An Analysis of Capsaicin, Dihydrocapsaicin, Vitamin C and Flavones in Different Tissues during the Development of Ornamental Pepper
Abstract
:1. Introduction
2. Results
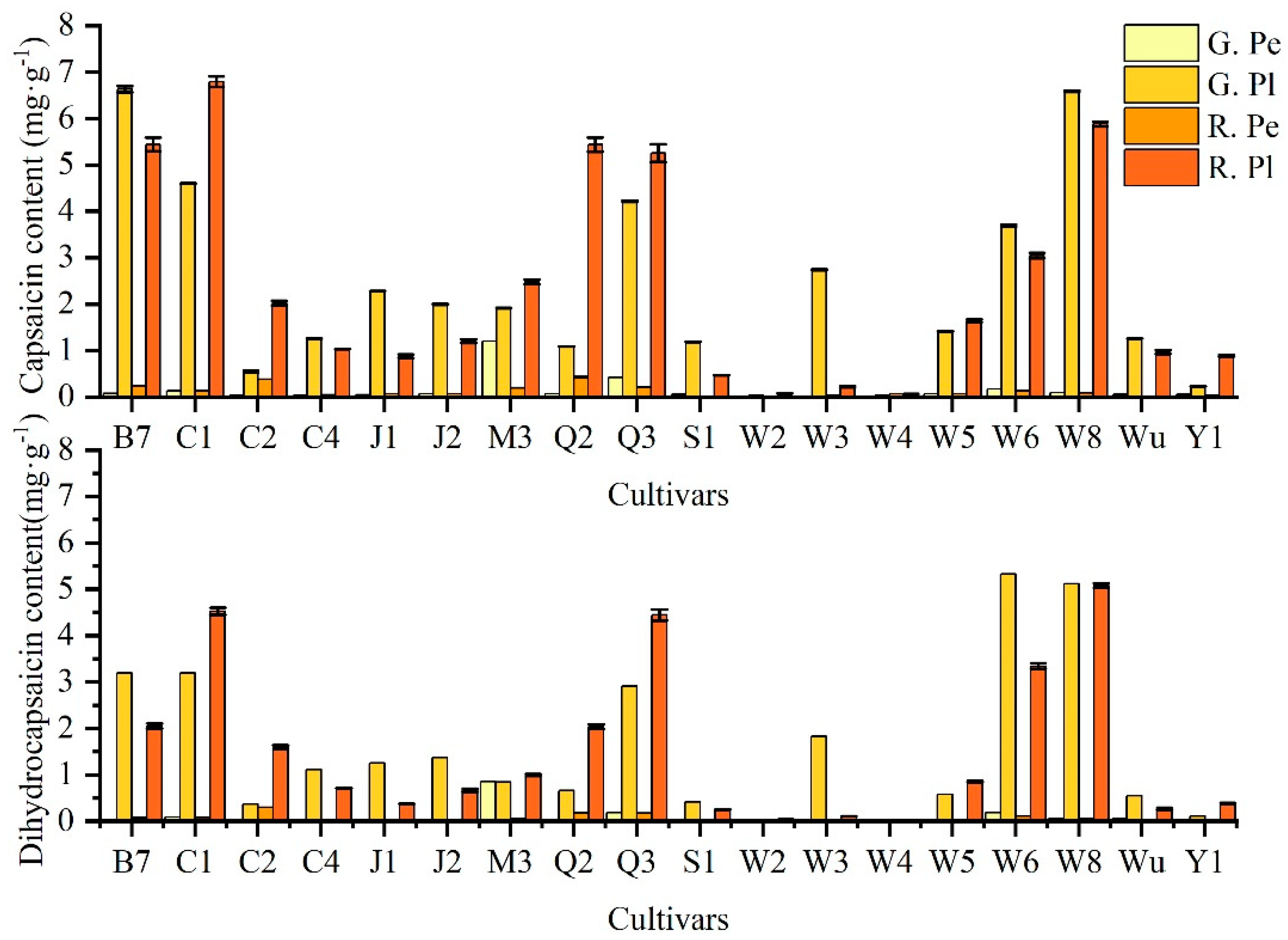
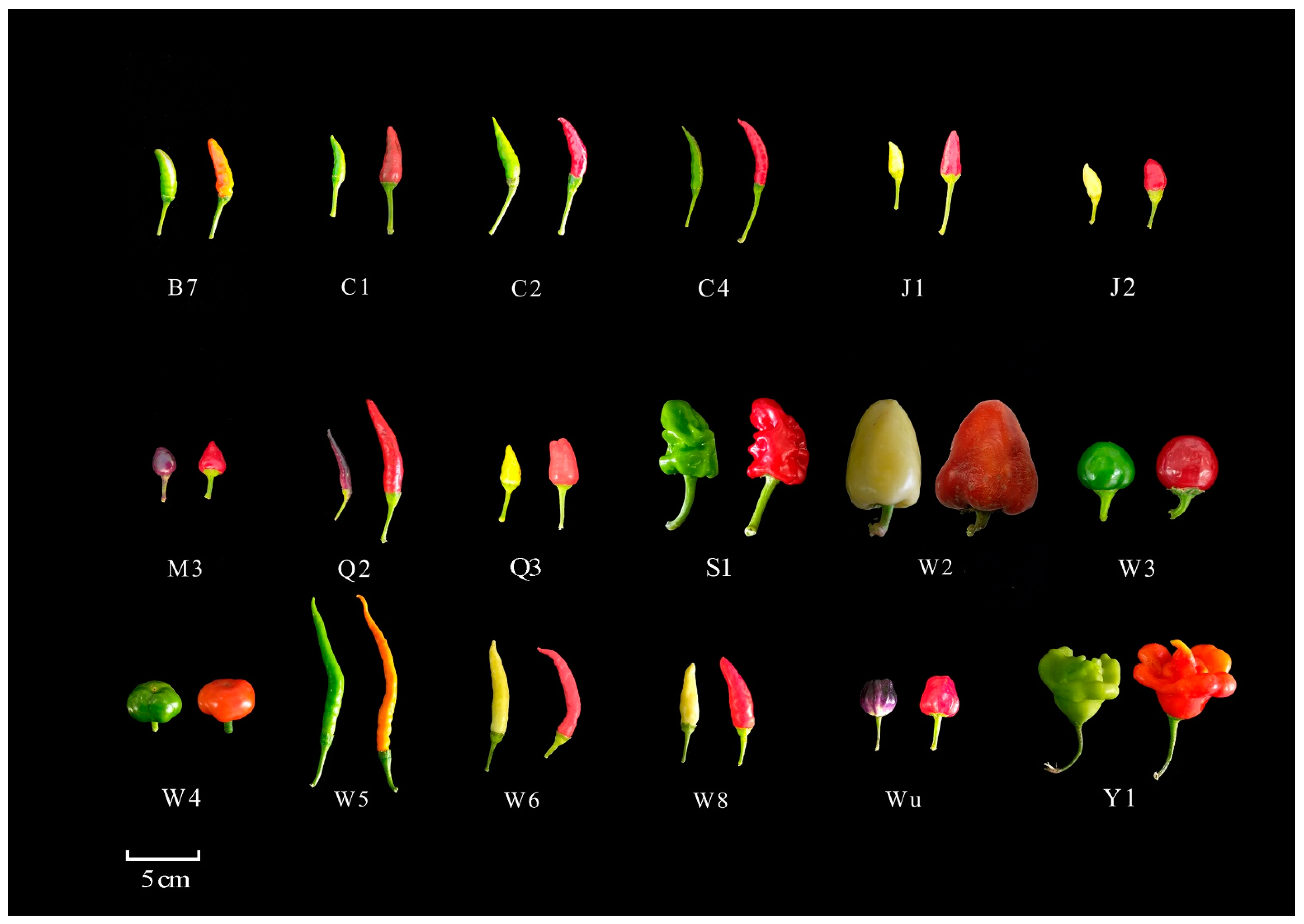
2.1. Analysis of Capsaicin and Dihydrocapsaicin Contents in Different Ornamental Peppers
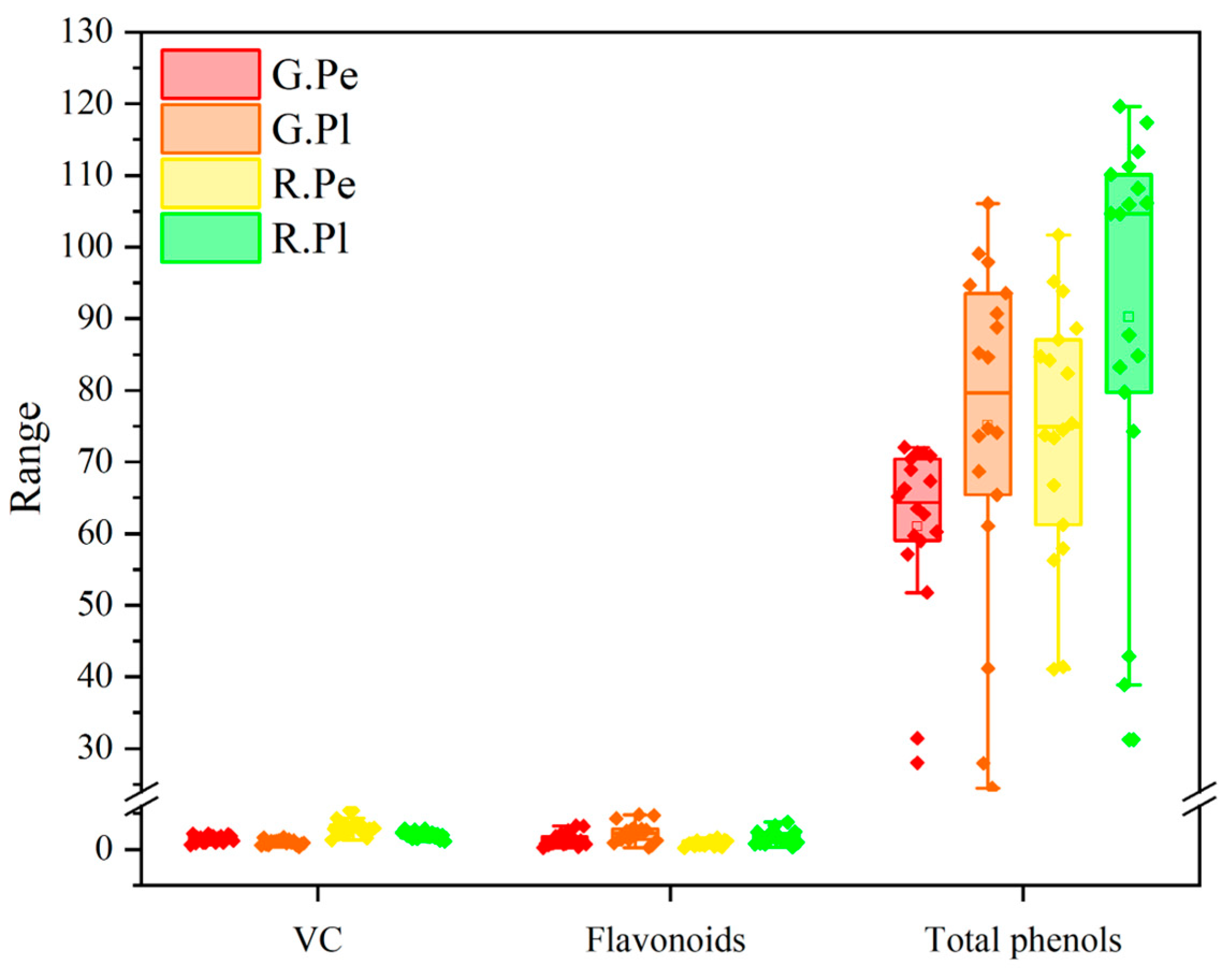
2.2. Analysis of Main Nutritional Quality of Different Ornamental Peppers
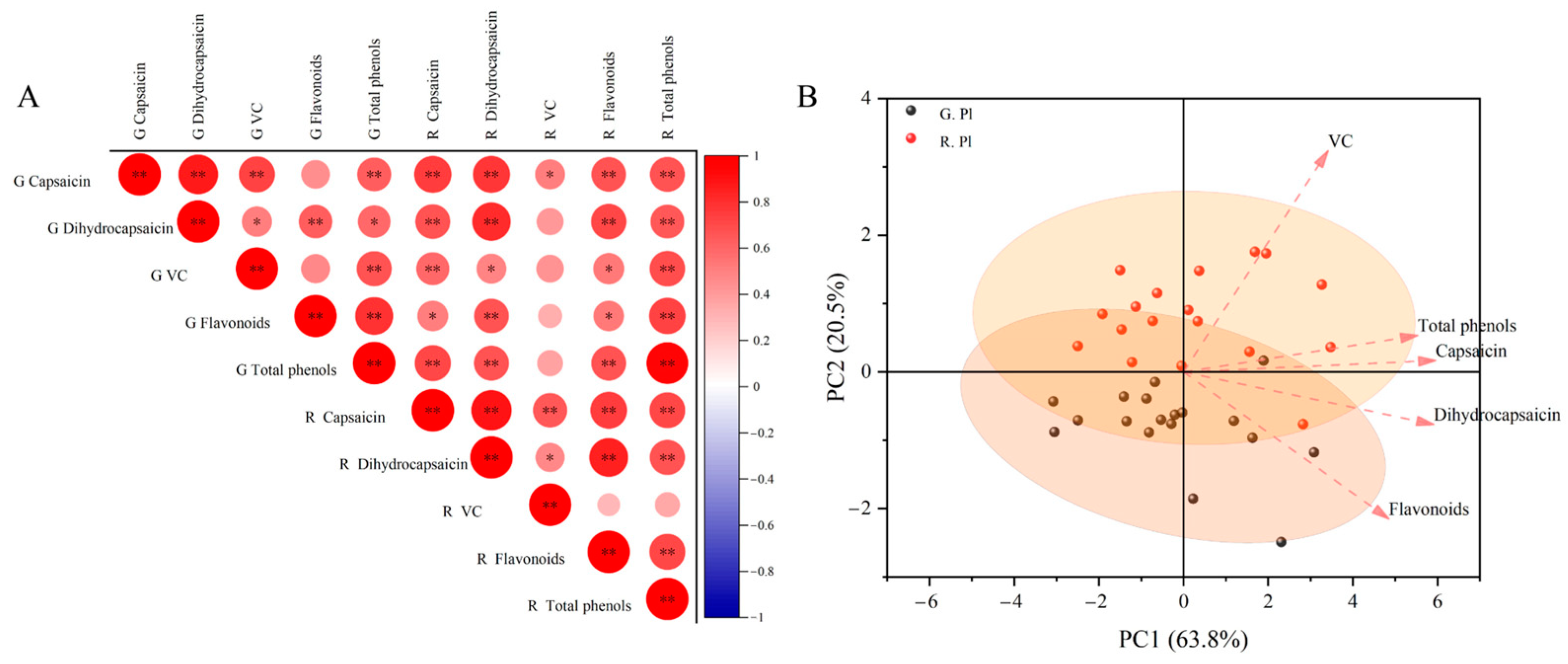
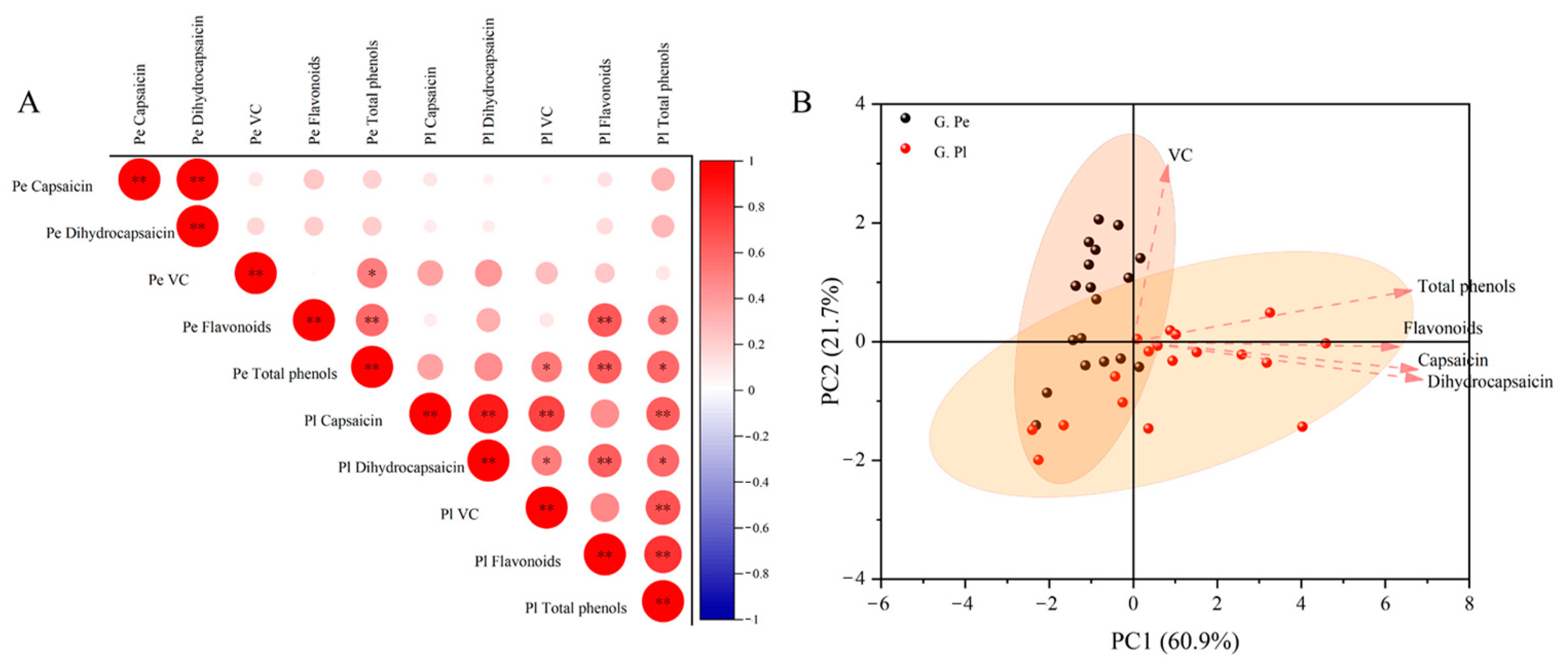
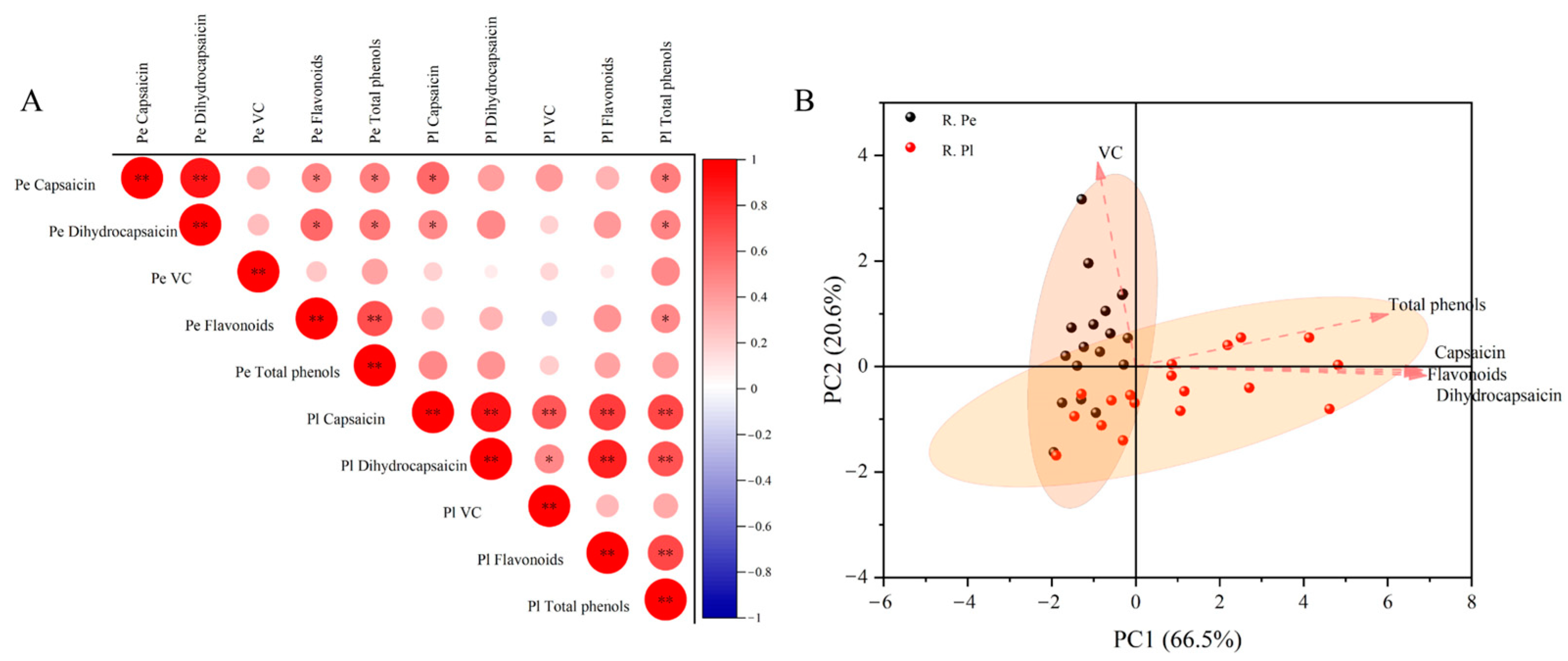
2.3. Correlation Analysis and Principal Component Analysis of Components in Different Developmental Stages of Ornamental Pepper
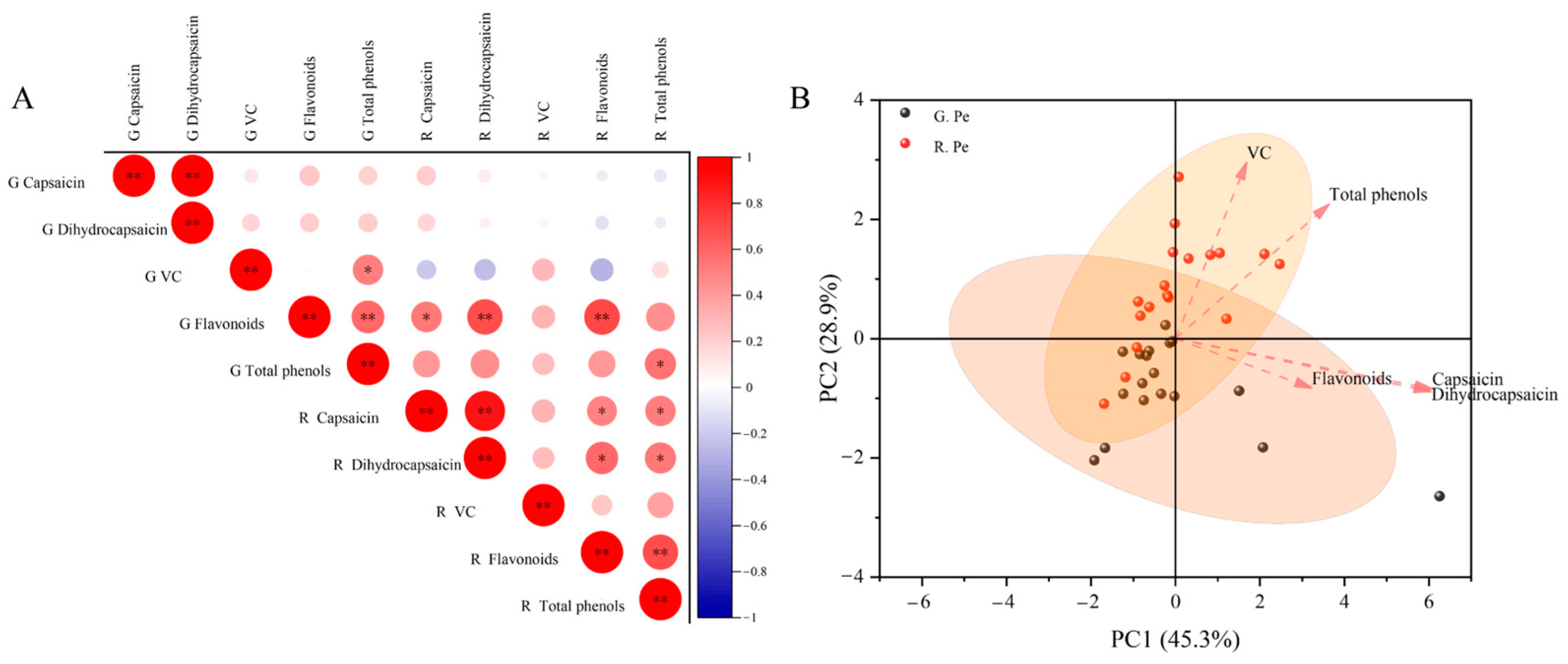
2.4. Correlation Analysis and Principal Component Analysis of Components in Different Tissue Parts of Ornamental Pepper
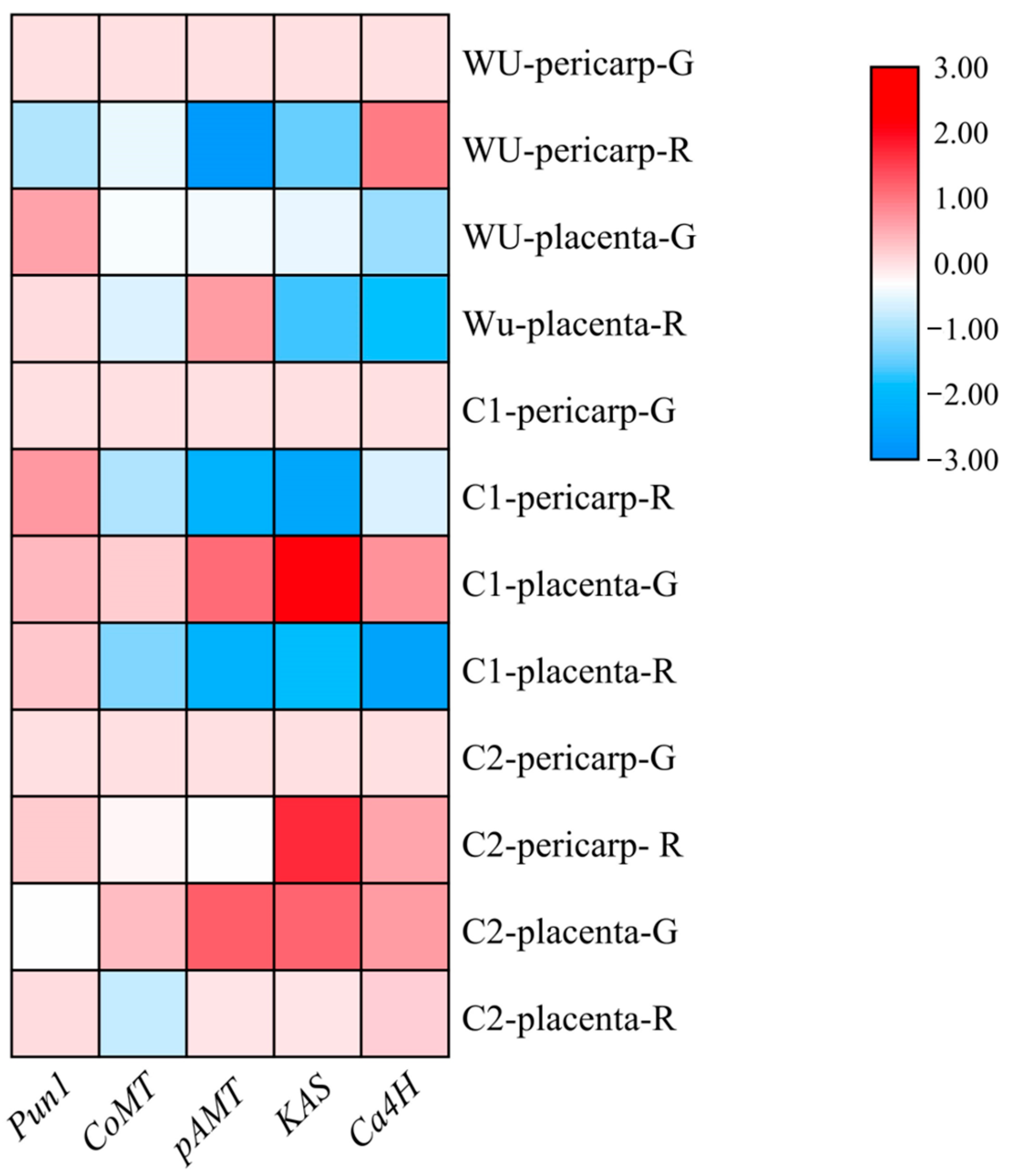
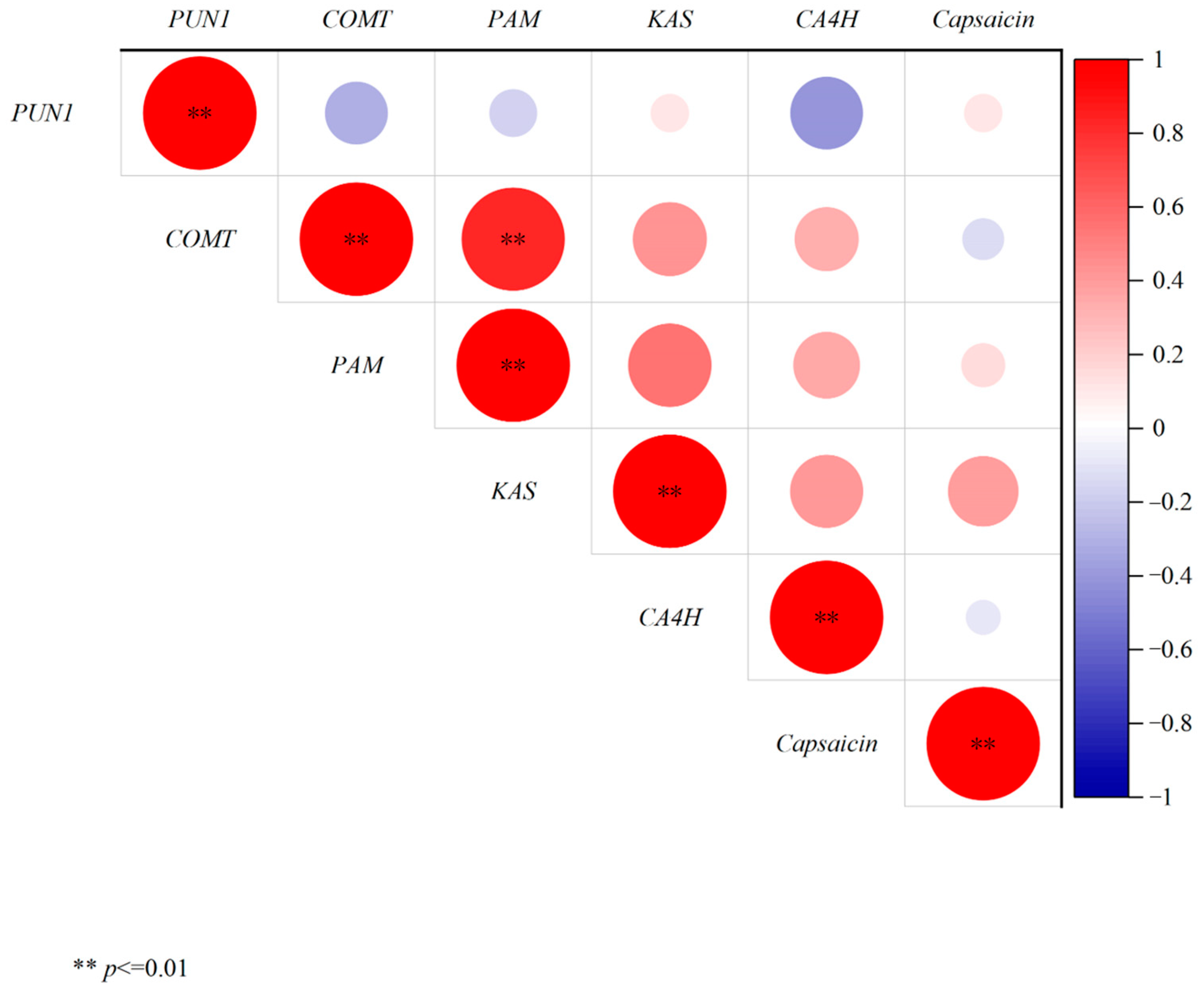
2.5. Analysis of Expression Patterns of Genes Related to Capsaicin Synthesis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Determination of Capsaicin and Dihydrocapsaicin
4.4. Determination of Nutrient Content
4.5. RNA Extraction, cDNA Synthesis and Real-Time RT-qPCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Bai, J.; Duan, X.; Wang, J. Accumulation characteristics of carotenoids and adaptive fruit color variation in ornamental pepper. Sci. Hortic. 2021, 275, 109699. [Google Scholar] [CrossRef]
- Li, J.; Ning, J.; Shuya, W.; Xin, M.; Yandong, X.; Zhaozhuang, L.; Guobin, Z.; Xueyun, Y.; Jian, L.; Yuan, Z.; et al. Comparing the morphological characteristics and nutritional composition of 23 pepper (Capsicum annuum L.) varieties. Eur. Food Res. Technol. 2022, 249, 963–974. [Google Scholar]
- Lois, O.T.; Jide, A.A. Comparison of nutritional, antioxidant vitamins and capsaicin contents in Capsicum annuum and C. frutescens. Int. J. Veg. Sci. 2020, 26, 190–207. [Google Scholar]
- Anandakumar, P.; Sattu, K.; Sundaram, J.; Gopalakrishnan, R.; Asokkumar, S.; Chandrashekar, N.; Subramanian, R.; Vanitha, M.; Devaki, T. The Anticancer Role of Capsaicin in Experimentallyinduced Lung Carcinogenesis. J. Pharmacopunct. 2015, 18, 19–25. [Google Scholar] [CrossRef]
- Hall, O.M.; Broussard, A.; Range, T.; Carroll Turpin, M.A.; Ellis, S.; Lim, V.M.; Cornett, E.M.; Kaye, A.D. Novel Agents in Neuropathic Pain, the Role of Capsaicin: Pharmacology, Efficacy, Side Effects, Different Preparations. Curr. Pain Headache Rep. 2020, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Sevilla, F.; Jiménez, A.; del Río, L.A.; Corpas, F.J.; Álvarez de Morales, P.; Camejo, D.M. Physiology of pepper fruit and the metabolism of antioxidants: Chloroplasts, mitochondria and peroxisomes. Ann. Bot. 2015, 116, 627–636. [Google Scholar] [CrossRef]
- Wätjen, A.; Huyskens-Keil, S.; Stöber, S. Nutritional Assessment of Indonesian Chilli Landraces (Capsicum chinense Jaqc.). IOP Conf. Ser. Earth Environ. Sci. 2021, 748, 012033. [Google Scholar] [CrossRef]
- Luján-Méndez, F.; Roldán-Padrón, O.; Castro-Ruíz, J.E.; López-Martínez, J.; García-Gasca, T. Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale. Cells 2023, 12, 2573. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary metabolites of Capsicum species and their importance in the human diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Mostafakamal, S.; Esra, A.Y.; Guleray, A.; Melek, E.; Raziye, K.; Metin, T.; Ertan, Y. Biosynthesis of capsaicinoids in pungent peppers under salinity stress. Physiol. Plant. 2023, 175, e13889. [Google Scholar]
- Sun, B.; Chen, C.; Song, J.; Zheng, P.; Wang, J.; Wei, J.; Cai, W.; Chen, S.; Cai, Y.; Yuan, Y.; et al. The Capsicum MYB31 regulates capsaicinoid biosynthesis in the pepper pericarp. Plant Physiol. Biochem. 2022, 176, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.B.; Lopes de Macêdo, I.Y.; Campos, H.M.; Gonçalves Moreno, E.K.; Batista Silva, M.F.; Raimundo de Oliveira Neto, J.; Feitosa Ramalho, R.R.; Nascimento, A.D.R.; Vaz, B.G.; Carlos da Cunha, L.; et al. Antioxidant activity of thirty-six peppers varieties and vasorelaxant of selected varieties. Food Biosci. 2021, 41, 100989. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Álvarez-Romero, M.; González-de-Peredo, A.V.; Ruíz-Rodríguez, A.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers (Capsicum annuum L.) throughout the Ripening of the Fruit at Two Different Stages of Plant Maturation. Agronomy 2023, 13, 435. [Google Scholar] [CrossRef]
- Materska, M. Bioactive phenolics of fresh and freeze-dried sweet and semi-spicy pepper fruits (Capsicum annuum L.). J. Funct. Foods 2014, 7, 269–277. [Google Scholar] [CrossRef]
- Kim, S.; Park, M.; Yeom, S.-I.; Kim, Y.-M.; Lee, J.M.; Lee, H.-A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.-T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Stewart, C., Jr.; Mazourek, M.; Stellari, G.M.; O’Connell, M.; Jahn, M. Genetic control of pungency in C. chinense via the Pun1 locus. J. Exp. Bot. 2007, 58, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, J.; Xie, J.; Gan, Y.; Coulter, J.A.; Yu, J.; Li, J.; Wang, J.; Zhang, X. Nitrogen Source Affects the Composition of Metabolites in Pepper (Capsicum annuum L.) and Regulates the Synthesis of Capsaicinoids through the GOGAT–GS Pathway. Foods 2020, 9, 150. [Google Scholar] [CrossRef]
- Mazourek, M.; Pujar, A.; Borovsky, Y.; Paran, I.; Mueller, L.; Jahn, M.M. A dynamic interface for capsaicinoid systems biology. Plant Physiol. 2009, 150, 1806–1821. [Google Scholar] [CrossRef]
- Garcés-Claver, A.; Fellman, S.M.; Gil-Ortega, R.; Jahn, M.; Arnedo-Andrés, M.S. Identification, validation and survey of a single nucleotide polymorphism (SNP) associated with pungency in Capsicum spp. TAG. Theor. Appl. Genet. Theor. Und Angew. Genet. 2007, 115, 907–916. [Google Scholar] [CrossRef]
- Sánchez, E.; Hellín, P.; Rodríguez-Burruezo, A.; Gomariz, J.; Garrido, I.; Cava, J.; Molina, M.V.; Fenoll, J.; Flores, P. Evaluation of traditional genotypes of pepper (Capsicum annuum) for vitamin C content. Acta Hortic. 2018, 1194, 979–984. [Google Scholar] [CrossRef]
- Agostini-Costa, T.d.S.; Gomes, I.d.S.; Melo, L.A.M.P.d.; Reifschneider, F.J.B.; Ribeiro, C.S.d.C. Carotenoid and total vitamin C content of peppers from selected Brazilian cultivars. J. Food Compos. Anal. 2017, 57, 73–79. [Google Scholar] [CrossRef]
- van Zonneveld, M.; Ramirez, M.; Williams, D.E.; Petz, M.; Meckelmann, S.; Avila, T.; Bejarano, C.; Ríos, L.; Peña, K.; Jäger, M.; et al. Screening Genetic Resources of Capsicum Peppers in Their Primary Center of Diversity in Bolivia and Peru. PLoS ONE 2015, 10, e0134663. [Google Scholar] [CrossRef]
- Lekala, C.S.; Madani, K.S.H.; Phan, A.D.T.; Maboko, M.M.; Fotouo, H.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Cultivar-specific responses in red sweet peppers grown under shade nets and controlled-temperature plastic tunnel environment on antioxidant constituents at harvest. Food Chem. 2019, 275, 85–94. [Google Scholar] [CrossRef]
- Yoshiyuki, T.; Mayuko, W.; Wakana, N.; Tanjuro, G.; Yuichi, Y.; KenIchiro, Y.; Sho, O.; Motoaki, D. Capsaicinoid biosynthesis in the pericarp of chili pepper fruits is associated with a placental septum-like transcriptome profile and tissue structure. Plant Cell Rep. 2021, 40, 1859–1874. [Google Scholar]
- Govindarajan, V.S. Capsicum production, technology, chemistry, and quality. Part 1: History, botany, cultivation, and primary processing. Crit. Rev. Food Sci. Nutr. 1985, 22, 109–176. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. The effect of ripening on the capsaicinoids composition of Jeromin pepper (Capsicum annuum L.) at two different stages of plant maturity. Food Chem. 2023, 399, 133979. [Google Scholar] [CrossRef]
- Iwai, K.; Suzuki, T.; Fujiwake, H. Formation and Accumulation of Pungent Principle of Hot Pepper Fruits, Capsaicin and Its Analogues, in Capsicum annuun var. annuun cv. Karayatsubusa at Different Growth Stages after Flowering. Agric. Biol. Chem. 2014, 43, 2493–2498. [Google Scholar]
- Lima, M.F.; Carvalho, S.I.C.; Ragassi, C.F.; Bianchetti, L.B.; Faleiro, F.G.; Reifschneider, F.J.B. Characterization of a pepper collection (Capsicum frutescens L.) from Brazil. Genet Mol. Res. 2017, 16, gmr16039704. [Google Scholar] [CrossRef]
- Oney Montalvo, J.E.; de Silva Madrigal, A.C.; Ramírez Sucre, M.O.; Rodríguez-Buenfil, I.M. Effect of the Soil and Ripening Stage in Capsicum chinense var. Jaguar on the Content of Carotenoids and Vitamins. Horticulturae 2021, 7, 442. [Google Scholar] [CrossRef]
- Karaman, K.; Pinar, H.; Ciftci, B.; Kaplan, M. Characterization of phenolics and tocopherol profile, capsaicinoid composition and bioactive properties of fruits in interspecies (Capsicum annuum X Capsicum frutescens) recombinant inbred pepper lines (RIL). Food Chem. 2023, 423, 136173. [Google Scholar] [CrossRef]
- Malik, A.A.; Malik, G.; Hussain, K.; Narayan, S.; Mufti, S.; Kumar, A.; Nazir, G.; Masoodi, U.; Magray, M. Characterization of Bioactive Compounds and Antioxidant Activity among Genetically Different Genotypes of Chilli (Capsicum annum L.). Int. J. Plant Soil Sci. 2022, 34, 384–393. [Google Scholar] [CrossRef]
- García-González, C.A.; Silvar, C. Phytochemical Assessment of Native Ecuadorian Peppers (Capsicum spp.) and Correlation Analysis to Fruit Phenomics. Plants 2020, 9, 986. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Li, Y.; Feng, X.; Song, L.; Gao, H.; Cao, B. Fruit morphological and nutritional quality features of goji berry (Lycium barbarum L.) during fruit development. Sci. Hortic. 2023, 308, 111555. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, Y.; Liu, Y.; Shi, Y.; Lin, X.; Wang, L.; Wen, P.; Hu, X.; Li, J. Comprehensive evaluation of aroma and taste properties of different parts from the wampee fruit. Food Chem. X 2023, 19, 100835. [Google Scholar] [CrossRef]
- Keyhaninejad, N.; Curry, J.; Romero, J.; O’Connell, M.A. Fruit specific variability in capsaicinoid accumulation and transcription of structural and regulatory genes in Capsicum fruit. Plant Sci. 2014, 215–216, 59–68. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakashima, F.; Kirii, E.; Goto, T.; Yoshida, Y.; Yasuba, K.I. Difference in capsaicinoid biosynthesis gene expression in the pericarp reveals elevation of capsaicinoid contents in chili peppers (Capsicum chinense). Plant Cell Rep. 2017, 36, 267–279. [Google Scholar] [CrossRef]
- Wei, Z.; Dan, W.; Liping, Z.; Chengzhi, Z.; Huangying, S.; Shanhan, C.; Zhiwei, W.; Jie, Z.; Pingwu, L. Identification and expression analysis of capsaicin biosynthesis pathway genes at genome level in Capsicum chinense. Biotechnol. Biotechnol. Equip. 2022, 36, 232–244. [Google Scholar]
- Kusaka, H.; Nakasato, S.; Sano, K.; Kobata, K.; Ohno, S.; Doi, M.; Tanaka, Y. An evolutionary view of vanillylamine synthase pAMT, a key enzyme of capsaicinoid biosynthesis pathway in chili pepper. Plant J. 2024, 117, 1453–1465. [Google Scholar] [CrossRef]
- Stewart, C., Jr.; Kang, B.C.; Liu, K.; Mazourek, M.; Moore, S.L.; Yoo, E.Y.; Kim, B.D.; Paran, I.; Jahn, M.M. The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J. 2005, 42, 675–688. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, K.; Yu, H.; Chen, S.; Xu, D.; Zhao, H.; Zhang, Z.; Yang, Y.; Gu, X.; Liu, X.; et al. Pepper variome reveals the history and key loci associated with fruit domestication and diversification. Mol. Plant 2022, 15, 1744–1758. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Y.; Bi, R.; Sun, N.; Yuan, W.; Li, Y.; Wang, J.; Guo, Q. Cloning and Expression of Pun1 Gene Controlling Pungency of Pepper (Capsicum spp.). Agric. Sci. Technol. 2016, 17, 2483. [Google Scholar]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. Silencing AT3 gene reduces the expression of pAmt, BCAT, Kas, and Acl genes involved in capsaicinoid biosynthesis in chili pepper fruits. Biol. Plant. 2015, 59, 477–484. [Google Scholar] [CrossRef]
- Kondo, F.; Hatakeyama, K.; Sakai, A.; Minami, M.; Nemoto, K.; Matsushima, K. The pungent-variable sweet chili pepper ‘Shishito’ (Capsicum annuum) provides insights regarding the relationship between pungency, the number of seeds, and gene expression involving capsaicinoid biosynthesis. Mol. Genet. Genom. 2021, 296, 591–603. [Google Scholar] [CrossRef]
- Ogawa, K.; Murota, K.; Shimura, H.; Furuya, M.; Togawa, Y.; Matsumura, T.; Masuta, C. Evidence of capsaicin synthase activity of the Pun1-encoded protein and its role as a determinant of capsaicinoid accumulation in pepper. BMC Plant Biol. 2015, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive characteristics and antioxidant activities of nine peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Ya, G. Discussion about NaNO2-Al(NO3)3-NaOH Colorimetry for Determination of Total Flavonoids. Chin. J. Pharm. Anal. 2002, 22, 97–98. [Google Scholar]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
| Genes | Accession No. | Primer Sequence | Length (bp) |
|---|---|---|---|
| Pun1 | GU300812.1 | F:GTGATGGTTGCTCTCTGCTT R:GTGGCGTAATGAGAGAACCA | 144 |
| CoMT | AF081214.1 | F:GAGCCTAACTCAAACAGAGGA R:GGTCAAGTTCTACGGTTGCTT | 101 |
| pAMT | AF085149.1 | F:TCTCTCTGGGCTTCCTCCAA R:TCTTCGGTTTCACCTGGCAA | 111 |
| KAS | AF085148.1 | F:ACGGACGCTTTTATTTCGGC R:CGGAGCCTTCACCAATGACA | 139 |
| Ca4H | AF088847.1 | F:CCGCTCACTGGAAGAAACC R:TCCTCCTACCAACACCGAA | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Duan, X.; An, Y.; He, J.; Li, J.; Xian, J.; Zhou, D. An Analysis of Capsaicin, Dihydrocapsaicin, Vitamin C and Flavones in Different Tissues during the Development of Ornamental Pepper. Plants 2024, 13, 2038. https://doi.org/10.3390/plants13152038
Wang J, Duan X, An Y, He J, Li J, Xian J, Zhou D. An Analysis of Capsaicin, Dihydrocapsaicin, Vitamin C and Flavones in Different Tissues during the Development of Ornamental Pepper. Plants. 2024; 13(15):2038. https://doi.org/10.3390/plants13152038
Chicago/Turabian StyleWang, June, Xudong Duan, Yu An, Jinyao He, Jiaxin Li, Jingqi Xian, and Daofen Zhou. 2024. "An Analysis of Capsaicin, Dihydrocapsaicin, Vitamin C and Flavones in Different Tissues during the Development of Ornamental Pepper" Plants 13, no. 15: 2038. https://doi.org/10.3390/plants13152038





