Plant-Growth-Promoting Rhizobacteria Improve Seeds Germination and Growth of Argania spinosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Bacteria Isolation
2.3. Screening for Phosphate Solubilization
2.4. Screening for Indole Acetic Acid (IAA) Production
2.5. Effect of Isolate on Maize Growth
2.6. In Vitro Effect of the Isolates on the Growth of Argan Seedlings
2.7. In Vivo Effect of PGPR Isolates on the Growth of Argan Seedlings
- i: initial measures
- f: final measures
2.8. Available Phosphate Production (P2O5)
2.9. Indole Acetic Acid (IAA) Production
2.10. Nitrogen Fixation
2.11. Hydrogen Cyanide Production
2.12. Siderophores Production
2.13. Ammonia Production
2.14. Bacterial Characterization
2.15. DNA Extraction, 16S rRNA Sequencing, and Analysis
2.16. Statistical Analysis
3. Results
3.1. Screening for Phosphorate Solubilization
3.2. Screening for Indole Acetic Acid (IAA) Production
3.3. Growth-Promotion Experiments
3.3.1. In Vitro Effect of the Isolates on the Growth of Maize Seedlings
3.3.2. Isolates Effect on the Argania spinosa Seeds In Vitro Germination
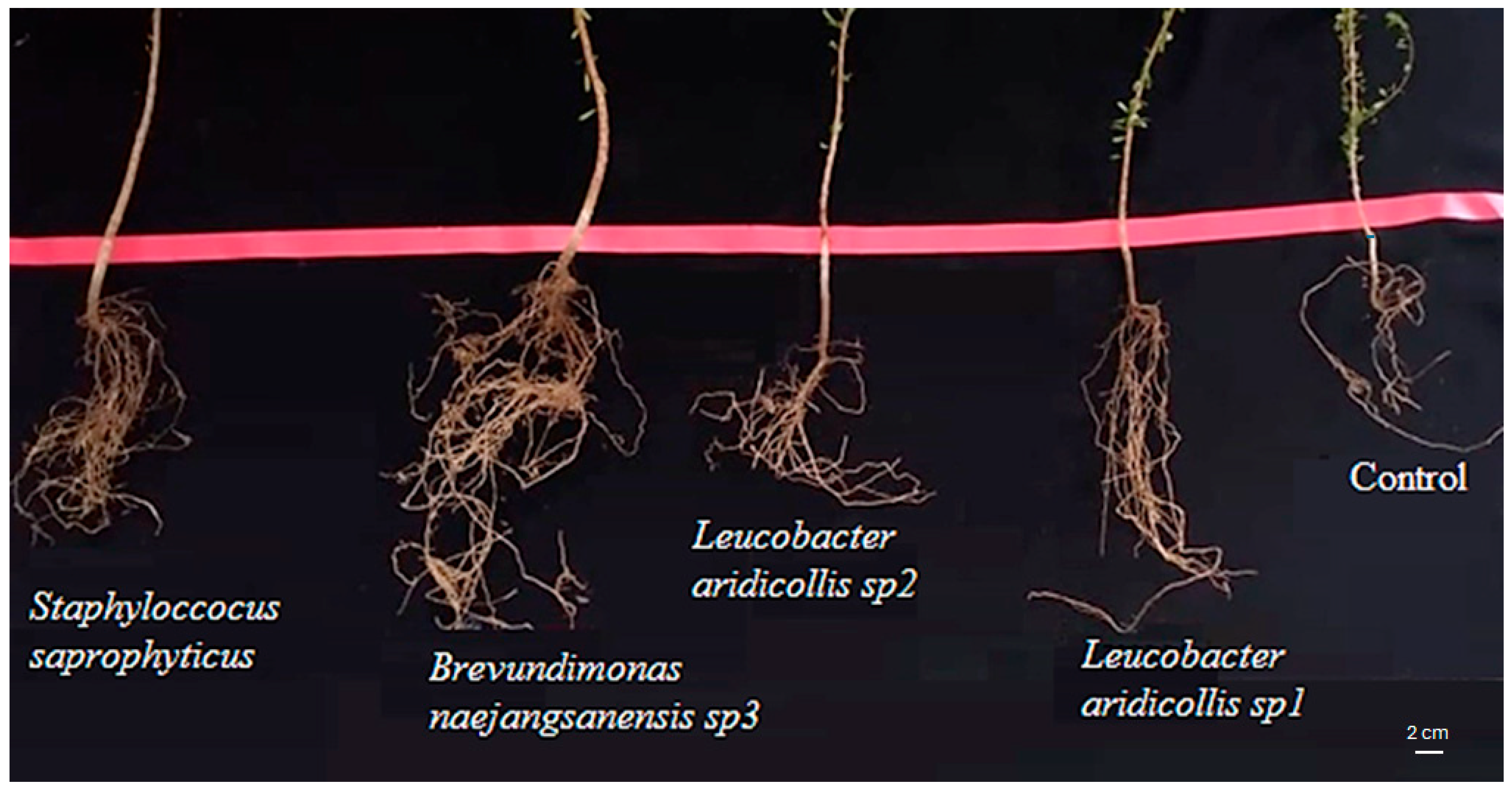
3.3.3. Effect of the Isolates on the Growth of Argania spinosa Seedlings under Greenhouse Conditions
3.4. PGPR Parameters
3.5. 16S rRNA Sequencing and Analysis of the Eight Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ait Aabd, N.A.; Bouharroud, R.; Tahiri, A.; Wifaya, A.; Mimouni, A.; El Mousadik, A. Genetic diversity and breeding of argan tree (Argania spinosa L. skeels). Adv. Plant Breed. Strateg. Nut Beverage Crops 2020, 4, 31–56. [Google Scholar] [CrossRef]
- Ait Aabd, N.A.; Tahiri, A.; Qessaoui, R.; Mimouni, A.; Bouharroud, R. Self- and Cross-Pollination in Argane Tree and their Implications on Breeding Programs. Cells 2022, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Ajerrar, A.; Zaafrani, M.; Qessaoui, R.; Aabd, N.A.; Bahadou, H.; Lahmyed, H.; Furze, J.N.; Chebli, B.; Mayad, E.H.; Bouharroud, R. Terrestrial arthropods diversity in the Argan Biosphere Reserve: Seasonal dynamics and ecological function roles. J. Saudi Soc. Agric. Sci. 2023, 22, 1–10. [Google Scholar] [CrossRef]
- Ait Aabd, N.A.; Abdelghani Tahiri, A.; Mimouni; Bouharroud, R. Study of compatibility and determination of the suitable pollinizer for Argane tree (Argania spinosa L.). bioRxiv 2021. bioRxiv:2021.05.23.445304. [Google Scholar] [CrossRef]
- Boukhalef, L.; Douch, A.N.; Bouqbis, L.; El Asbahani, A.; Touaf, M.; Ain-Lhout, F. Physiological and Morphological Response of Argania spinosa (L.) Skeels to a Competing Liana: Case Study under Arid Climate. Int. J. Plant Biol. 2022, 14, 1–13. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Elmostapha, O.; Hanane, D.; Rachid, B.; Lahcen, O. Application of Arbuscular Mycorrhizal Fungi Isolates from Semi-arid Mediterranean Ecosystems as Biofertilizers in Argan Tree Development. J. Soil Sci. Plant Nutr. 2021, 22, 944–955. [Google Scholar] [CrossRef]
- Qessaoui, R.; Bouharroud, R.; Benhima, R.; Mayad, E.H.; Chebli, B.; Serghini, M.A. Effects of plant growth promoting rhizobacteria (PGPR) on Citrus macrophylla rootstock. Moroc. J. Agric. Sci. 2020, 1, 79–83. [Google Scholar]
- Qessaoui, R.; Elaalaoui, M.; Chafiki, S.; Alouani, M.; Chabbi, N.; Abd, N.A.; Tahiri, A.; Bouharroud, R. Pseudomonas siderophores: Production, spectrophotometry detection and Botrytis suppression. Not. Sci. Biol. 2024, 16, 11555. [Google Scholar] [CrossRef]
- Pandurang, K.P. Plant Growth Promoting Rhizobacteria (PGPR): A Review. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 882–886. [Google Scholar] [CrossRef]
- Qessaoui, R.; Zanzan, M.; Ajerrar, A.; Lahmyed, H.; Boumair, A.; Tahzima, R.; Alouani, M.; Mayad, E.H.; Chebli, B.; Walters, S.A.; et al. Pseudomonas Isolates as Potential Biofungicides of Green Mold (Penicillium digitatum) on Orange Fruit. Int. J. Fruit Sci. 2022, 22, 142–150. [Google Scholar] [CrossRef]
- Qessaoui, R.; Lahmyed, H.; Ajerrar, A.; Furze, J.; Timothy, P.; Alouani, M.; Chebli, B.; Mayad, E.; Bouharroud, R. Solutions rhizosphériques: Isolats de Pseudomonas contre Botrytis cinerea de la tomate. Afr. Mediterr. Agric. J. Al Awamia 2021, 131, 50–72. [Google Scholar] [CrossRef]
- Pérez-García, L.-A.; Sáenz-Mata, J.; Fortis-Hernández, M.; Navarro-Muñoz, C.E.; Palacio-Rodríguez, R.; Preciado-Rangel, P. Plant-Growth-Promoting Rhizobacteria Improve Germination and Bioactive Compounds in Cucumber Seedlings. Agronomy 2023, 13, 315. [Google Scholar] [CrossRef]
- Bichara, L.; Taoufiq, K.; El Biari, K.; Oufdou, K.; Faghire, M. Germination of Alfalfa in the rhizosphere of the Argan tree soil: In vitro study of the effect of water and salt stress. J. Anal. Sci. Appl. Biotechnol. 2024, 6, 4805. [Google Scholar] [CrossRef]
- Mapelli, F.; Riva, V.; Vergani, L.; Choukrallah, R.; Borin, S. Unveiling the Microbiota Diversity of the Xerophyte Argania spinosa L. Skeels Root System and Residuesphere. Microb Ecol. 2020, 80, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Bouizgarne, B.; Lanoot, B.; Loqman, S.; Sproer, C.; Klenk, H.-P.; Swings, J.; Ouhdouch, Y. Streptomyces marokkonensis sp. nov., isolated from rhizosphere soil of Argania spinosa L. Int. J. Syst. Evol. Microbiol. 2009, 59, 2857–2863. [Google Scholar] [CrossRef]
- Biol, T.J.; Koçak, F.Ö. Turkish Journal of Biology Identification of Streptomyces strains isolated from Humulus lupulus rhizosphere and determination of plant growth promotion potential of selected strains. J. Biol. 2019, 43, 391–403. [Google Scholar] [CrossRef]
- Aragosa, A.; Specchia, V.; Frigione, M. PHB Produced by Bacteria Present in the Argan Field Soil: A New Perspective for the Synthesis of the Bio-Based Polymer. Proceedings 2020, 69, 5. [Google Scholar] [CrossRef]
- Aragosa, A.; Saccomanno, B.; Specchia, V.; Frigione, M. A Novel Sphingomonas sp. Isolated from Argan Soil for the Polyhydroxybutyrate Production from Argan Seeds Waste. Polymers 2023, 15, 512. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef]
- Sayyed, R.Z. Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Berlin/Heidelberg, Germany, 2019; Volume 13. [Google Scholar] [CrossRef]
- Choubane, S.; Cheba, B.A.; Benourrad, A. Screening and Phenotypic Diversity of Amylase Producing Rhizospheric Bacteria from Some North African Plants. Procedia Technol. 2016, 22, 1197–1204. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, R.S.; Alabouvette, C. Antagonistic Activity of Selected Isolates of Fluorescent Pseudomonas Against Fusarium oxysporum f. sp. ciceri. Asian J. Plant Sci. 2007, 6, 446–454. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Qessaoui, R.; Bouharroud, R.; Furze, J.N.; El Aalaoui, M.; Akroud, H.; Amarraque, A.; Van Vaerenbergh, J.; Tahzima, R.; Mayad, E.H.; Chebli, B. Applications of New Rhizobacteria Pseudomonas Isolates in Agroecology via Fundamental Processes Complementing Plant Growth. Sci. Rep. 2019, 9, 12832. [Google Scholar] [CrossRef] [PubMed]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Saadaoui, N.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Alenezi, F.N.; Luptakova, L.; Boulahouat, S.; Belbahri, L. Semi-Arid-Habitat-Adapted Plant-Growth-Promoting Rhizobacteria Allows Efficient Wheat Growth Promotion. Agronomy 2022, 12, 2221. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Erie, KS, USA, 1954.
- Chamkhi, I.; Ibnyasser, J.Z.A.; Chetou, S.; Geistlinger, J.; Saidi, R.; Zeroual, Y.; Kouisni, L.; Bargaz, A.; Ghoulam, C. Siccibacter colletis as a member of the plant growth-promoting rhizobacteria consortium to improve faba-bean growth and alleviate phosphorus deficiency stress. Front. Sustain. Food Syst. 2023, 7, 1134809. [Google Scholar] [CrossRef]
- Mir, M.I.; Hameeda, B.; Quadriya, H.; Kumar, B.; Ilyas, N.; Kee Zuan, A.T.; El Enshasy, H.A.; Dailin, D.J.; Kassem, H.S.; Gafur, A.; et al. Multifarious Indigenous Diazotrophic Rhizobacteria of Rice (Oryza sativa L.) Rhizosphere and Their Effect on Plant Growth Promotion. Front. Nutr. 2022, 8, 781764. [Google Scholar] [CrossRef] [PubMed]
- Stella, M.; Suhaimi, M.; Matthews, S.; Masduki, S. Selection of suitable growth medium for free-living diazotrophs isolated from compost. J. Trop. Agric. Fd. Sc 2010, 38, 211–219. [Google Scholar]
- Lorck, H. Production of Hydrocyanic Acid by Bacteria. Physiol. Plant 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Patel, K.; Parmar, S.; Vaghela, H.; Muley, N.; Dhandhukia, P.; Thakker, J.N. Elucidating multifaceted urease producing marine Pseudomonas aeruginosa BG as a cogent PGPR and bio-control agent. Plant Growth Regul. 2014, 75, 253–263. [Google Scholar] [CrossRef]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-Priming of Soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to Improve Seed Germination and the Initial Seedling Growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, E.; Franchi, E.; Giovannini, L.; Brescia, F.; Sillo, F.; Fusini, D.; Pietrini, I.; Centritto, M.; Balestrini, R. Diverse plant promoting bacterial species differentially improve tomato plant fitness under water stress. Front. Plant Sci. 2023, 14, 1297090. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.K.; Wang, Z.; Wang, F.-Y.; Li, C.-N.; Lan, T.-J.; Singh, R.K.; Singh, P.; Yang, L.-T.; Li, Y.-R. Intercropping in Sugarcane Cultivation Influenced the Soil Properties and Enhanced the Diversity of Vital Diazotrophic Bacteria. Sugar Tech. 2016, 19, 136–147. [Google Scholar] [CrossRef]
- Kumar, V.; Gera, R. Isolation of a multi-trait plant growth promoting Brevundimonas sp. and its effect on the growth of Bt-cotton. 3 Biotech 2013, 4, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Bekkar, A.A.; Zaim, S. Newly isolated Brevundimonas naejangsanensis as a biocontrol agent against Fusarium redolens the causal of Fusarium yellows of chickpea. Folia Microbiol. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.D.; Rigobelo, E.C. Growth-Promoting Potential of Rhizobacteria Isolated From Sugarcane. Front. Sustain. Food Syst. 2021, 5, 596269. [Google Scholar] [CrossRef]
- Zapata-Sifuentes, G.; Hernandez-Montiel, L.G.; Saenz-Mata, J.; Fortis-Hernandez, M.; Blanco-Contreras, E.; Chiquito-Contreras, R.G.; Preciado-Rangel, P. Plant Growth-Promoting Rhizobacteria Improve Growth and Fruit Quality of Cucumber under Greenhouse Conditions. Plants 2022, 11, 1612. [Google Scholar] [CrossRef]
- Raj, S.N.; Shetty, N.; Shetty, H. Seed bio-priming withPseudomonas fluorescensisolates enhances growth of pearl millet plants and induces resistance against downy mildew. Int. J. Pest Manag. 2004, 50, 41–48. [Google Scholar] [CrossRef]
- Gholami, A.; Shahsavani, S.; Nezarat, S. The Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Germination, Seedling Growth and Yield of Maize. Int. J. Agric. Biosyst. Eng. 2009, 3, 9–14. [Google Scholar]
- Mehboob, I.; Naveed, M.; Zahir, Z.; Ashraf, M. Potential of Rhizobia for Sustainable Production of Non-Legumes; Springer: Berlin/Heidelberg, Germany, 2012; Volume 9, pp. 659–704. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S.; Jamil, N.; Iqbal, R.; Ali, B.; Ercisli, S.; et al. Multifaceted Impacts of Plant-Beneficial Pseudomonas spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296–22315. [Google Scholar] [CrossRef] [PubMed]

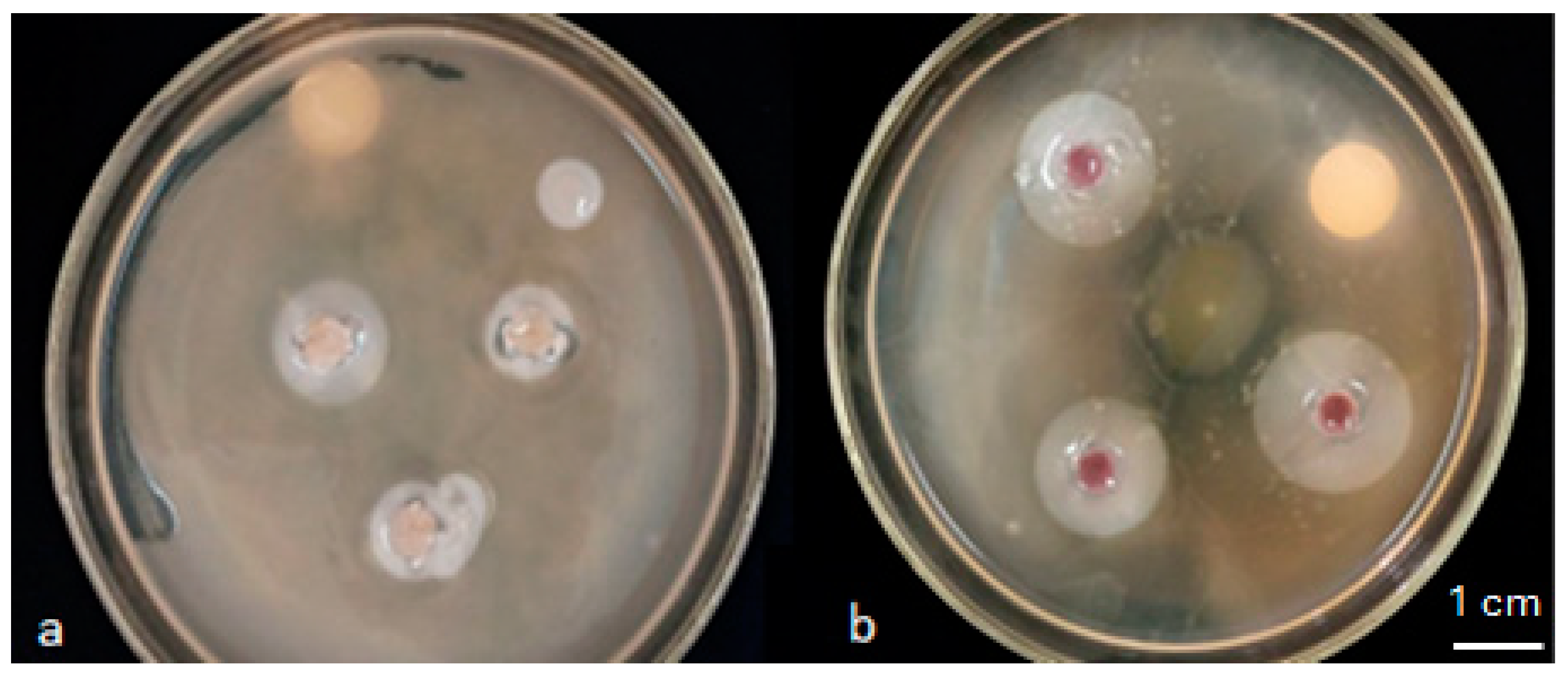

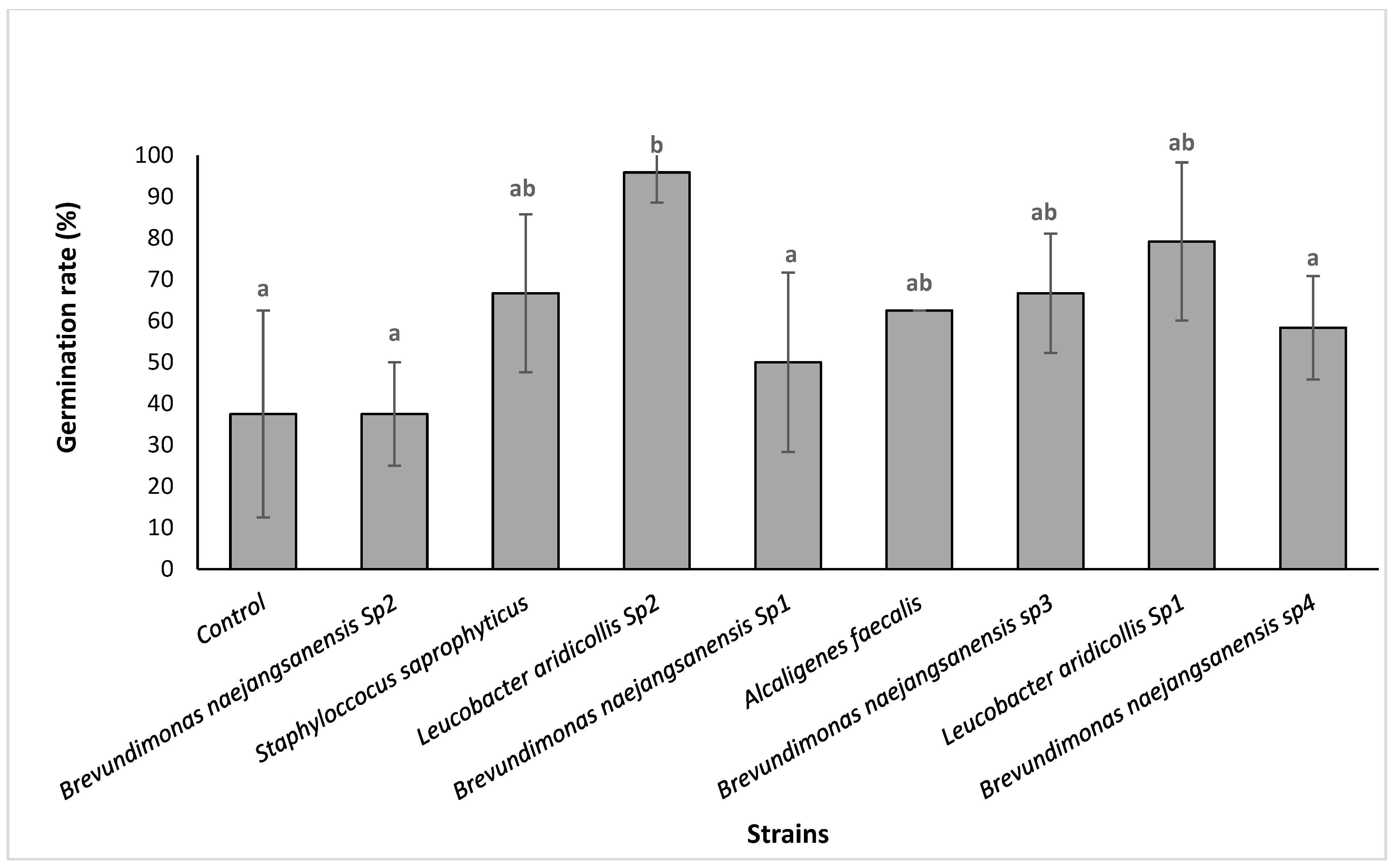
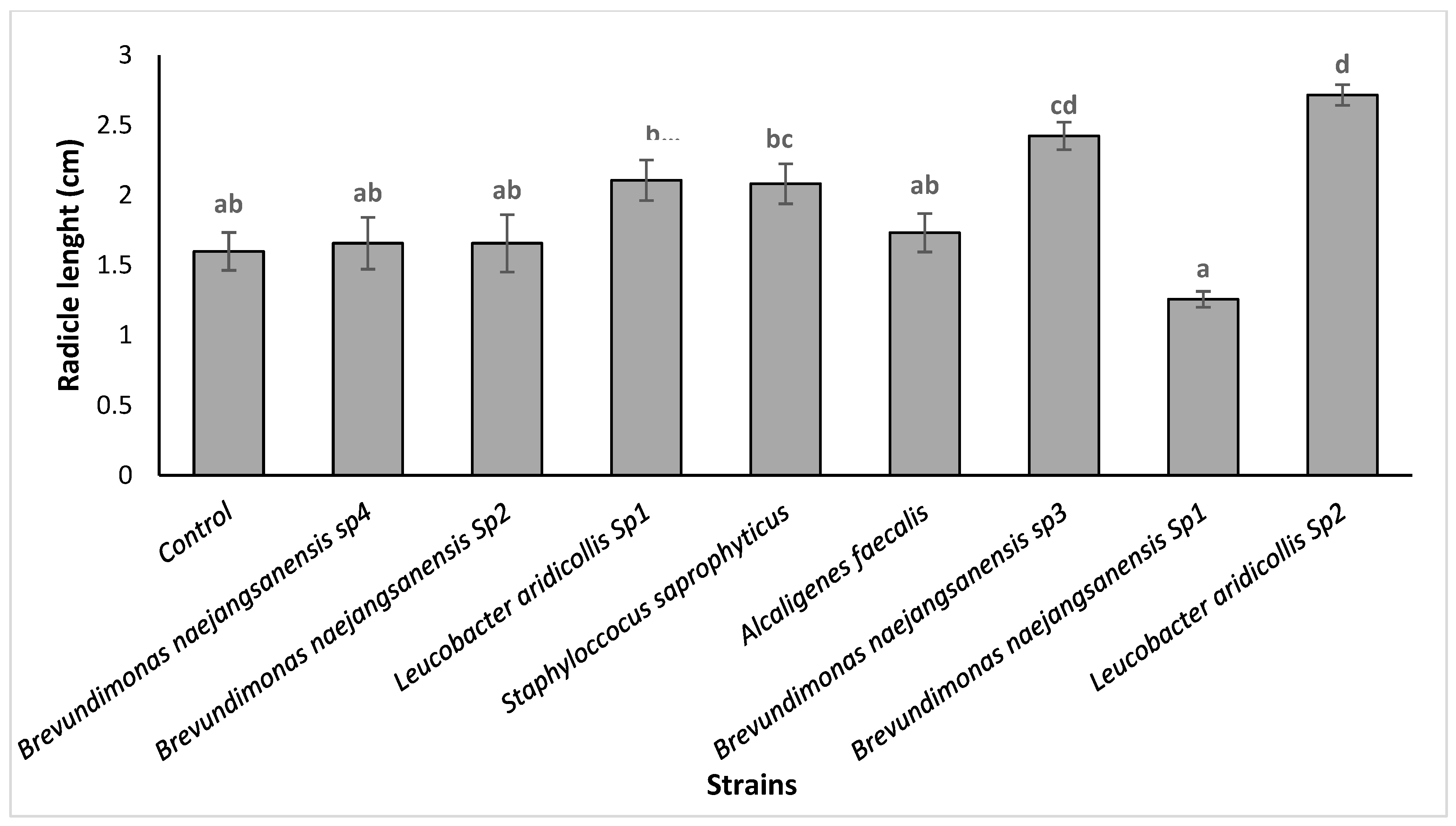



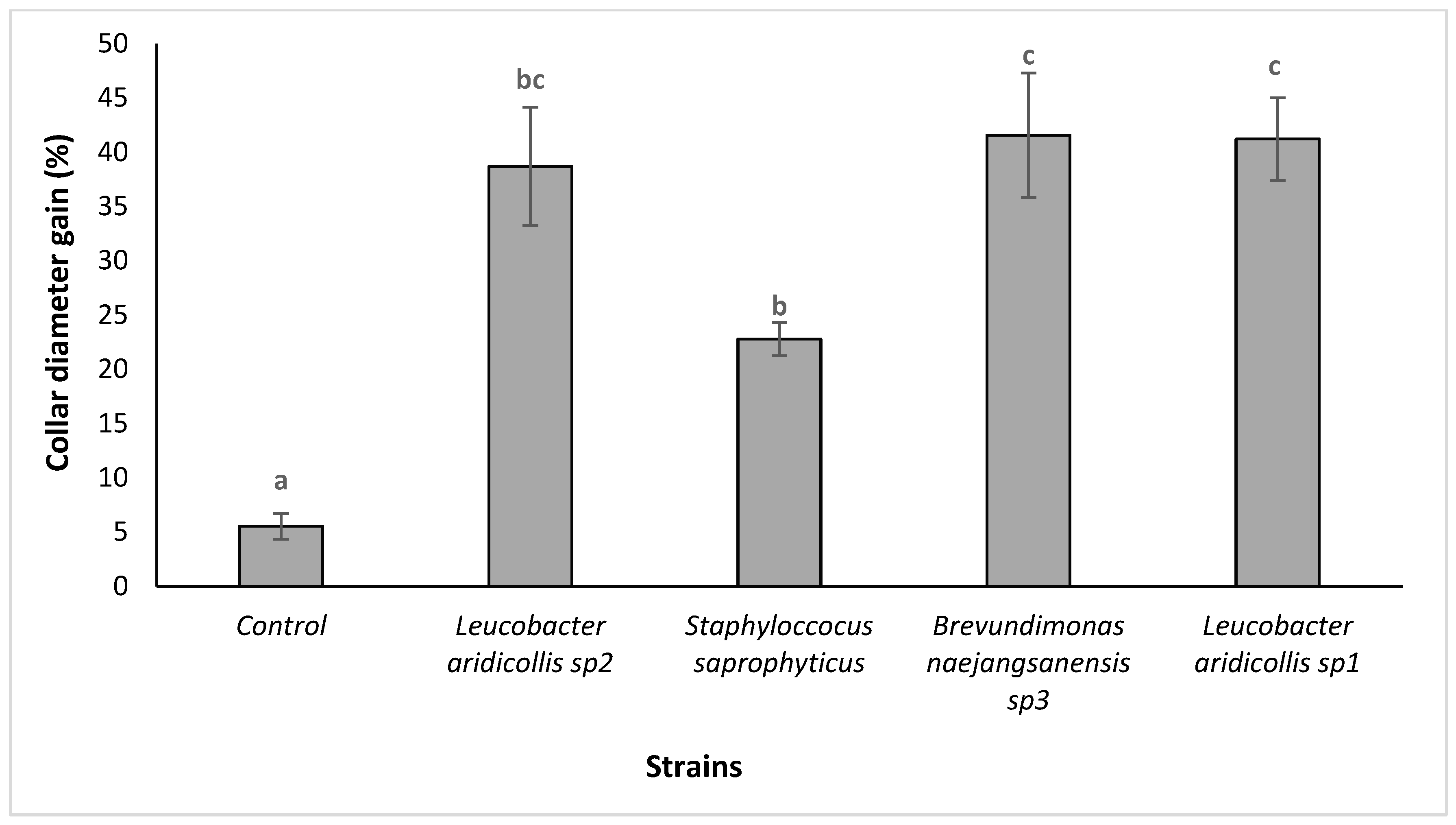


| Isolates | P-Solubilisation Halo (cm) | IAA Production | Isolates | P-Solubilisation Halo (cm) | IAA Production |
|---|---|---|---|---|---|
| CN001 | 1.40 ± 0.00 | + | CN083 | 0.86 ± 0.09 | − |
| CN002 | 1.63 ± 0.12 | + | CN099 | 0.90 ± 0.00 | − |
| CN003 | 0.56 ± 0.14 | + | CN076 | 0.96 ± 0.05 | − |
| CN004 | 1.87 ± 0.05 | + | CN061 | 1.07 ± 0.09 | − |
| CN005 | 1.33 ± 0.17 | + | CN118 | 1.07 ± 0.17 | − |
| CN006 | 1.43 ± 0.05 | + | CN101 | 1.10 ± 0.14 | − |
| CN007 | 1.83 ± 0.12 | + | CN120 | 1.13 ± 0.09 | − |
| CN008 | 1.47 ± 0.17 | + | CN119 | 1.17 ± 0.24 | − |
| CN009 | 2.23 ± 0.05 | + | CN074 | 1.20 ± 0.08 | − |
| CN010 | 2.90 ± 0.08 | + | CN114 | 1.23 ± 0.05 | − |
| CN011 | 1.40 ± 0.00 | + | CN088 | 1.27 ± 0.05 | − |
| CN012 | 1.27 ± 0.05 | + | CN097 | 1.30 ± 0.08 | − |
| CN013 | 1.40 ± 0.08 | + | CN102 | 1.33 ± 0.05 | − |
| CN014 | 1.30 ± 0.00 | + | CN100 | 1.37 ± 0.05 | − |
| CN015 | 1.73 ± 0.09 | + | CN064 | 1.40 ± 0.08 | − |
| CN016 | 1.27 ± 0.12 | + | CN098 | 1.47 ± 0.05 | − |
| CN017 | 0.87 ± 0.09 | + | CN089 | 1.53 ± 0.05 | − |
| CN018 | 2.00 ± 0.16 | + | CN071 | 1.55 ± 0.74 | − |
| CN019 | 2.00 ± 0.00 | + | CN108 | 1.67 ± 0.24 | − |
| CN020 | 1.90 ± 0.00 | + | CN086 | 1.80 ± 0.00 | − |
| CN021 | 1.40 ± 0.08 | + | CN052 | 1.86 ± 0.05 | − |
| CN022 | 1.15 ± 0.05 | + | CN080 | 1.90 ± 0.90 | − |
| CN023 | 1.30 ± 0.08 | + | CN069 | 2.00 ± 0.24 | − |
| CN024 | 2.00 ± 0.16 | + | CN115 | 2.03 ± 0.05 | − |
| CN025 | 1.10 ± 0.00 | + | CN109 | 2.83 ± 0.12 | − |
| CN096 | 0.80 ± 0.08 | − | |||
| CN029 | 0.83 ± 0.17 | − |
| Strains | Strains | P Solubilization (µg/mL) | IAA Production (µg/mL) | HCN Production | N2 Fixation | Ammonia Production | Siderophore Production |
|---|---|---|---|---|---|---|---|
| CN011 | Staphyloccocus saprophyticus | 49.11 ± 0.76 | 137.14 ± 0.33 | − | − | + | + |
| CN006 | Brevundimonas naejangsanensis sp3 | 36.21 ± 0.11 | 27.83 ± 0.49 | − | − | + | + |
| CN009 | Leucobacter aridicollis sp2 | 42.30 ± 0.77 | 97.47 ± 1.65 | + | − | + | + |
| CN008 | Leucobacter aridicollis sp1 | 41.96 ± 0.89 | 60.29 ± 4.66 | − | + | + | + |
| Isolates | CN011 | CN006 | CN009 | CN008 | |
|---|---|---|---|---|---|
| Strains | Staphyloccocus saprophyticus | Brevundimonas naejangsanensis sp3 | Leucobacter aridicollis sp2 | Leucobacter aridicollis sp1 | |
| Colony color | Yellow | White | White | White | |
| Colony texture | Smooth | Smooth | Smooth | Smooth | |
| Gram stain | − | + | + | + | |
| Catalase | + | + | + | + | |
| Oxidase | + | − | − | − | |
| Motility | − | − | − | − | |
| Shape | Cocci | Bacilli | Bacilli | Bacilli | |
| Temperature | 4 °C | − | + | + | − |
| 10 °C | − | + | + | + | |
| 30 °C | + | + | + | + | |
| 55 °C | + | − | − | − | |
| NaCl | 5 g/L | + | + | + | + |
| 10 g/L | + | + | + | + | |
| 20 g/L | + | + | + | + | |
| 40 g/L | + | + | + | − | |
| pH | 4 | + | + | + | + |
| 5 | + | + | + | + | |
| 7 | + | + | + | + | |
| 10 | + | + | + | + | |
| 12 | + | + | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabbi, N.; Chafiki, S.; Telmoudi, M.; Labbassi, S.; Bouharroud, R.; Tahiri, A.; Mentag, R.; El Amri, M.; Bendiab, K.; Hsissou, D.; et al. Plant-Growth-Promoting Rhizobacteria Improve Seeds Germination and Growth of Argania spinosa. Plants 2024, 13, 2025. https://doi.org/10.3390/plants13152025
Chabbi N, Chafiki S, Telmoudi M, Labbassi S, Bouharroud R, Tahiri A, Mentag R, El Amri M, Bendiab K, Hsissou D, et al. Plant-Growth-Promoting Rhizobacteria Improve Seeds Germination and Growth of Argania spinosa. Plants. 2024; 13(15):2025. https://doi.org/10.3390/plants13152025
Chicago/Turabian StyleChabbi, Naima, Salahddine Chafiki, Maryem Telmoudi, Said Labbassi, Rachid Bouharroud, Abdelghani Tahiri, Rachid Mentag, Majda El Amri, Khadija Bendiab, Driss Hsissou, and et al. 2024. "Plant-Growth-Promoting Rhizobacteria Improve Seeds Germination and Growth of Argania spinosa" Plants 13, no. 15: 2025. https://doi.org/10.3390/plants13152025





