tomato DC3000 (PsPto) | Bacterial speck | C | 22, 28 | EDS1, PAD4, RPS2, RPS4, RPM1 | (−) | HR suppression (ETI) | [64] |
| PsPto | Bacterial speck | C | 42 (1 h), 37 (2 h) | FLS2 | (−) | Reduced tolerance (PTI) | [65] | |
| PsPto | Bacterial speck | C | 28, 30 | ZAR1, RPS2 | (−) | HR suppression (ETI) | [66] | |
| PsPto | Bacterial speck | C | 28, 30 | SR1/CAMTA3 | (−) | Reduced tolerance through compromised stomata closing (apoplastic immunity) | [67] | |
| Ralstonia solanacearum GMI1000 | Bacterial wilt | C | 27, 30 | RPS4/RRS1-R | (−) | Reduced tolerance (ETI) | [68] | |
| SSL4-SSL5 | (+) | Stable HS resistance | ||||||
| Rice (21–28 °C) | Xanthomonas oryzae pv. oryzae | Bacterial blight | C | 29, 35 | Xa3, Xa4, Xa5, Xa10 | (−) | Resistance less effective (QDR) | [69] |
| C, F | 29–35 (C) 29–33 (F) | Xa7 | (+) | Lines with Xa7 showed tolerance (ETI) | [69,70] | |||
| X. oryzae pv. oryzae | Bacterial blight | C | 29, 35 | Xa4, Xa7 | (+) | Line with Xa4 + Xa7 showed tolerance (ETI) | [71] | |
| X. oryzae pv. oryzae | Bacterial blight | C, F | ≥35, 42 (60 h) | SGS3a/b, ARF3a/b, ARF3la/lb | (±) | SGS3a/b: (+HS) and (−Pathogen resistance) ARF3a/b, ARF3la/lb: (−HS) and (+Pathogen resistance) | [72] | |
| Tomato (21–27 °C) | Ralstonia solanacearum | Bacterial wilt | C | 24, 32 | Polygenic resistance | (−) | Resistance inhibition | [73] |
| R. solanacearum | Bacterial wilt | C | 28, 34, 37 (high humidity >80%) | ITP5, trans-zeatin | (+) | Cytokinin-mediated resistance (ETI, PTI) | [74] | |
| PsPto | Bacterial speck | C | 26, 31 | Increased ABA, JA, spermine accumulation | (+) | Showed tolerance under mild HS | [75] | |
| Pepper (28 °C) | R. solanacearum | Bacterial wilt | C | 28, 37 | AGL8, SWC4 | (+) | Stable HS resistance (ETI, PTI) | [76] |
| R. solanacearum | Bacterial wilt | C | 28, 37 (high humidity >80%) | ITP5, trans-zeatin | (+) | Cytokinin-mediated resistance (ETI, PTI) | [74] | |
| R. solanacearum | Bacterial wilt | C | 28, 37 (high humidity >80%) | KAN3, HSF8 | (+) | KAN3-HSF8 form a complex to confer immunity and HS tolerance (ETI, PTI) | [77] | |
| R. solanacearum | Bacterial wilt | C | 28, 37 (high humidity >80%) | MLO1, PUB21 | (+) | MLO1-PUB21 form a complex and function distinctly in a temperature-dependent manner (stable resistance at 37 °C) | [78] | |
| Tobacco (22–28 °C) | R. solanacearum | Bacterial wilt | C | 28, 37 (high humidity >80%) | ITP5, trans-zeatin | (+) | Cytokinin-mediated resistance (ETI, PTI) | [74] |
| Fungi | ||||||||
| Rice (21–28 °C) | Magnaporthe oryzae | Blast | C | 38, 35 | Several R gene loci | (−) | Reduced resistance (QDR) | [79] |
| M. oryzae | Blast | C | 38, 35 | Pik-h, Pita2, Pii, Pi9, Piz-t | (+) | Enhanced tolerance (QDR) | [79] | |
| M. oryzae | Blast | C | 38, 35 | Pi54 | (+) | Enhanced tolerance (ETI) | [80] | |
| M. oryzae | Blast | C | 14, 22, 28, 33 | MYC2, MYC22, CEBiP | (−) | Suppression of JA signaling | [81] | |
| M. oryzae | Blast | F | ≥35, 42 (60 h) | SGS3a/b, ARF3a/b, ARF3la/lb | (±) | SGS3a/b: (+HS) and (−Pathogen resistance). ARF3a/b, ARF3la/lb: (−HS) and (−Pathogen resistance). | [72] | |
| Rhizoctonia solani | Sheath blight | T-FACE | Ambient and +2 | Malondialdehyde (MDA) levels | (−) | Reduced tolerance | [82] | |
| Fusarium fujikuroi | Bakanae | C | 22/18, 26/22, 30/26 (day/night) | prx98, MAPKK, GLP 8-7, NDR1/HIN1 13 | (−) | Reduced tolerance | [83] | |
| Chickpea (10–22 °C) | Macrophomina phaseolina | Dry root rot | C | 22/10, 35/25 (day/night) | - | (−) | Reduced tolerance | [84] |
| M. phaseolina | Dry root rot | C | 25, 35 | Endochitinase, CHI-III | (−) | Reduced tolerance (PR proteins) | [85] | |
| Coffee (18–21 °C) | Hemileia vastatrix | Leaf rust | C | 23/18, 27/22 (day/night) | Shade and high nitrogen (N) | (−) | Reduced tolerance under HS; shade and high N enhanced tolerance | [86] |
| American chestnut (16–27 °C) | Phytophthora cinnamomi | Root rot | F | <10, >12 (soil temp.) | Process-based Forest landscape model, biomass | (−) | Reduced tolerance | [87] |
| Chestnut (16–27 °C) | P. cinnamomi | Ink disease | C | 30, 35, 45 | Secondary metabolite compounds | (−) | Reduced resistance (metabolic aspects) | [88] |
| Common wheat (15–25 °C) | Puccinia graminis f. sp. tritici | Stem rust | C | 19, 26 | Sr6 | (−) | Reduced resistance (ETI) | [89,90] |
| Blumeria graminis f. sp. tritici | Powdery mildew | C | 15, 25 | Pm1, Pm8, Pm4a, Pm4b | (+) | Stable or enhanced resistance (QDR) | [91] | |
| B. graminis f. sp. tritici | Powdery mildew | C | 15, 18, 22, 26, 30 | Sr14, Sr9b | (+) | Reduced symptoms (ETI) | [92] | |
| Puccinia recondita (P. triticina) | Leaf rust | C | 15, 25 | Several R gene loci | (+) | Stable resistance (QDR) | [93] | |
| P. recondita (P. triticina) | Leaf rust | C | 18, 22, 25 | LrZH22 | (+) | Enhanced tolerance (QDR) | [94] | |
| Puccinia striiformis f. sp. tritici | Stripe rust | C | 4–20, 10–30 (diurnal) | Several R gene loci | (+) | HTAP tolerance (QDR) | [95] | |
| P. striiformis f. sp. tritici | Stripe rust | C | 10, 15, 20 | TaXa21 | (+) | HTSP tolerance (PTI, QDR) | [96,97] | |
| P. striiformis f. sp. tritici | Stripe rust | C | 4–20, 10–30 (diurnal) | Yr79 | (+) | HTAP tolerance (QDR) | [98] | |
| P. striiformis f. sp. tritici | Stripe rust | C | 4–20, 10–30 (diurnal) | Yr62 | (+) | HTAP tolerance (QDR) | [99] | |
| P. striiformis f. sp. tritici | Stripe rust | C | 4–20, 10–30 (diurnal) | Yr59 | (+) | HTAP tolerance (QDR) | [100] | |
| P. striiformis f. sp. tritici | Stripe rust | C | 4–20, 10–30 (diurnal) | Yr52 | (+) | HTAP tolerance (QDR) | [101] | |
| Durum wheat (15–25 °C) | P. striiformis f. sp. tritici | Stripe rust | C, F | 10–25, 10–35 (diurnal) | Yr36 | (+) | HTAP tolerance (QDR) | [102,103] |
| Oat (20–25 °C) | P. striiformis f. sp. tritici | Stripe rust | C | 15, 20, 25, 30 | B, E, F, H | (−) | Reduced resistance (ETI) | [104] |
| P. striiformis f. sp. tritici | Stripe rust | C | 15, 20, 25, 30 | A, D | (+) | Stable resistance (ETI) | [104] | |
| Barley (18–20 °C) | Bipolaris sorokiniana | Common root rot, seedling, and head blight, black point, leaf spot | C | 20, 49 (water bath for 20 s) | SOD, BI-1, DHAR Cyt, PR-1b | (−) | Reduced resistance (ETI) | [105] |
| Blumeria graminis f. sp. hordei | Powdery mildew | C | 28, 35 (30 s to 5 days) | BI-1, PR-1b, RBOHF2 | (±) | (−) Susceptible lines showed severe symptoms. (+) Resistant line showed stable resistance (ETI) | [106] | |
| B. graminis f. sp. hordei | Powdery mildew | C | 35, 49 | mlo5, Mlg, Mla12 | (−) | Reduced resistance (ETI) | [107,108,109] | |
| Oomycetes | ||||||||
| Arabidopsis (22 °C) | Peronospora parasitica | Downy mildew | C | 22, 28 | SNC1 | (−) | Compromised resistance (ETI, PTI) | [110] |
| Phytophthora sojae | Root and stem rot | C | 25, 33 | Several R gene loci | (−) | Reduced resistance (QDR) | [111] | |
| Soybean (19–25 °C) | P. sojae | Root and stem rot | C | 25, 33 | Rps1-c, Rps2, Rps5 | (+) | Stable resistance (QDR) | [111] |
| Pepper | P. sojae | Blight and fruit rot | C | 25, 37 | Multiple WRKY and HSP genes | (−) | QDR | [112] |
| Sweet basil (10–25 °C) | Peronospora belbahrii | Downy mildew | C | 20, >25, 26–31 | - | (+) | Preheat-treated plants showed stable thermotolerance | [113] |
| Viruses | ||||||||
| Tobacco (22–28 °C) | Tobacco mosaic virus | Mosaic | C | 28 | Necrosis (N) | (−) | HR suppression (ETI) | [114] |
| Tobacco (22–28 °C) | Potato virus X genes | Mosaic | C | 28, 30 | Rx | (−) | HR suppression (ETI) | [64] |
| Potato (15–20 °C) | Potato virus Y | Mosaic | C | 18, 24 | Necrosis y (Ny) | (−) | HR suppression (ETI) | [115] |
| Potato virus Y | Mosaic | C | 22, 28 | PR, HSP genes | (±) | (−) Susceptible lines showed severe symptoms. (+) Resistant line showed stable resistance | [116] | |
| Rice (21–28 °C) | Rice stripe virus | Stripe | C | 35–37, 38 | Stvb-i | (+) | Stable resistance | [117] |
| Tomato (21–27 °C) | Tomato yellow leaf curl virus | Leaf curl | C | 42–43 (2 h), 40–45/20–25 (diurnal) | HSFA2, HSFB1, Hsp17, Apx1, Apx2, Hsp90 | (+) | Stable resistance | [118] |
| Capsicum (25 °C) | Capsicum chlorosis virus | Chlorosis | C | 25, 35 | RNA silencing-related genes | (+) | Recovery (antiviral RNA silencing activation) | [119] |
| Common wheat (15–25 °C) | Wheat streak mosaic virus | Streak mosaic | C | 18, 24 | Wsm1, Wsm2, Wsm3 | (±) | Wsm1/Wsm2: (−) Symptoms observed; Only Wsm3; (+) Stable resistance | [120] |
| Wheat streak mosaic virus | Streak mosaic | C | 20, 32 | Signaling chemical compounds | (−) | Reduced resistance (metabolic aspects) | [121,122] | |
| Triticum mosaic virus | Streak mosaic | C | 18, 24 | Wsm3 | (+) | Stable resistance | [120] | |
| Pepper | Paprika mild mottle virus | Chlorosis and mottling | C | 24, 30 | L 1a locus resistance gene | (−) | HR suppression (ETI) | [123] |
| Tobacco mild green mosaic virus | Mosaic | C | 22, 32 | L 1- L 2, L 1a | (−) | L 1- L 2: HR suppression (ETI) L 1a: Stable resistance (ETI) | [123] | |
| Common bean | Bean pod mottle virus | Bean pod mottle | C | 20, 25, 30, 35 | R-BPMV | (±) | (−) Susceptible lines showed severe symptoms. (+) Resistant line showed stable resistance (ETI) | [124] |
| Nematodes | ||||||||
| Tomato (21–27 °C) | Meloidogyne incognita | Roo-knot | C, F | 22, 26, 32 | Mi-1 | (−) | Reduced resistance (ETI) | [125,126] |
| M. incognita | Roo-knot | C, F | 22, 26, 32 | Mi-9 | (+) | Stable resistance (ETI) | [125] | |
| M. incognita | Roo-knot | C | 25, 32 | Mi-7, Mi-8 | (−) | Reduced resistance (ETI) | [127] | |
| Meloidogyne javanica, M. incognita, M. arenaria | Roo-knot | C | 24, 32 | Mi-1 | (−) | HR suppression (ETI) | [128] | |
| Pepper (20–25 °C) | Meloidogyne spp. | Roo-knot | C | 32, 42 | Me1, Me3 | (+) | Stable resistance (ETI) | [129] |
| Insects | ||||||||
| Tomato (14–25 °C) | Helicoverpa zia (Corn earworm) | Leaf feeding | C | 25/14, 30/18, 35/22 | Insect: GOX Plant: TPI, PPO | (−) | Accelerated insect growth, compromised plant growth, and recovery | [130] |
| Tomato (18–28 °C) | Manduca sexta (hornworm) | Leaf feeding | C | 28/18, 38/28, | HSP90, COI1 | (−) | Wound-induced JA signaling inhibited growth | [131] |
| Tomato (19–26 °C) | Spodoptera littoralis (noctuid moth) | Leaf feeding | C | 20, 25 | HS impact on Trichoderma sp. and stress responses of tomato by insects | (+) | T. afroharzianum T22 enhanced plant resistance against insects fed on detached leaves (no living plant used) | [132] |
| Macrosiphum euphorbiae (aphid) | Phloem feeding | C | 20, 25 | HS impact on Trichoderma sp. and stress responses of tomato by insects | (+) | T. afroharzianum T22 enhanced plant resistance against insects fed on detached leaves (no living plant used) | [132] | |
| Oregano mint (18–24 °C) | Trialeurodes vaporariorum (greenhouse whitefly) | Phloem feeding | C | 24/18, 45 (5 min) | Volatile compound emissions (lipoxygenase, terpene, benzenoid) | (+) | Heat tolerance, quick recovery from infestation | [133] |
| Maize (18–22 °C) | Diabrotica balteata (banded cucumber beetle) | Root feeding | C | 17.8, 20.8 (soil temp.) | Soluble sugars, soluble proteins, benzoxazinoids | (−) | Increased root damage but decreased herbivore survival depending on soil moisture | [134] |
| Potato (15–25 °C) | Macrosiphum euphorbiae (aphid) | Phloem feeding | C | 25/15, 30/20 | Stomatal conductance | (+) | Reduced survival and fecundity of insects | [135] |
| Soybean (24–26 °C) | Aphis glycines (aphid) | Phloem feeding | C | 24–26, 35/20 (diurnal) | Lifespan and fecundity of insects | (+) | Insects fed on detached leaves showed reduced fitness (no living plant used) | [136] |
| Plant | Plant-Beneficial Microbes | Details | Ref. |
|---|---|---|---|
| Japonica rice | Paecilomyces formosus | Reduced endogenous JA and AA, enhanced total protein amounts | [215] |
| Wheat | Bacillus amyliliquefaciens, Pseudomonas fluorescens, Pantoea agglomerans, Pantoea agglomerans | Several common plant protective SMs notably accumulated during HS | [216] |
| Klebsiella sp. | Protection against salt and HS; reduced ethylene production and regulation of ion transporters | [217] | |
| Bacillus cereus, Pseudomonas putida | Increased PGP activity (root, shoot fresh and dry weight, chlorophyll contents) under HS | [218] | |
| Bacillus amyloliquefaciens UCMB5113 Abd El- | Increased plant growth (root, shoot fresh and dry weight, chlorophyll contents) under HS | [216] | |
| Pseudomonas brassicacearum, Bacillus thuringiensis, Bacillus subtilis | Increase HSP26 protein, GABA, and chlorophyll content, modulated metabolic pathways | [219] | |
| Bacillus amyloliquefaciens UCMB5113, Azospirillum brasilense | Augmented antioxidant activities, higher AA and protein content | [220] | |
| Bacillus amyloliquefaciens UCMB5113, Azospirillum brasilense | Reduced ROS, higher expression of HSFs and HSPs | [218] | |
| Lactobacillus agilis, Lactobacillus plantarum, Lactobacillus acidophilus | Increased PGP activity, higher chlorophyll content, enhanced antioxidant levels | [221] | |
| Thermotolerant bacterial isolates | Increased PGP activity | [222] | |
| Tomato | Bacillus cereus | Increased number of flowers and fruits, increased chlorophyll, proline, antioxidants | [223] |
| Paraburkholderia phytofirmans | Alleviate the harmful effects of HS by PGP | [224] | |
| Bacillus safensis | Significantly improved PGP activity and chlorophyll content, antioxidant enzyme production | [225] | |
| PGP bacteria synthetic consortium | ACC-deaminase production, ethylene accumulation, PGP | [226] | |
| Bacillus cereus | Reduced ABA, increased SA and antioxidant enzyme activities, and increased APX, SOD, and GSH levels | [227] | |
| Potato | Pseudomonas sp. PsJN | Promoted plant growth | [228] |
| Sorghum | Azospirillum brasilense NO40, Pseudomonas sp. AKM-P6 | Enhanced tolerance in seedling stage to HS | [229,230] |
| Bacillus cereus TCR17 | Reduced ROS stress via upregulation of CAT, APX1, SOD, and HSPs | [231] | |
| Pseudomonas sp. strain AKM-P6 | Induced stress-related protein production, Preserved membrane integrity, higher levels of sugars, AA, and chlorophyll | [232] | |
| Cabbage | Bacillus aryabhattai H26-2, Bacillus siamensis H30-3 | Higher ABA in leaf, biocontrol activity against soft rot, reduced stomatal opening | [233] |
| Soybean | Bradyrhizobium diazoefficiens USDA110 | Survival in starvation | [234] |
| Aeromonas hydrophilla, Serratia liquefaciens, Serratia proteamaculans | Increased yields by genistein (antioxidant isoflavone) | [235] | |
| Bacillus cereus SA1 | Increased SA, reduced ABA, upregulation of APX, HSP, LAX3, SOD, and AKT2 genes, elevated GSH AA content, increased K gradients | [236] | |
| Legumes | Rhizobium sp. | HSP of 63–74 kDa | [237] |
| Alfalfa | Shinorizobium meliloti | Affect symbiosis during HS condition | [238] |
| Legume | Rhizobium sp. | HSP of 63–74 kDa | [215] |
| Grapevine | PGP bacteria synthetic consortium | Maintained leaf turgidity, lower osmolyte content, enhanced antioxidant mechanisms | [239] |
| Avocado, tomato | Pseudomonas-based consortium | PGP activity | [240] |
| Arabidopsis | Serendipita indica | PGP effect | [241] |
| Paraburkholderia phytofirmans PsJN | PGP and HS amelioration | [242] | |
| Rapeseed, camelina | Pseudomonas spp. | PGP activity through carbon reallocation | [243] |
| Maize | Bacillus spp. (AH-08, AH-67, SH-16), Pseudomonas spp. SH-29 | PGP and HS tolerance during seedling/early vegetative growth | [244] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelake, R.M.; Wagh, S.G.; Patil, A.M.; Červený, J.; Waghunde, R.R.; Kim, J.-Y. Heat Stress and Plant–Biotic Interactions: Advances and Perspectives. Plants 2024, 13, 2022. https://doi.org/10.3390/plants13152022
Shelake RM, Wagh SG, Patil AM, Červený J, Waghunde RR, Kim J-Y. Heat Stress and Plant–Biotic Interactions: Advances and Perspectives. Plants. 2024; 13(15):2022. https://doi.org/10.3390/plants13152022
Chicago/Turabian StyleShelake, Rahul Mahadev, Sopan Ganpatrao Wagh, Akshay Milind Patil, Jan Červený, Rajesh Ramdas Waghunde, and Jae-Yean Kim. 2024. "Heat Stress and Plant–Biotic Interactions: Advances and Perspectives" Plants 13, no. 15: 2022. https://doi.org/10.3390/plants13152022







