Morphological Changes to Fruit Development Induced by GA3 Application in Sweet Cherry (Prunus avium L.)
Abstract
:1. Introduction
2. Results
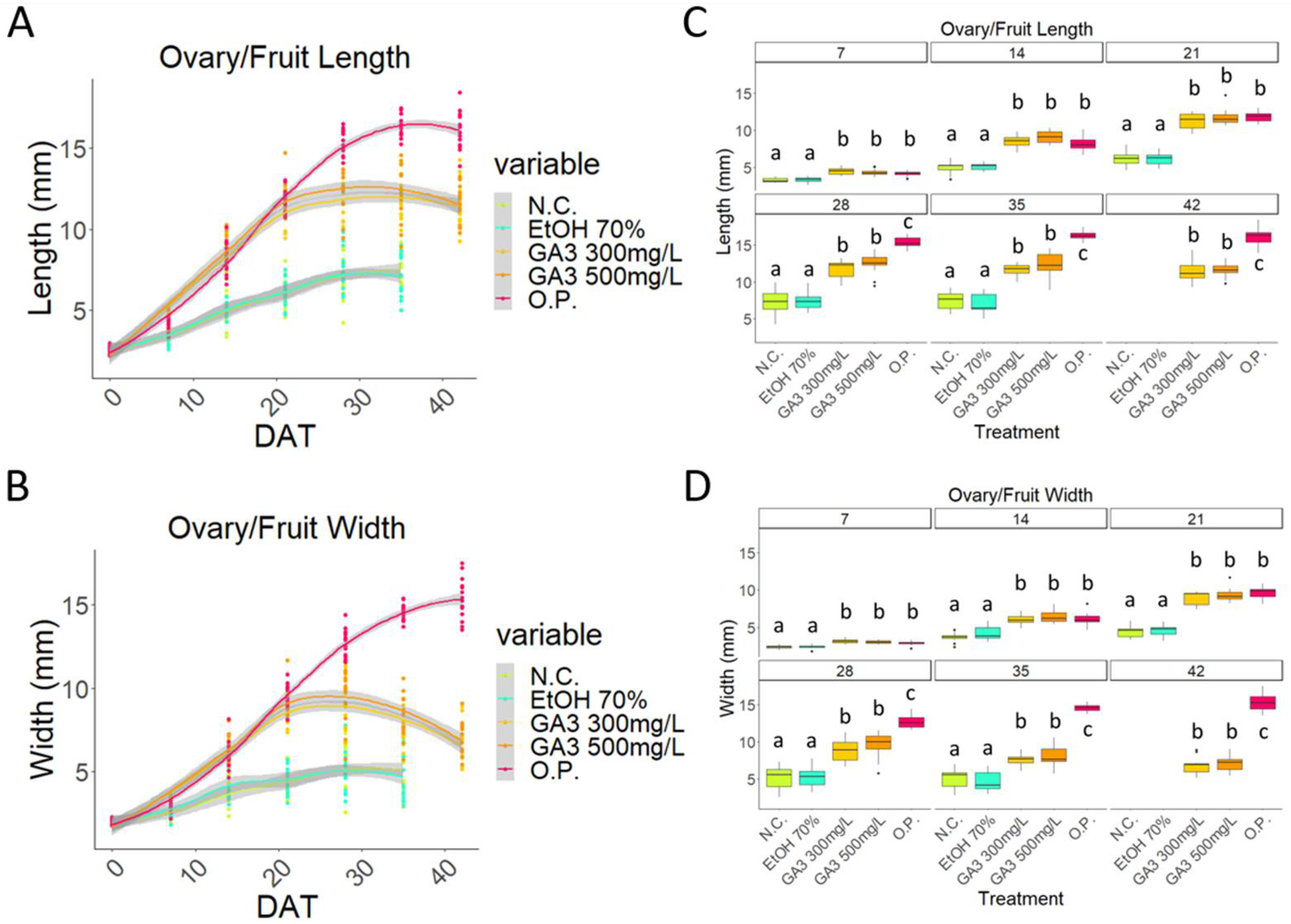
2.1. Exogenous Application of Gibberellins Induces Putative Fruit Development in Sweet Cherry
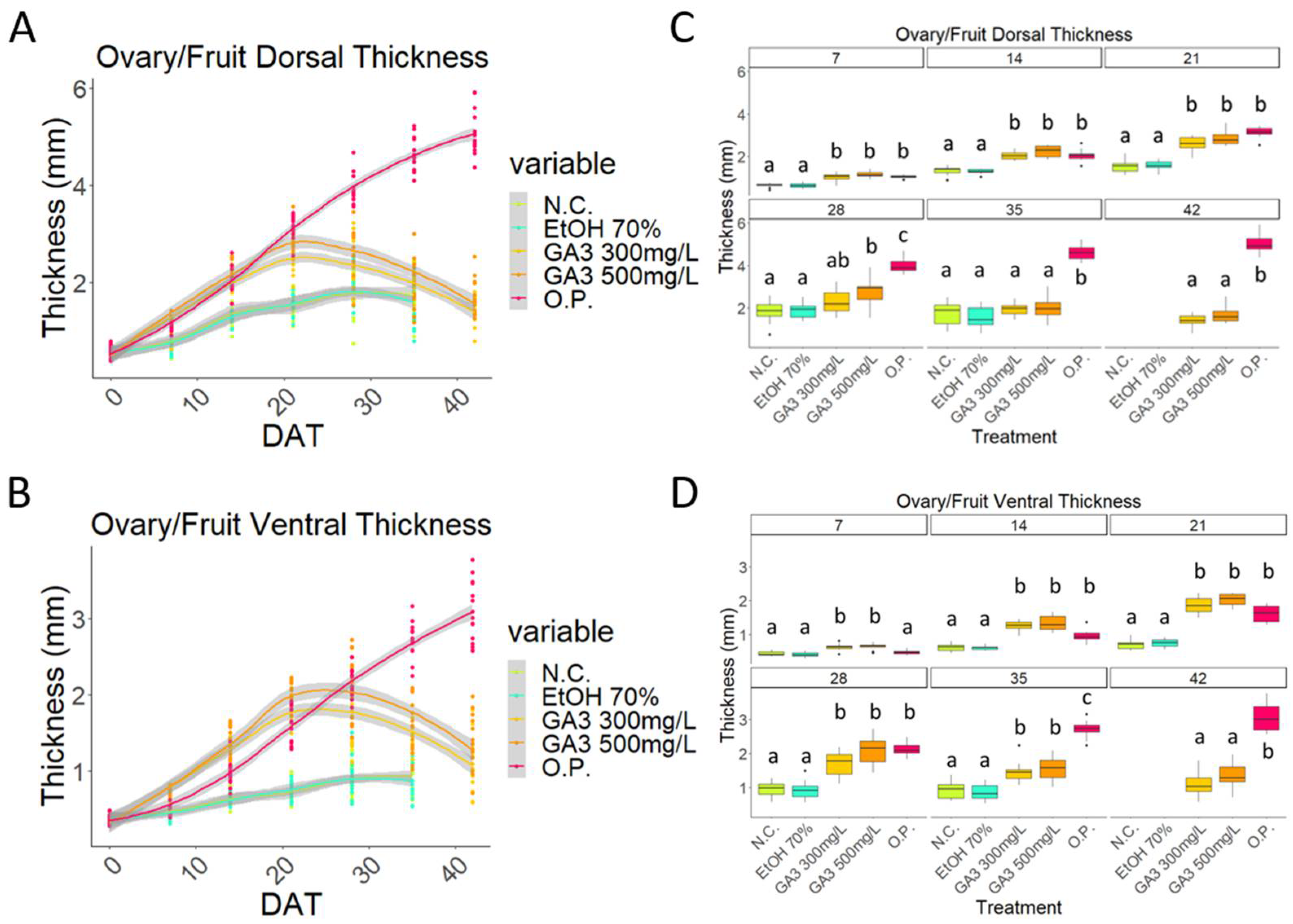
2.2. Initial Development of GA3-Treated Samples Follows That Observed in the Open-Pollinated Samples
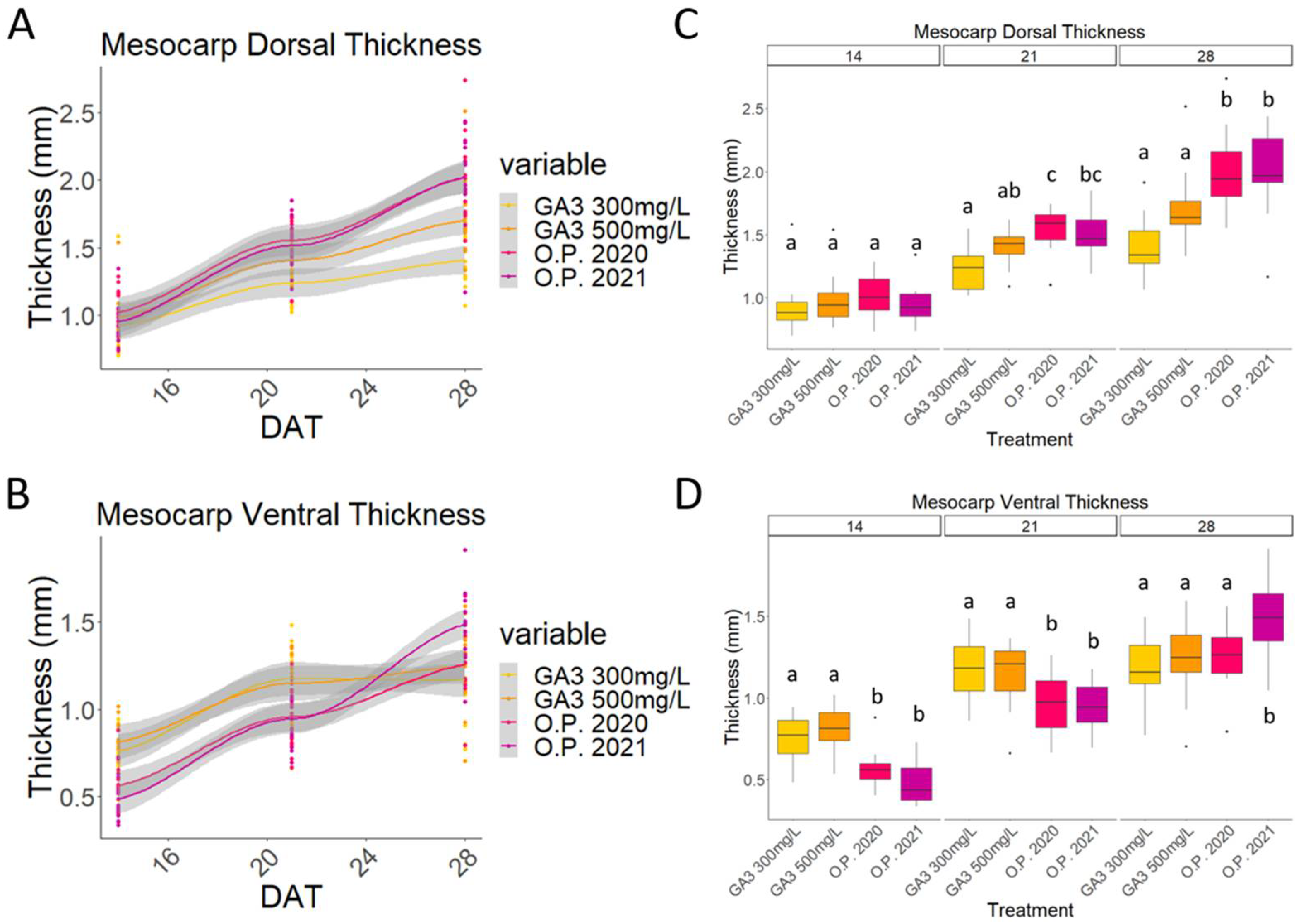
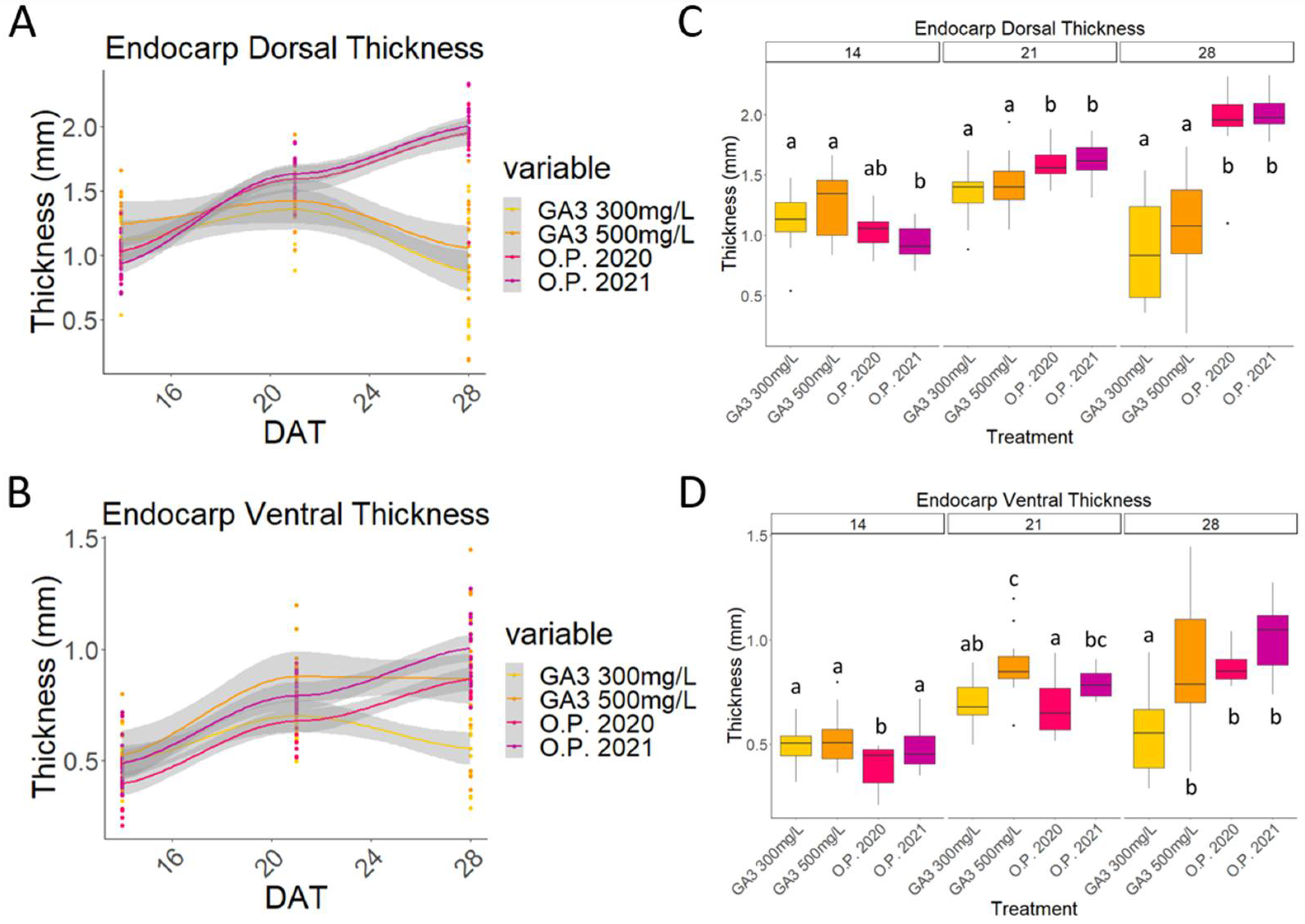
2.3. Mesocarp and Endocarp Development Fits the Pattern of a Double Sigmoid Curve
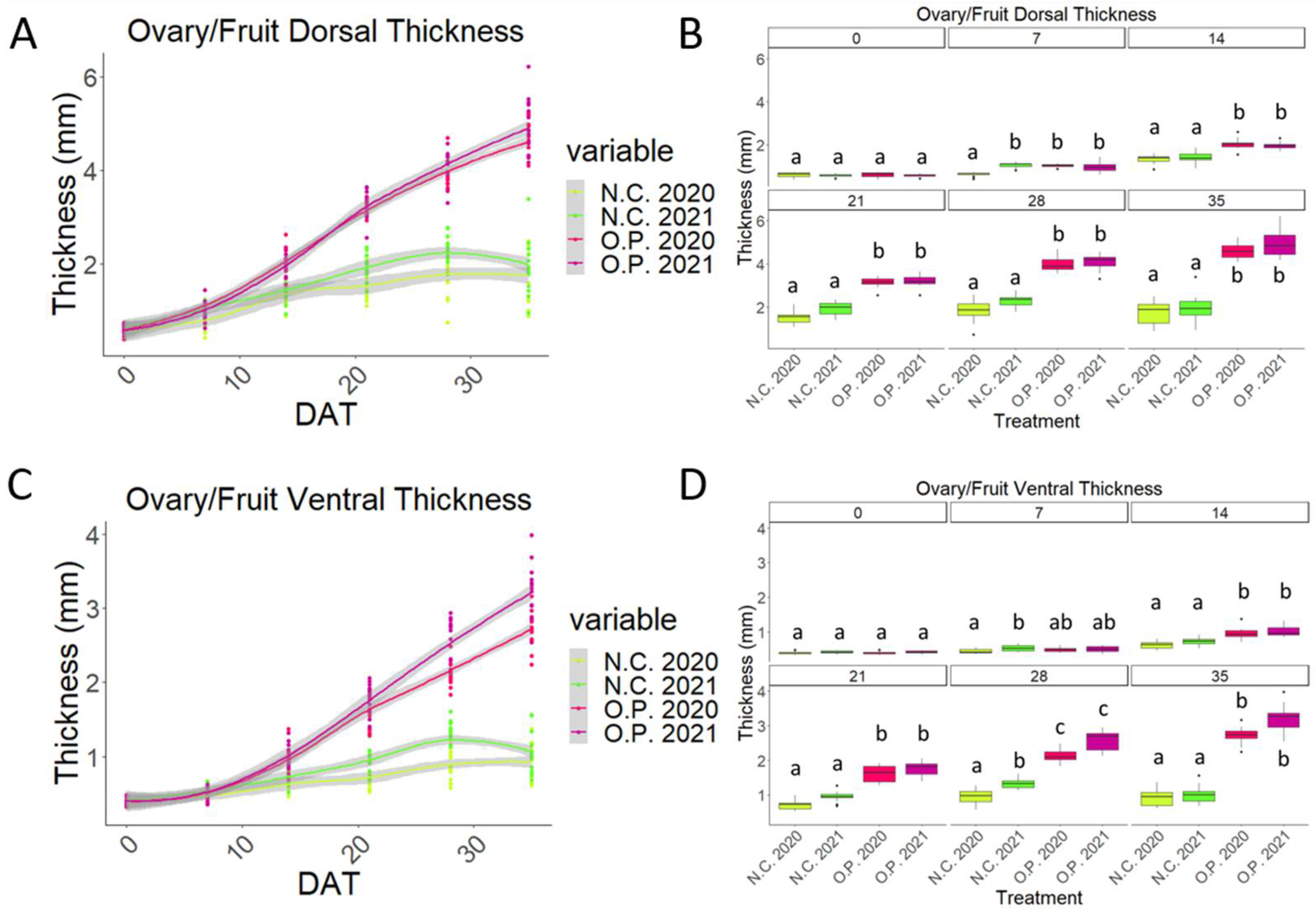
2.4. Impact of Environmental Differences in 2020 and 2021 on the Development of Cherry Fruit cv Regina
3. Discussion
3.1. GA3 Treatment Is Sufficient to Initiate Fruit Set in Sweet Cherry
3.2. The Arrest of the Growth of the GA3-Treated Developing Putative Fruits Begins after Twenty-One Days
3.3. Annual Environmentally Induced Variation in Fruit Expansion over Two Growing Seasons
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Growth Conditions
5.2. Hormone Solution Preparation and Treatments
5.3. Treatment Procedures
5.4. Sampling and Storage
5.5. Samples Photo Shooting and Measurements
5.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faust, M.; Surányi, D. Origin and dissemination of cherry. Hortic. Rev. 1997, 19, 263–317. [Google Scholar]
- Quero-García, J.; Schuster, M.; López-Ortega, G.; Charlot, G. Sweet cherry Varieties and Improvement; CABI International: Wallingford, UK, 2017; pp. 60–94. [Google Scholar]
- Vignati, E.; Lipska, M.; Dunwell, J.M.; Caccamo, M.; Simkin, A.J. Fruit development in sweet cherry. Plants 2022, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Bujdosó, G.; Hrotkó, K. Cherry Production; CABI International: Wallingford, UK, 2017; pp. 1–13. [Google Scholar]
- Ganopoulou, M.; Michailidis, M.; Angelis, L.; Ganopoulos, I.; Molassiotis, A.; Xanthopoulou, A.; Moysiadis, T. Could causal discovery in proteogenomics assist in understanding gene-protein relations? A perennial fruit tree case study using sweet cherry as a model. Cells 2022, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Kappel, F.; Fisher-Fleming, B.; Hogue, E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 1996, 31, 443–446. [Google Scholar] [CrossRef]
- Zheng, X.; Yue, C.; Gallardo, K.; McCracken, V.; Luby, J.; McFerson, J. What attributes are consumers looking for in sweet cherries? Evidence from choice experiments. Agric. Resour. Econ. Rev. 2016, 45, 124–142. [Google Scholar] [CrossRef]
- Guyer, D.E.; Sinha, N.K.; Chang, T.S.; Cash, J.N. Physiochemical and sensory characteristics of selected Michigan sweet cherry (Prunus avium L.) cultivars. J. Food Qual. 1993, 16, 355–370. [Google Scholar] [CrossRef]
- Cliff, M.A.; Dever, M.C.; Hall, J.W.; Giraud, B. Development and evaluation of multiple regression models for predicting sweet cherry liking. Food Res. Int. 1996, 28, 583–589. [Google Scholar] [CrossRef]
- Lyngstad, L.; Sekse, L. Economic aspects of developing a high sweet cherry product in Norway. ActaHortic. 1995, 379, 313–320. [Google Scholar] [CrossRef]
- Sekse, L.; Lyngstad, L. Strategies for maintaining high quality in sweet cherries during harvesting, handling and marketing. Acta Hortic. 1996, 410, 351–355. [Google Scholar] [CrossRef]
- Wermund, U.; Fearne, A. Key challenges facing the cherry supply chain in the UK. Acta Hortic. 2000, 536, 613–624. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Vignati, E.; Lipska, M.; Dunwell, J.M.; Caccamo, M.; Simkin, A.J. Options for the generation of seedless cherry, the ultimate snacking product. Planta 2022, 256, 90. [Google Scholar] [CrossRef]
- Gustafson, F.G. Parthenocarpy: Natural and artificial. Bot. Rev. 1942, 8, 599–654. [Google Scholar] [CrossRef]
- Picarella, M.E.; Mazzucato, A. The occurrence of seedlessness in higher plants; Insights on roles and mechanisms of parthenocarpy. Front. Plant Sci. 2019, 9, 1997. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, W.W.; Mills, J.J. Hormones and parthenocarpic fruit set: A literature survey. Hort. Abstr. 1981, 51, 661–698. [Google Scholar]
- Sjut, V.; Bangerth, F. Induced parthenocarpy—A way of changing the levels of endogenous hormones in tomato fruits (Lycopersicon esculentum Mill.) 1. Extractable hormones. Plant Growth Regul. 1982, 1, 243–251. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Hedden, P. Gibberellin biosynthesis in higher plants. Annu. Plant Rev. 2016, 49, 37–72. [Google Scholar]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Zang, Y.-X.; Chun, I.-J.; Zhang, L.-L.; Hong, S.-B.; Zheng, W.-W.; Xu, K. Effect of gibberellic acid application on plant growth attributes, return bloom, and fruit quality of rabbiteye blueberry. Sci. Hortic. 2016, 200, 13–18. [Google Scholar] [CrossRef]
- Garmendia, A.; Beltrán, R.; Zornoza, C.; García-Breijo, F.J.; Reig, J.; Merle, H. Gibberellic acid in Citrus spp. flowering and fruiting: A systematic review. PLoS ONE 2019, 14, e0223147. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez, Y.T.R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Yarushnykov, V.V.; Blanke, M.M. Alleviation of frost damage to pear flowers by application of gibberellin. Plant Growth Regul. 2005, 45, 21–27. [Google Scholar] [CrossRef]
- Santos, R.C.d.; Pereira, M.C.T.; Mendes, D.S.; Sobral, R.R.S.; Nietsche, S.; Mizobutsi, G.P.; Santos, B.H.C.d. Gibberellic acid induces parthenocarpy and increases fruit size in the ‘Gefner’ custard apple (‘Annona cherimola’ × ‘Annona squamosa’). Aust. J. Crop Sci. 2016, 10, 314–321. [Google Scholar] [CrossRef]
- Galimba, K.D.; Bullock, D.G.; Dardick, C.; Liu, Z.; Callahan, A.M. Gibberellic acid induced parthenocarpic ‘Honeycrisp’ apples (Malus domestica) exhibit reduced ovary width and lower acidity. Hortic. Res. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lamikanra, O.; Leong, S. Induction of seedlessness in ‘triumph’ muscadine grape (Vitis rotundifolia michx.) by applying gibberellic acid. Hortic. Sci. 1997, 32, 89–90. [Google Scholar] [CrossRef]
- Or, E.; Oren, O.; Halaly-Basha, T.; Koilkonda, P.; Shi, Z.; Zheng, C.; Acheampong, A.K. Gibberellin induced shot berry formation in cv. early sweet is a direct consequence of high fruit set. Hortic. Res. 2020, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Gultom, T.; Silitonga, D.Y. Effect of hormones gibberelin (GA3) to produce parthenocarpy fruit on tomato tree (Solanum betaceum, Cav). IOP Conf. Ser. Mater. Sci. Eng. 2018, 420, 012074. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Reig, C.; Martínez-Fuentes, A.; Iglesias, D.J.; Muñoz-Fambuena, N.; Bermejo, A.; Germanà, M.A.; Primo-Millo, E.; Agustí, M. Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant Sci. 2016, 247, 13–24. [Google Scholar] [CrossRef]
- Qian, C.; Ren, N.; Wang, J.; Xu, Q.; Chen, X.; Qi, X. Effects of exogenous application of CPPU, NAA and GA(4+7) on parthenocarpy and fruit quality in cucumber (Cucumis sativus L.). Food Chem. 2018, 243, 410–413. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Liu, J.; Liu, F.; Zhai, R.; Zhu, C.; Wang, H.; Ma, F.; Xu, L. Histological, hormonal and transcriptomic reveal the changes upon gibberellin-induced parthenocarpy in pear fruit. Hortic. Res. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Wang, T.; Li, J.; Yang, Q.; Qian, M.; Teng, Y. Effects of exogenous application of GA4+7 and N-(2-chloro-4-pyridyl)-N′-phenylurea on induced parthenocarpy and fruit quality in Pyrus pyrifolia ‘Cuiguan’. Plant Growth Regul. 2015, 76, 251–258. [Google Scholar] [CrossRef]
- Crane, J.C.; Primer, P.E.; Campbell, R.C. Gibberellin induced parthenocarpy in Prunus. Proc. Am. Soc. Hortic. Sci. 1960, 75, 129–137. [Google Scholar]
- Wen, B.; Song, W.; Sun, M.; Chen, M.; Mu, Q.; Zhang, X.; Wu, Q.; Chen, X.; Gao, D.; Wu, H. Identification and characterization of cherry (Cerasus pseudocerasus G. Don) genes responding to parthenocarpy induced by GA3 through transcriptome analysis. BMC Genet. 2019, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Tukey, H.B.; Young, J.O. Histological study of the developing fruit of the sour cherry. Bot. Gaz. 1939, 100, 723–749. [Google Scholar] [CrossRef]
- Singh, D.P.; Jermakow, A.M.; Swain, S.M. Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 2002, 14, 3133–3147. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Reid, J.B.; Kamiya, Y. Gibberellins are required for embryo growth and seed development in pea. Plant J. 1997, 12, 1329–1338. [Google Scholar] [CrossRef]
- Tukey, H.B. Growth of the embryo, seed, and pericarp of the sour cherry (Prunus cerasus) in relation to season of fruit ripening. Proc. Am. Soc. Hortic. Sci. 1934, 31, 125–144. [Google Scholar]
- Lilleland, O.; Newsome, L. A growth study of the cherry fruit. Proc. Am. Soc. Hortic. Sci. 1934, 32, 291–299. [Google Scholar]
- Lilleland, O. Growth study of the peach fruit. Proc. Am. Soc. Hortic. Sci. 1935, 33, 269–279. [Google Scholar]
- Lilleland, O. Growth study of the plum fruit-I. The growth and changes in chemical composition of the climax plum. Proc. Am. Soc. Hortic. Sci. 1933, 30, 203–208. [Google Scholar]
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.D.; Köhler, C. Bridging the generation gap: Communication between maternal sporophyte, female gametophyte and fertilization products. Curr. Opin. Plant Biol. 2016, 29, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.C. Family life at close quarters: Communication and constraint in angiosperm seed development. Protoplasma 2010, 247, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, F.-M.; Götz, K.-P. Performance of models for the beginning of sweet cherry blossom under current and changed climate conditions. Agric. For. Meteorol. 2016, 218–219, 85–91. [Google Scholar] [CrossRef]
- Carrera, E.; Ruiz-Rivero, O.; Peres, L.E.; Atares, A.; Garcia-Martinez, J.L. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012, 160, 1581–1596. [Google Scholar] [CrossRef]
- Martí, C.; Orzáez, D.; Ellul, P.; Moreno, V.; Carbonell, J.; Granell, A. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007, 52, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Livne, S.; Lor, V.S.; Nir, I.; Eliaz, N.; Aharoni, A.; Olszewski, N.E.; Eshed, Y.; Weiss, D. Uncovering DELLA-independent gibberellin responses by characterizing new tomato procera mutants. Plant Cell 2015, 27, 1579–1594. [Google Scholar] [CrossRef]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usadel, B.; Salts, Y.; Barg, R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 2017, 15, 634–647. [Google Scholar] [CrossRef]
- Takisawa, R.; Nakazaki, T.; Nunome, T.; Fukuoka, H.; Kataoka, K.; Saito, H.; Habu, T.; Kitajima, A. The parthenocarpic gene Pat-k is generated by a natural mutation of SlAGL6 affecting fruit development in tomato (Solanum lycopersicum L.). BMC Plant Biol. 2018, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Israeli, A.; Ori, N.; Sun, T.-p. The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, R.; Xiao, J.; Qian, C.; Wang, T.; Li, H.; Ouyang, B.; Ye, Z. A single-base deletion mutation in SlIAA9 gene causes tomato (Solanum lycopersicum) entire mutant. J. Plant Res. 2007, 120, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Torres-Pérez, R.; Mauri, N.; Diestro, N.; Cabezas, J.A.; Marchal, C.; Lacombe, T.; Ibáñez, J.; Tornel, M.; Carreño, J.; et al. The major origin of seedless grapes Is associated with a missense mutation in the MADS-Box gene VviAGL11. Plant Physiol. 2018, 177, 1234–1253. [Google Scholar] [CrossRef]
- Yao, J.-L.; Dong, Y.-H.; Morris, B.A.M. Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor. Proc. Natl. Acad. Sci. USA 2001, 98, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Ocarez, N.; Mejía, N. Suppression of the D-class MADS-box AGL11 gene triggers seedlessness in fleshy fruits. Plant Cell Rep. 2016, 35, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Chaïb, J.; Martinez-Zapater, J.M.; Thomas, M.R.; Torregrosa, L. Mis-expression of a PISTILLATA-like MADS box gene prevents fruit development in grapevine. Plant J. 2013, 73, 918–928. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertifcation, Land Degradation, Sustainableland Management, Food Security, and Greenhouse Gas Fuxes in Terrestrial Ecosystems; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019. [Google Scholar]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.L.T.; Lamarque, J.F.; Matsumoto, K.; Montzka, S.A.; Raper, S.C.B.; Riahi, K.; et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213. [Google Scholar] [CrossRef]
- NASA. Global Climate Change: Vital Signs of the Planet. Available online: https://climate.nasa.gov/413ppmquotes (accessed on 1 June 2022).
- IPCC. IPCC: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- Doddrell, N.H.; Lawson, T.; Raines, C.A.; Wagstaff, C.; Simkin, A.J. Feeding the world: Impacts of elevated [CO2] on nutrient content of greenhouse grown fruit crops and options for future yield gains. Hortic. Res. 2023, 10, uhad026. [Google Scholar] [CrossRef]
- Herrero, M.; Rodrigo, J.; Wünsch, A. Flowering, Fruit Set and Development; CABI International: Wallingford, UK, 2017; pp. 14–35. [Google Scholar]
- Rutkowski, K.; Łysiak, G.P. Weather conditions, orchard age and nitrogen fertilization influences yield and quality of ‘Łutówka’ Sour cherry fruit. Agriculture 2022, 12, 2008. [Google Scholar] [CrossRef]
- Gou, C.; Zhu, P.; Meng, Y.; Yang, F.; Xu, Y.; Xia, P.; Chen, J.; Li, J. Evaluation and genetic analysis of parthenocarpic germplasms in cucumber. Genes 2022, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Eeraerts, M.; Borremans, L.; Smagghe, G.; Meeus, I. A growers’ perspective on crop pollination and measures to manage the pollination service of wild pollinators in sweet cherry cultivation. Insects 2020, 11, 372. [Google Scholar] [CrossRef]
- Eeraerts, M.; Smsagghe, G.; Meeus, I. Pollinator diversity, floral resources and semi-natural habitat, instead of honey bees and intensive agriculture, enhance pollination service to sweet cherry. Agric. Ecosyst. Environ. 2019, 284, 106586. [Google Scholar] [CrossRef]
- Cong, L.; Yue, R.; Wang, H.; Liu, J.; Zhai, R.; Yang, J.; Wu, M.; Si, M.; Zhang, H.; Yang, C.; et al. 2,4-D-induced parthenocarpy in pear is mediated by enhancement of GA4 biosynthesis. Physiol. Plant. 2019, 166, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Akšić, M.F.; Colić, S.; Meland, M.; Natić, M. Sugar and polyphenolic diversity in floral nectar of cherry. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 755–773. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignati, E.; Caccamo, M.; Dunwell, J.M.; Simkin, A.J. Morphological Changes to Fruit Development Induced by GA3 Application in Sweet Cherry (Prunus avium L.). Plants 2024, 13, 2052. https://doi.org/10.3390/plants13152052
Vignati E, Caccamo M, Dunwell JM, Simkin AJ. Morphological Changes to Fruit Development Induced by GA3 Application in Sweet Cherry (Prunus avium L.). Plants. 2024; 13(15):2052. https://doi.org/10.3390/plants13152052
Chicago/Turabian StyleVignati, Edoardo, Mario Caccamo, Jim M. Dunwell, and Andrew J. Simkin. 2024. "Morphological Changes to Fruit Development Induced by GA3 Application in Sweet Cherry (Prunus avium L.)" Plants 13, no. 15: 2052. https://doi.org/10.3390/plants13152052






