Chlorogenic Acid as a Promising Tool for Mitigating Chilling Injury: Cold Tolerance and the Ripening Effect on Tomato Fruit (Solanum lycopersicum L.)
Abstract
1. Introduction
2. Results and Discussion
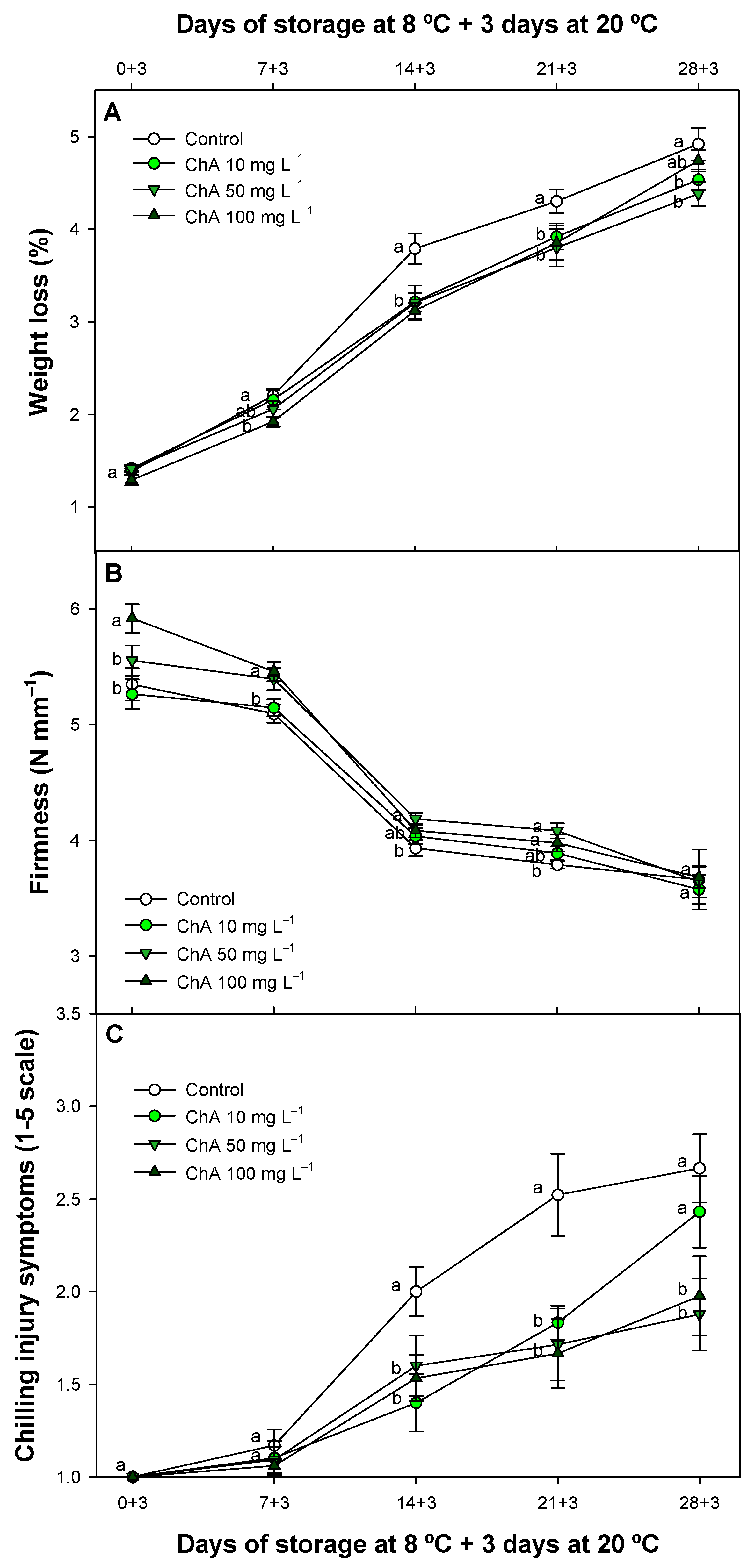
2.1. Chlorogenic Acid Impact on Weight Loss Fruit Softening and Chilling Injury Incidence
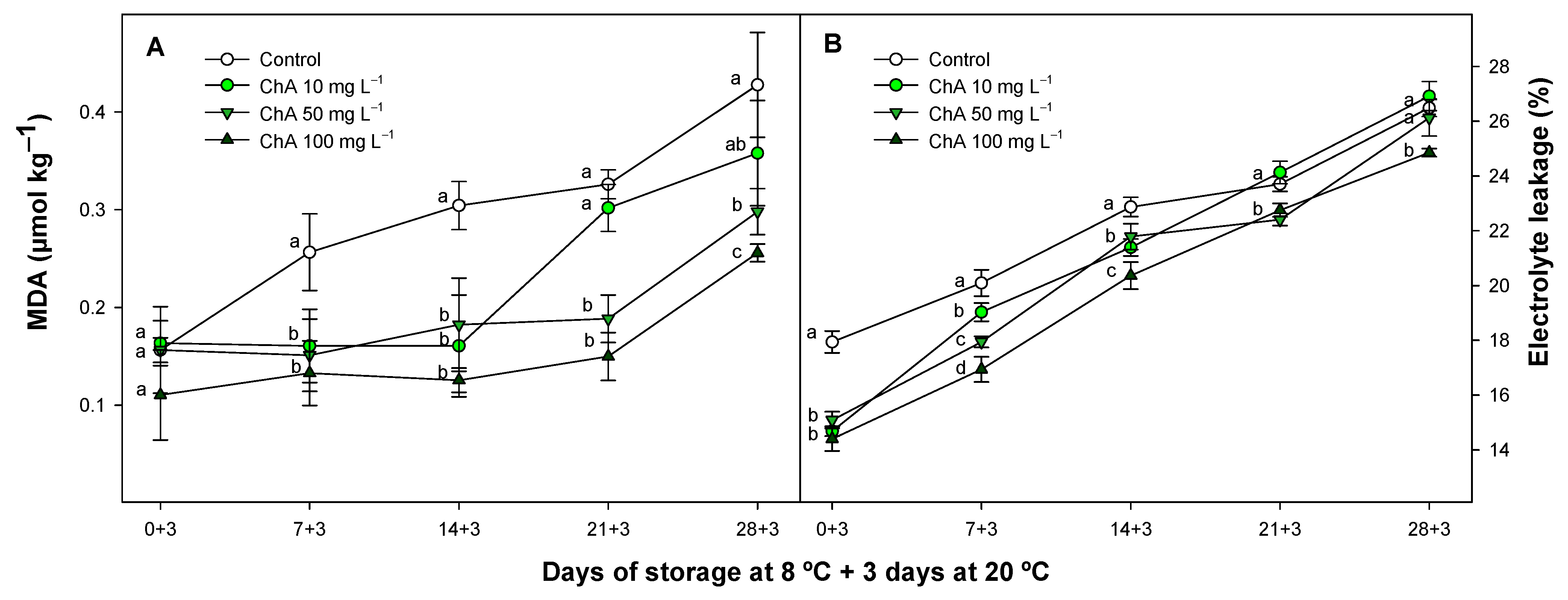
2.2. Effect of Chlorogenic Acid on Malondialdehyde Content and Electrolyte Leakage
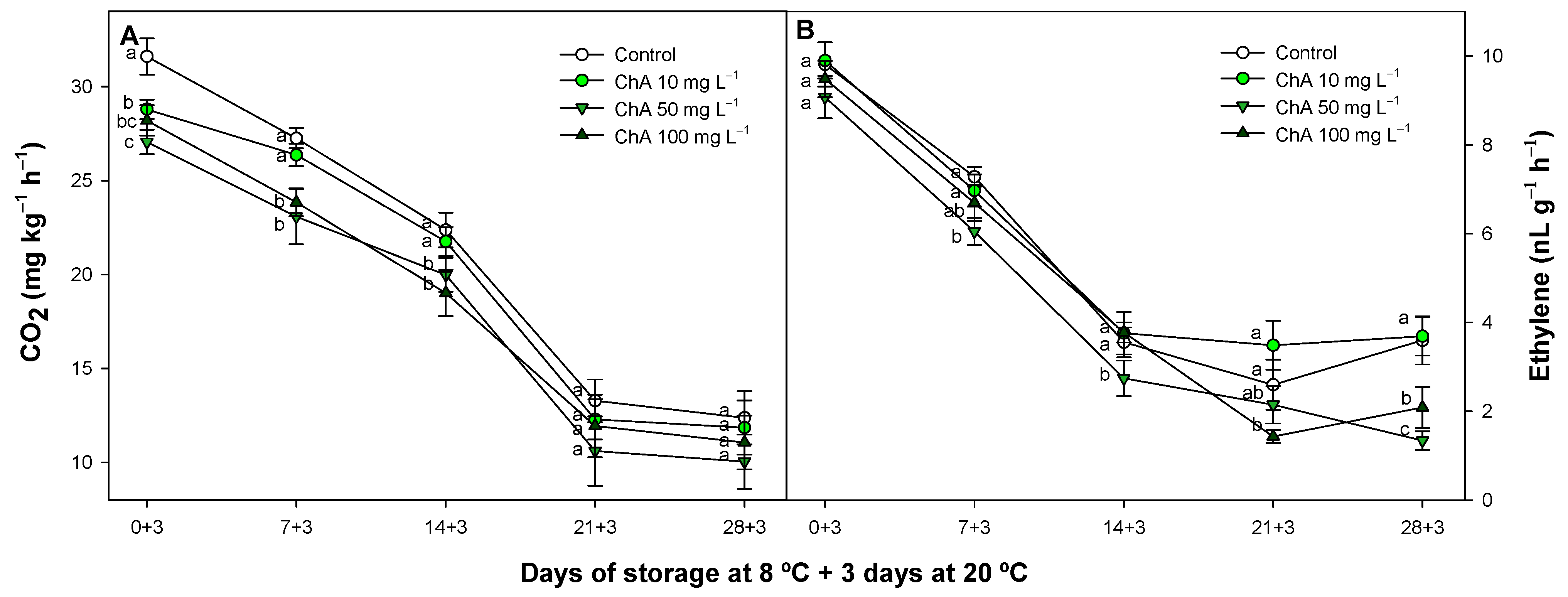
2.3. Effect of Chlorogenic Acid on Respiration and Ethylene Production
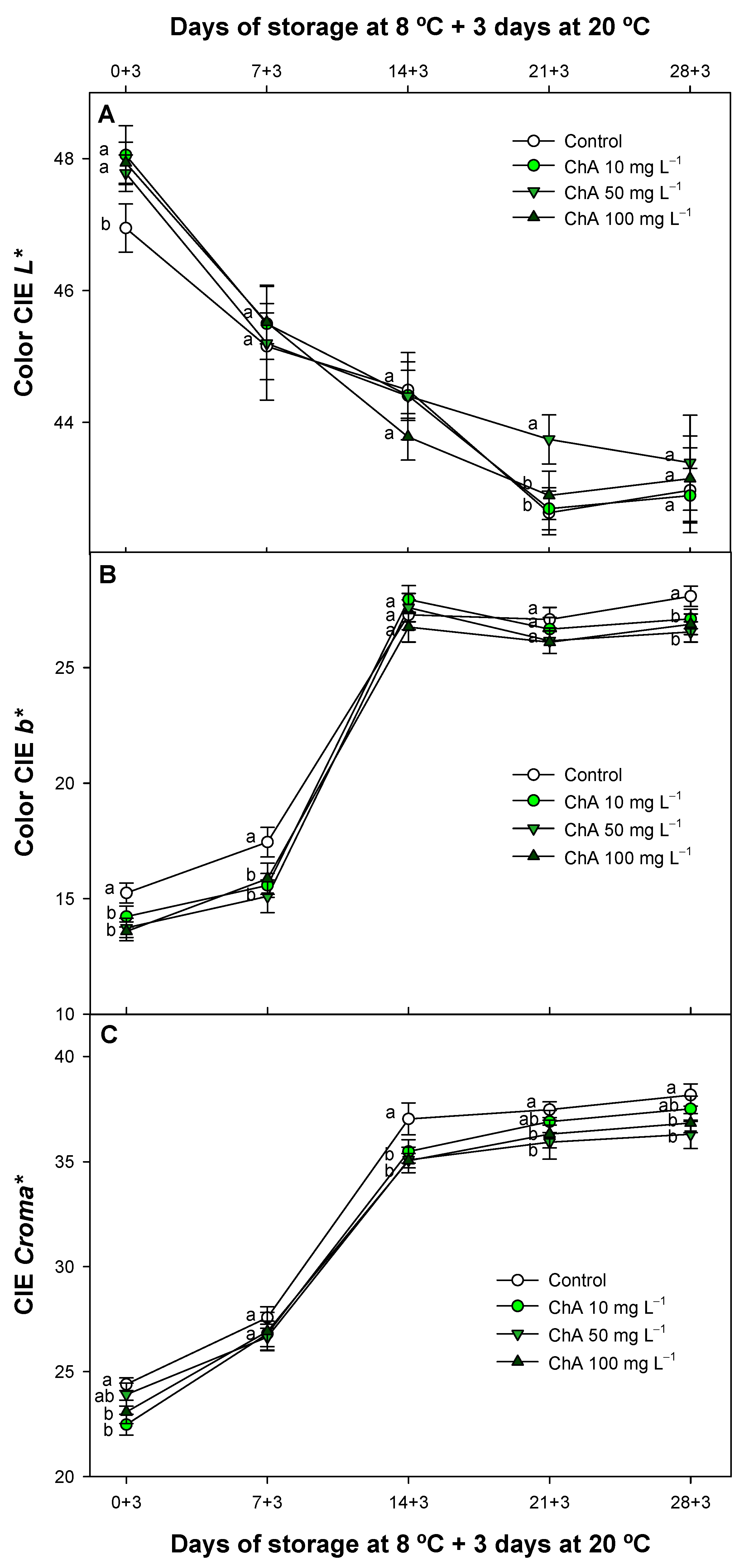
2.4. Effect of Chlorogenic Acid on Fruit Color Parameters
2.5. Effect of Chlorogenic Acid on Phenolic Compounds
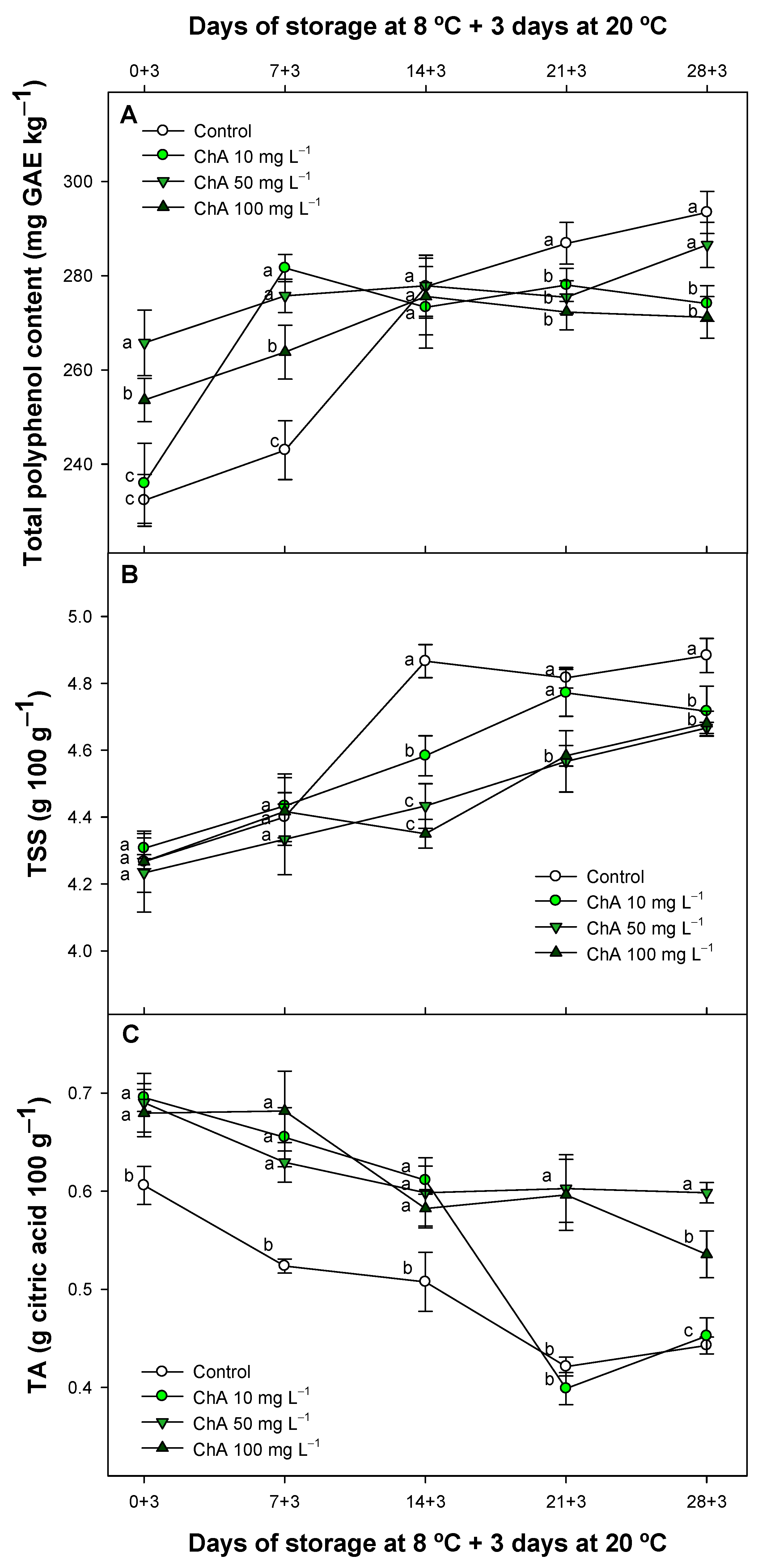
2.6. Effect of Chlorogenic Acid on Total Polyphenol Content Total Soluble Solids and Titratable Acidity throughout Storage
3. Materials and Methods
3.1. Plant Material and Experimental Design
3.2. Postharvest Quality Traits
3.3. Polyphenolic Content
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, V.; Parrotta, L.; Romi, M.; Del Duca, S.; Cai, G. Tomato biodiversity and drought tolerance: A multilevel review. Int. J. Mol. Sci. 2023, 24, 10044. [Google Scholar] [CrossRef]
- Cruz-Mendívil, A.; Lopez-Valenzuela, J.A.; Calderón-Vázquez, C.L.; Vega-García, M.O.; Reyes-Moreno, C.; Valdez-Ortiz, A. Transcriptional changes associated with chilling tolerance and susceptibility in ‘Micro-Tom’ tomato fruit using RNA-Seq. Postharvest Biol. Technol. 2015, 99, 141–151. [Google Scholar] [CrossRef]
- Park, M.-H.; Sangwanangkul, P.; Choi, J.-W. Reduced chilling injury and delayed fruit ripening in tomatoes with modified atmosphere and humidity packaging. Sci. Hortic. 2018, 231, 66–72. [Google Scholar] [CrossRef]
- Guillén, F.; Castillo, S.; Zapata, P.J.; Martínez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP treatment in tomato fruit. 2. Effect of cultivar and ripening stage at harvest. Postharvest Biol. Technol. 2006, 42, 235–242. [Google Scholar] [CrossRef]
- Guillén, F.; Castillo, S.; Zapata, P.J.; Martinez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP treatment in tomato fruit. 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol. Technol. 2007, 43, 23–27. [Google Scholar] [CrossRef]
- Law, D.M.; Davies, P.J.; Mutschler, M.A. Polyamine-induced prolongation of storage in tomato fruits. Plant Growth Regul. 1991, 10, 283–290. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Tao, N.; Yan, T.; Wang, Q.; Li, Q. Nitric oxide is essential to keep the postharvest quality of fruits and vegetables. Horticulturae 2023, 9, 135. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Effect of postharvest visible spectrum LED lighting on quality and bioactive compounds of tomatoes during shelf life. LWT 2023, 174, 114420. [Google Scholar] [CrossRef]
- Yoon, K.N.; Yoon, Y.S.; Hong, H.J.; Yeom, S.J.; Park, J.H.; Song, B.S.; Eun, J.B.; Kim, J.-K. Extending shelf life and analyzing dosimetric and detection techniques in postharvest tomatoes (Solanum lycopersicum) via X-ray irradiation. LWT 2024, 201, 116230. [Google Scholar] [CrossRef]
- Domínguez, I.; Lafuente, M.T.; Hernández-Muñoz, P.; Gavara, R. Influence of modified atmosphere and ethylene levels on quality attributes of fresh tomatoes (Lycopersicon esculentum Mill.). Food Chem. 2016, 209, 211–219. [Google Scholar] [CrossRef]
- Alvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Castillejo, N.; Martínez, J.A.; Artés-Hernández, F. Development of an antifungal active packaging containing thymol and an ethylene scavenger. Validation during storage of cherry tomatoes. Food Packag. Shelf Life 2021, 29, 100734. [Google Scholar] [CrossRef]
- Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Salvador Castillo, D.V.; Serrano, M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill.) quality. J. Sci. Food Agric. 2008, 88, 1287–1293. [Google Scholar] [CrossRef]
- Gultom, T.; Silitonga, D.Y. Effect of hormones gibberellin (Ga3) to produce parthenocarpy fruit on tomato tree (Solanum betaceum, Cav). IOP Conf. Ser. Mater. Sci. Eng. 2018, 420, 012074. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Fu, X.; Zhu, B.; Chen, F.; Liu, B.; Mao, L.; Luo, Z.; Li, L.; Ying, T. Understanding of exogenous auxin in regulating sucrose metabolism during postharvest tomato fruit ripening. Postharvest Biol. Technol. 2022, 189, 111913. [Google Scholar] [CrossRef]
- Baek, M.W.; Choi, H.R.; Lee, H.C.; Lee, J.H.; Lee, O.H.; Hong, J.S.; Jeong, C.S.; Tilahun, S. Preharvest methyl jasmonate and salicylic acid treatments improve the nutritional qualities and postharvest storability of tomato. Sci. Hortic. 2023, 321, 112332. [Google Scholar] [CrossRef]
- Min, D.; Li, Z.; Ai, W.; Li, J.; Zhou, J.; Zhang, X.; Mu, D.; Li, F.; Li, X.; Guo, Y. The co-regulation of ethylene biosynthesis and ascorbate-glutathione cycle by methyl jasmonate contributes to aroma formation of tomato fruit during postharvest ripening. J. Agric. Food Chem. 2020, 68, 10822–10832. [Google Scholar] [CrossRef]
- Shahab, M.; Roberto, S.R.; Adnan, M.; Fahad, S.; Koyama, R.; Saleem, M.H. Phenolic compounds as a quality determinant of grapes: A critical review. J. Plant Growth Regul. 2023, 42, 5325–5331. [Google Scholar] [CrossRef]
- Le Gall, G.; DuPont, M.S.; Mellon, F.A.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E.; Colquhoun, I.J. Characterization and content of flavonoid glycosides in genetically modified tomato (Lycopersicon esculentum) fruits. J. Agric. Food Chem. 2003, 51, 2438–2446. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M.J. Content of chalconaringenin and chlorogenic acid in cherry tomatoes is strongly reduced during postharvest ripening. J. Agric. Food Chem. 2005, 53, 7251–7256. [Google Scholar] [CrossRef]
- Cao, X.; Islam, M.N.; Chitrakar, B.; Duan, Z.; Xu, W.; Zhong, S. Effect of combined chlorogenic acid and chitosan coating on antioxidant, antimicrobial, and sensory properties of snakehead fish in cold storage. Food Sci. Nutr. 2020, 8, 973–981. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Roussos, P.; Kostelenos, G.; Vemmos, S. Chlorogenic acid: A possible cofactor in the rooting of ‘Kalamata’ olive cultivar. J. Plant Growth Regul. 2021, 40, 2017–2027. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Qu, H.; Yun, Z.; Wang, H.; Huang, Z.; Huang, H.; Jiang, Y. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Cheng, D.; Zeng, X.; Cao, J.; Jiang, W. Evidences for chlorogenic acid—A major endogenous polyphenol involved in regulation of ripening and senescence of apple fruit. PLoS ONE 2016, 11, e0146940. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Hu, M.; Gao, Z.; Li, M.; Yun, Z.; Pan, Y.; Zhang, Z.; Jiang, Y. Apple polyphenols delay senescence and maintain edible quality in litchi fruit during storage. Postharvest Biol. Technol. 2019, 157, 110976. [Google Scholar] [CrossRef]
- Bai, X.Y.; Yang, Z.M.; Shen, W.J.; Shao, Y.Z.; Zeng, J.K.; Li, W. Polyphenol treatment delays the browning of litchi pericarps and promotes the total antioxidant capacity of litchi fruit. Sci. Hortic. 2022, 291, 110563. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Huang, Q.; Li, A.R.; Gan, Z.Y.; Zeng, J.K.; Kai, W.B.; Chen, C.Y.; Chen, J.Y. Apple polyphenols delay postharvest senescence and quality deterioration of ‘Jinshayou’ pummelo fruit during storage. Front. Plant Sci. 2023, 13, 1117106. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M.; Fonseca, J.M.; Kitinoja, L. Postharvest losses and waste. In Postharvest Technology of Perishable Horticultural Commodities; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Baninaiem, E.; Dastjerdi, A.M. Enhancement of storage life and maintenance of quality in tomato fruits by preharvest salicylic acid treatment. Front. Sustain. Food Syst. 2023, 7, 1180243. [Google Scholar] [CrossRef]
- Li, F.; Yan, Y.; Gu, C.; Sun, J.; Han, Y.; Huangfu, Z.; Song, F.; Chen, J. Preparation and characterization of phenolic acid-chitosan derivatives as an edible coating for enhanced preservation of Saimaiti apricots. Foods 2022, 11, 3548. [Google Scholar] [CrossRef]
- Shu, C.; Zhang, W.; Zhao, H.; Cao, J.; Jiang, W. Chlorogenic acid treatment alleviates the adverse physiological responses of vibration injury in apple fruit through the regulation of energy metabolism. Postharvest Biol. Technol. 2020, 159, 110997. [Google Scholar] [CrossRef]
- Du, H.; He, L. Synergistic improvement of antioxidant and antibacterial properties of carbon quantum complexes with zinc doping and chlorogenic acid for longan preservation. Food Chem. 2024, 439, 138169. [Google Scholar] [CrossRef]
- Razali, Z.; Somasundram, C.; Nurulain, S.Z.; Kunasekaran, W.; Alias, M.R. Postharvest quality of cherry tomatoes coated with mucilage from dragon fruit and irradiated with UV-C. Polymers 2021, 13, 2919. [Google Scholar] [CrossRef]
- Liu, G.; Chen, B.; Liu, H.; Wang, X.; Zhang, Y.; Wang, C.; Liu, C.; Zhong, Y.; Qiao, Y. Effects of hydroxyethyl cellulose and sulfated rice bran polysaccharide coating on quality maintenance of cherry tomatoes during cold storage. Foods 2023, 12, 3156. [Google Scholar] [CrossRef]
- Padda, M.S.; Picha, D.H. Effect of low temperature storage on phenolic composition and antioxidant activity of sweetpotatoes. Postharvest Biol. Technol. 2008, 47, 176–180. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Zeng, X.; Li, Z.; Qin, B.; He, N. New perspective of GABA as an inhibitor of formation of advanced lipoxidation end-products: Its interaction with malondialdehyde. J. Biomed. Nanotechnol. 2010, 6, 318–324. [Google Scholar] [CrossRef]
- Aghdam, S.M. Mitigation of postharvest chilling injury in tomato fruit by prohexadione calcium. J. Food Sci. Technol. 2013, 50, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Charoenphun, N.; Pham, N.H.; Rattanawut, J.; Venkatachalam, K. Exogenous application of melatonin on the preservation of physicochemical and enzymatic qualities of pepper fruit from chilling injury. Horticulturae 2024, 10, 550. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Serrano, M.; Martínez-Romero, D.; Ruiz-Aracil, M.C.; Valero, D.; Guillén, F. The simultaneous use of 1-methylcyclopropene and methyl jasmonate vapor as an innovative strategy for reducing chilling injury and maintaining pomegranate fruit quality at suboptimal temperatures. Foods 2024, 13, 60. [Google Scholar] [CrossRef]
- Fan, P.; Huber, D.J.; Su, Z.; Hu, M.; Gao, Z.; Li, M.; Shi, X.; Zhang, Z. Effect of postharvest spray of apple polyphenols on the quality of fresh-cut red pitaya fruit during shelf life. Food Chem. 2018, 243, 19–25. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Fatty acid composition and antioxidant system in relation to susceptibility of loquat fruit to chilling injury. Food Chem. 2011, 127, 1777–1783. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.; Zheng, Y. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Valdenegro, M.; Fuentes, L.; Bernales, M.; Huidobro, C.; Monsalve, L.; Hernández, I.; Schelle, M.; Simpson, R. Antioxidant and fatty acid changes in pomegranate peel with induced chilling injury and browning by ethylene during long storage times. Front. Plant Sci. 2022, 13, 771094. [Google Scholar] [CrossRef] [PubMed]
- Concellón, A.; Zaro, M.J.; Chaves, A.R.; Vicente, A.R. Changes in quality and phenolic antioxidants in dark purple American eggplant (Solanum melongena L. cv. Lucía) as affected by storage at 0 °C and 10 °C. Postharvest Biol. Technol. 2012, 66, 35–41. [Google Scholar] [CrossRef]
- López-López, M.E.; López-Valenzuela, J.A.; Delgado-Vargas, F.; López-Angulo, G.; Carrillo-López, A.; Ayón-Reyna, L.E.; Vega-García, M.O. A treatment combining hot water with calcium lactate improves the chilling injury tolerance of mango fruit. HortScience 2018, 53, 217–223. [Google Scholar] [CrossRef]
- Vittani, L.; Populin, F.; Stuerz, S.; Buehlmann, A.; Khomenko, I.; Biasioli, F.; Bühlmann-Schütz, S.; Vrhovsek, U.; Masuero, D.; Zanella, A.; et al. Comparative transcriptome and metabolite survey reveal key pathways involved in the control of the chilling injury disorder superficial scald in two apple cultivars, ‘Granny Smith’ and ‘Ladina’. Front. Plant Sci. 2023, 14, 1150046. [Google Scholar] [CrossRef] [PubMed]
- Medina-Santamarina, J.; Serrano, M.; Ruiz-Aracil, M.C.; Ilea, M.I.M.; Martínez-Romero, D.; Guillén, F. A synergistic effect based on the combination of melatonin with 1-Methylcyclopropene as a new strategy to increase chilling tolerance and general quality in zucchini fruit. Foods 2022, 11, 2784. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Jiao, W.; Cao, J.; Jiang, W. Effects of chlorogenic acid on capacity of free radicals scavenging and proteomic changes in postharvest fruit of nectarine. PLoS ONE 2017, 12, e0182494. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Liu, Y.; Lu, X.; Li, S.; Cui, J.; Qi, J.; Yu, W. Involvement of energy and cell wall metabolisms in chilling tolerance improved by hydrogen sulfide in cold-stored tomato fruits. Plant Cell Rep. 2024, 43, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Min, D.; Li, Z.; Fu, X.; Zhao, X.; Wang, J.; Zhang, X.; Li, F.; Li, X. Effects of chilling acclimation and methyl jasmonate on sugar metabolism in tomato fruits during cold storage. Sci. Hortic. 2021, 289, 110495. [Google Scholar] [CrossRef]
- Mabunda, E.; Mafeo, T.; Mathaba, N.; Buthelezi, D.; Satekge, T. Effects of putrescine postharvest dips and refrigerated storage temperature on quality attributes and shelf-life of ‘Solo’ papaya fruit. J. Hortic. Postharvest Res. 2023, 6, 93–206. [Google Scholar] [CrossRef]
- Darré, M.; Zaro, M.J.; Guijarro-Fuertes, M.; Careri, L.; Concellón, A. Melatonin combined with wax treatment enhances tolerance to chilling injury in red bell pepper. Metabolites 2024, 14, 330. [Google Scholar] [CrossRef]
- Gago, C.; Guerreiro, A.; Souza, M.; Martins, N.; Fonseca, D.; Cabrita, M.J.; Miguel, M.G.; Antunes, M.D. effectiveness of sodium alginate and carnauba wax nanoemulsions with lemongrass essential oil on the quality of ‘hass’ avocado fruit from early, middle, and late harvest season during prolonged cold storage. Sci. Hortic. 2024, 333, 113237. [Google Scholar] [CrossRef]
- Nunes, C.N.; Emond, J.P. Relationship between weight loss and visual quality of fruits and vegetables. Proc. Fla. State Hortic. Soc. 2007, 120, 235–245. [Google Scholar]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Paolo, D.; Bianchi, G.; Scalzo, R.L.; Morelli, C.F.; Rabuffetti, M.; Speranza, G. The chemistry behind tomato quality. Nat. Prod. Commun. 2018, 13, 1225–1232. [Google Scholar] [CrossRef]
- Xi, Y.; Fan, X.; Zhao, H.; Li, X.; Cao, J.; Jiang, W. Postharvest fruit quality and antioxidants of nectarine fruit as influenced by chlorogenic acid. LWT 2017, 75, 537–544. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Medina-Remon, A.; Casals-Ribes, I.; Lamuela-Raventos, R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012, 130, 222–227. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Song, J.; Tian, M.; Li, R.; Cui, X. Phenolic compound identification in tomato fruit by UPLC-QTOF-MS. LWT 2023, 182, 114791. [Google Scholar] [CrossRef]
- López-Téllez, J.M.; del Pilar Cañizares-Macías, M. Evaluation of tomato (Lycopersicon esculentum P. Mill.) by-product extracts obtained by different extraction methods as exploitation strategy of high-value polyphenols. Food Bioproc. Technol. 2024, 13, 1743–1756. [Google Scholar] [CrossRef]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical investigation and antioxidant properties of unripe tomato cultivars (Solanum lycopersicum L.). Food Chem. 2024, 438, 137863. [Google Scholar] [CrossRef] [PubMed]
- Gude, K.M.; Rajashekar, C.B.; Cunningham, B.; Kang, Q.; Wang, W.; Lee, M.; Rivard, C.L.; Pliakoni, E.D. Effect of high tunnel coverings on antioxidants of breaker and light red tomatoes at harvest and during ripening. Agronomy 2020, 10, 1639. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT Food Sci. Technol. 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. p-Coumaric acid induces antioxidant capacity and defense responses of sweet cherry fruit to fungal pathogens. Front. Plant Sci. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Palumbo, M.; Cefola, M.; Pace, B.; Ricci, I.; Siano, F.; Amato, G.; Stocchero, M.; Cozzolino, R. Volatile metabolites to assess the onset of chilling injury in fresh-cut nectarines. Foods 2024, 13, 1047. [Google Scholar] [CrossRef]
- Populin, F.; Vittani, L.; Zanella, A.; Stuerz, S.; Folie, I.; Khomenko, I.; Biasioli, F.; Scholz, M.; Masuero, D.; Vrhovsek, U.; et al. Transcriptome and metabolic survey disclose the mode of action of static and dynamic low oxygen postharvest storage strategies to prevent the onset of superficial scald disorder in fruit of ‘Granny Smith’ apple cultivar. Postharvest Biol. Technol. 2023, 205, 112492. [Google Scholar] [CrossRef]
- Menaka, M.; Asrey, R.; Singh, D.; Patel, V.B.; Meena, N.K.; Vinod, B.R.; Ahamad, S. Preserving functional properties and inhibiting postharvest peel browning in guava during cold storage via 24-epibrassinolide application. Postharvest Biol. Technol. 2024, 216, 113033. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in polyphenols contents and antioxidant capacities of organically and conventionally cultivated tomato (Solanum lycopersicum L.) fruits during ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef] [PubMed]
- Pék, Z.; Helyes, L.; Lugasi, A. Color changes and antioxidant content of vine and postharvest-ripened tomato fruits. HortScience 2010, 45, 466–468. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of postharvest UV-C irradiation on phenolic acids, flavonoids, and key phenylpropanoid pathway genes in tomato fruit. Sci. Hortic. 2018, 241, 107–114. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Vega-Álvarez, M.; Landeros Sánchez, A.; López-Angulo, G.; Salazar-Salas, N.Y.; Quintero-Soto, M.F.; Pineda-Hidalgo, K.V.; López-Valenzuela, J.A. Metabolic changes associated with chilling injury tolerance in tomato fruit with hot water pretreatment. J. Food Biochem. 2022, 46, e14056. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Bodbodak, S. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- Zakriya, M.; Hussain, A.; Mahdi, A.A.; Yasmeen, F.; Kausar, T.; Rehman, A.; Yaqub, S.; Fatima, P.; Noreen, S.; Kabir, K.; et al. Effect of different types of ethylene scavengers used in different combinations, on the post-harvest quality and phytochemicals retention of tomatoes (Solanum lycopersicum L.). Chem. Biol. Technol. Agric. 2023, 10, 90. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Wang, L.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [CrossRef]
- Boonyaritthongchai, P.; Supapvanich, S. Effects of methyl jasmonate on physicochemical qualities and internal browning of ‘queen’ pineapple fruit during cold storage. Hortic. Environ. Biotechnol. 2017, 58, 479–487. [Google Scholar] [CrossRef]
- Sangsoy, K.; Beckles, D.M.; Terdwongworakul, A.; Luengwilai, K. Discriminating pineapple batches for susceptibility to postharvest internal browning. Sci. Hortic. 2022, 300, 111069. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, C.; Shao, T.; Jiang, X.; Zhao, H.; Ai, W. Postharvest hot water dipping and hot water forced convection treatments alleviate chilling injury for zucchini fruit during cold storage. Sci. Hortic. 2019, 249, 219–227. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, C.; Yi, P.; Li, L.; Wu, G.; Huang, F.; Huang, M.; Gan, T. The effects of combined 1-methylcyclopropene and melatonin treatment on the quality characteristics and active oxygen metabolism of mango fruit during storage. Foods 2023, 12, 1979. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. Food Chem. Toxicol. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Lezoul, N.E.H.; Belkadi, M.; Habibi, F.; Guillén, F. Extraction processes with several solvents on total bioactive compounds in different organs of three medicinal plants. Molecules 2020, 25, 4672. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilea, M.I.M.; Zapata, P.J.; Fernández-Picazo, C.; Díaz-Mula, H.M.; Castillo, S.; Guillén, F. Chlorogenic Acid as a Promising Tool for Mitigating Chilling Injury: Cold Tolerance and the Ripening Effect on Tomato Fruit (Solanum lycopersicum L.). Plants 2024, 13, 2055. https://doi.org/10.3390/plants13152055
Ilea MIM, Zapata PJ, Fernández-Picazo C, Díaz-Mula HM, Castillo S, Guillén F. Chlorogenic Acid as a Promising Tool for Mitigating Chilling Injury: Cold Tolerance and the Ripening Effect on Tomato Fruit (Solanum lycopersicum L.). Plants. 2024; 13(15):2055. https://doi.org/10.3390/plants13152055
Chicago/Turabian StyleIlea, Mihaela Iasmina Madalina, Pedro Javier Zapata, Christian Fernández-Picazo, Huertas María Díaz-Mula, Salvador Castillo, and Fabián Guillén. 2024. "Chlorogenic Acid as a Promising Tool for Mitigating Chilling Injury: Cold Tolerance and the Ripening Effect on Tomato Fruit (Solanum lycopersicum L.)" Plants 13, no. 15: 2055. https://doi.org/10.3390/plants13152055
APA StyleIlea, M. I. M., Zapata, P. J., Fernández-Picazo, C., Díaz-Mula, H. M., Castillo, S., & Guillén, F. (2024). Chlorogenic Acid as a Promising Tool for Mitigating Chilling Injury: Cold Tolerance and the Ripening Effect on Tomato Fruit (Solanum lycopersicum L.). Plants, 13(15), 2055. https://doi.org/10.3390/plants13152055









