Effects of AMF on Maize Yield and Soil Microbial Community in Sandy and Saline Soils
Abstract
1. Introduction
2. Results
2.1. The Effects of AMF on Corn Root Growth and Yield Formation
2.1.1. Impact of AMF on Maize Root Growth
2.1.2. Impact of AMF on Maize Yield Formation
2.1.3. Impact of AMF on Maize Grain Quality
2.2. Effects of AMF on Rhizosphere Soil Physicochemical Properties and Microbial Community Structure
2.2.1. Impact of AMF on Soil Physicochemical Properties
2.2.2. Impact of AMF on Soil Microbial Alpha Diversity
2.2.3. Impact of AMF on Beta Diversity of Soil Microorganisms
2.2.4. Impact of AMF on Soil Microbial Community Structure
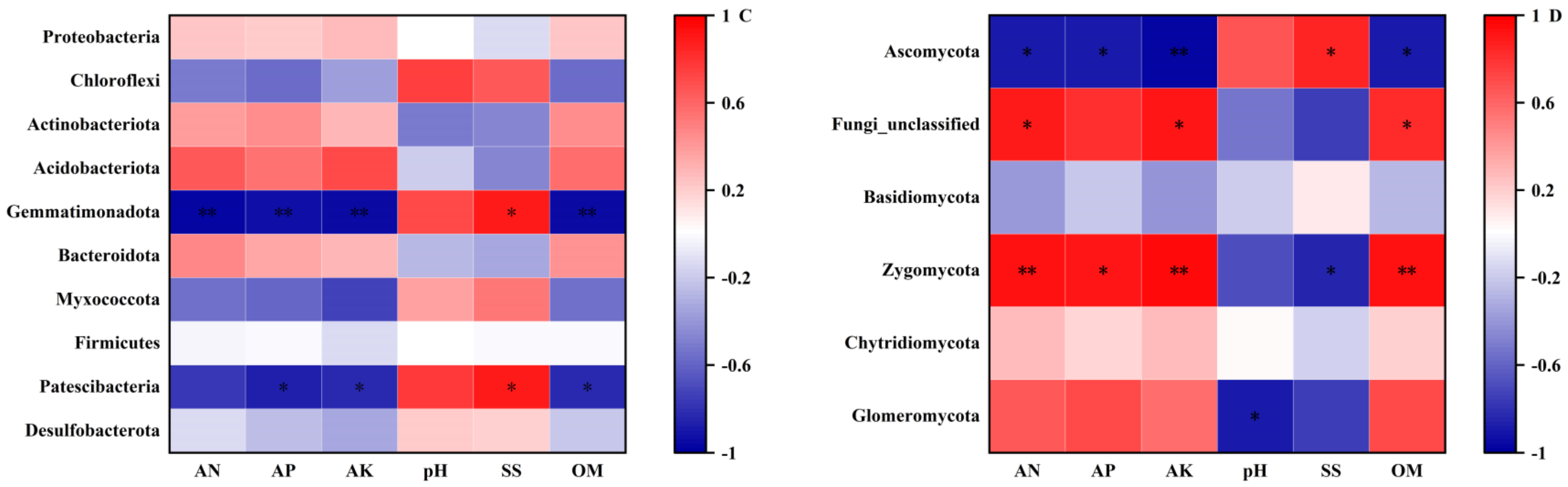
2.2.5. Correlation Analysis between Soil Microbial Community and Soil Environmental Factors
3. Discussion
4. Materials and Methods
4.1. Experimental Site Overview and Experimental Materials
4.2. Experimental Design
4.3. Sample Collection and Analysis
4.3.1. Determination of Soil Physicochemical Parameters
4.3.2. Soil Microbial Community Analysis
4.3.3. Measurement of Rhizosphere Configuration
4.3.4. Determination of Maize Yield
4.3.5. Determination of Maize Grain Quality
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hossain, A.; Krupnik, T.J.; Timsina, J.; Mahboob, M.G.; Chaki, A.K.; Farooq, M.; Bhatt, R.; Fahad, S.; Hasanuzzaman, M. Agricultural land degradation: Processes and problems undermining future food security. In Environment, Climate, Plant and Vegetation Growth; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–61. [Google Scholar]
- Zhang, J. Soil environmental deterioration and ecological rehabilitation. In Study of Ecological Engineering of Human Settlements; Springer: Singapore, 2020; pp. 41–82. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Stutz, J.C.; Copeman, R.; Martin, C.A.; Morton, J.B. Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa. Can. J. Bot. 2000, 78, 237–245. [Google Scholar]
- Sharmah, D.; Jha, D.K. Diversity of arbuscular mycorrhizal fungi in undisturbed forest, slash-and-burn field, and monoculture forest of Indo-Burma megadiverse region. Braz. J. Bot. 2014, 37, 339–351. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.-C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Alam Bhat, M.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Wang, Y.; Zhang, Q.; Luo, J.; Jiang, X.; Liu, W.; Zhao, X. Effects of arbuscular mycorrhizal fungi on improvement of degraded landscape soil in an ionized rare earth mining area, subtropical China. Soil Sci. Soc. Am. J. 2022, 86, 275–292. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Li, Y.; Huang, T.; Xie, K.; Zhou, J.; Li, X. Effects of interactions between arbuscular mycorrhizal fungi and bacteria on the growth of Lotus corniculatus L.: From the perspective of regulating rhizosphere fungal community. Pedosphere 2024, 34, 411–423. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Noceto, P.-A.; Bettenfeld, P.; Boussageon, R.; Hériché, M.; Sportes, A.; van Tuinen, D.; Courty, P.-E.; Wipf, D. Arbuscular mycorrhizal fungi, a key symbiosis in the development of quality traits in crop production, alone or combined with plant growth-promoting bacteria. Mycorrhiza 2021, 31, 655–669. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [Google Scholar] [CrossRef]
- Yuan, M.M.; Kakouridis, A.; Starr, E.; Nguyen, N.; Shi, S.; Pett-Ridge, J.; Nuccio, E.; Zhou, J.; Firestone, M. Fungal-bacterial cooccurrence patterns differ between arbuscular mycorrhizal fungi and nonmycorrhizal fungi across soil niches. mBio 2021, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. The Application of Arbuscular Mycorrhizal Fungi as Microbial Biostimulant, Sustainable Approaches in Modern Agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hao, Y.; Wu, X.L.; Dai, F.J.; Abd-Allah, E.F.; Wu, Q.S.; Liu, S.R. Arbuscular mycorrhizal fungi improve drought tolerance of tea plants via modulating root architecture and hormones. Plant Growth Regul. 2024, 102, 13–22. [Google Scholar] [CrossRef]
- Yao, Q.; Lin, F.X.; Chen, J.Z.; Lei, X.T.; Zhu, H.H. Responses of citrus seedlings and a leguminous herb, Stylosanthes gracilis, to arbuscular mycorrhizal fungal inoculation. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Citrus and Other Tropical and Subtropical, Seoul, Republic of Korea, 13 August 2006; pp. 63–67. [Google Scholar]
- Zhang, J.; Bi, Y.; Song, Z.; Xiao, L.; Christie, P. Arbuscular mycorrhizal fungi alter root and foliar responses to fissure-induced root damage stress. Ecol. Indic. 2021, 127, 107800. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Guo, M.; Qu, L.; Biere, A. Effects of arbuscular mycorrhizal fungi on plant growth and herbivore infestation depend on availability of soil water and nutrients. Front. Plant Sci. 2023, 14, 1101932. [Google Scholar] [CrossRef]
- Li, Y.; Laterrière, M.; Lay, C.-Y.; Klabi, R.; Masse, J.; St-Arnaud, M.; Yergeau, É.; Lupwayi, N.Z.; Gan, Y.; Hamel, C. Effects of arbuscular mycorrhizal fungi inoculation and crop sequence on root-associated microbiome, crop productivity and nutrient uptake in wheat-based and flax-based cropping systems. Appl. Soil Ecol. 2021, 168, 104136. [Google Scholar] [CrossRef]
- Ushio, M.; Fujiki, Y.; Hidaka, A.; Kitayama, K. Linkage of root physiology and morphology as an adaptation to soil phosphorus impoverishment in tropical montane forests. Funct. Ecol. 2015, 29, 1235–1245. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Santos, G.F.; Carvalho, I.R.; Fernandes, M.d.S.; Carnevale, A.B.; Lopes, K.; Prado, R.L.F.; Lautenchleger, F.; Pereira, A.I.d.A.; Curvêlo, C.R.d.S. Inoculation of Azospirillum brasilense in corn. Communications 2020, 10, 037–045. [Google Scholar]
- Qin, Z.; Zhang, H.; Feng, G.; Christie, P.; Zhang, J.; Li, X.; Gai, J. Soil phosphorus availability modifies the relationship between AM fungal diversity and mycorrhizal benefits to maize in an agricultural soil. Soil Biol. Biochem. 2020, 144, 107790. [Google Scholar] [CrossRef]
- Massa, N.; Cesaro, P.; Todeschini, V.; Capraro, J.; Scarafoni, A.; Cantamessa, S.; Copetta, A.; Anastasia, F.; Gamalero, E.; Lingua, G.; et al. Selected autochthonous rhizobia, applied in combination with AM fungi, improve seed quality of common bean cultivated in reduced fertilization condition. Appl. Soil Ecol. 2020, 148, 103507. [Google Scholar] [CrossRef]
- Liu, X.Q.; Xie, M.M.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.S. Arbuscular mycorrhizal fungi and rhizobia synergistically promote root colonization, plant growth, and nitrogen acquisition. Plant Growth Regul. 2023, 100, 691–701. [Google Scholar] [CrossRef]
- Bao, X.; Zou, J.; Zhang, B.; Wu, L.; Yang, T.; Huang, Q. Arbuscular mycorrhizal fungi and microbes interaction in rice mycorrhizosphere. Agronomy 2022, 12, 1277. [Google Scholar] [CrossRef]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Haq, M.Z.U.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular mycorrhizal fungi symbiosis to enhance plant–soil interaction. Sustainability 2022, 14, 7840. [Google Scholar] [CrossRef]
- Wei, L.L.; Lu, C.Y.; Ding, J.; Yu, S. Functional relationships between arbuscular mycorrhizal symbionts and nutrient dynamics in plant-soil-microbe system. Acta Ecol. Sin. 2016, 36, 4233–4243. [Google Scholar]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, I.F.; Campolino, M.L.; de Oliveira, R.G.; de Paula Lana, U.G.; Gomes, E.A.; de Sousa, S.M. Agronomic Practices for Optimizing the AMF Abundance and Diversity for Sustainable Food Production. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Springer Nature: Singapore, 2024; pp. 55–76. [Google Scholar]
- Zhang, M.; Liang, G.; Ren, S.; Li, L.; Li, C.; Li, Y.; Yu, X.; Yin, Y.; Liu, T.; Liu, X. Responses of soil microbial community structure, potential ecological functions, and soil physicochemical properties to different cultivation patterns in cucumber. Geoderma 2023, 429, 116237. [Google Scholar] [CrossRef]
- Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Wu, H.; Li, M.; Yan, L.; Zhang, K.; Wang, J.; Kang, X. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between plant roots, rhizosphere microorganisms, and nitrogen and its special focus on rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Miransari, M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biotechnol. 2011, 89, 917–930. [Google Scholar] [CrossRef]
- Brito, I.; Carvalho, M.; Goss, M.J. Managing the functional diversity of arbuscular mycorrhizal fungi for the sustainable intensification of crop production. Plants People Planet 2021, 3, 491–505. [Google Scholar] [CrossRef]
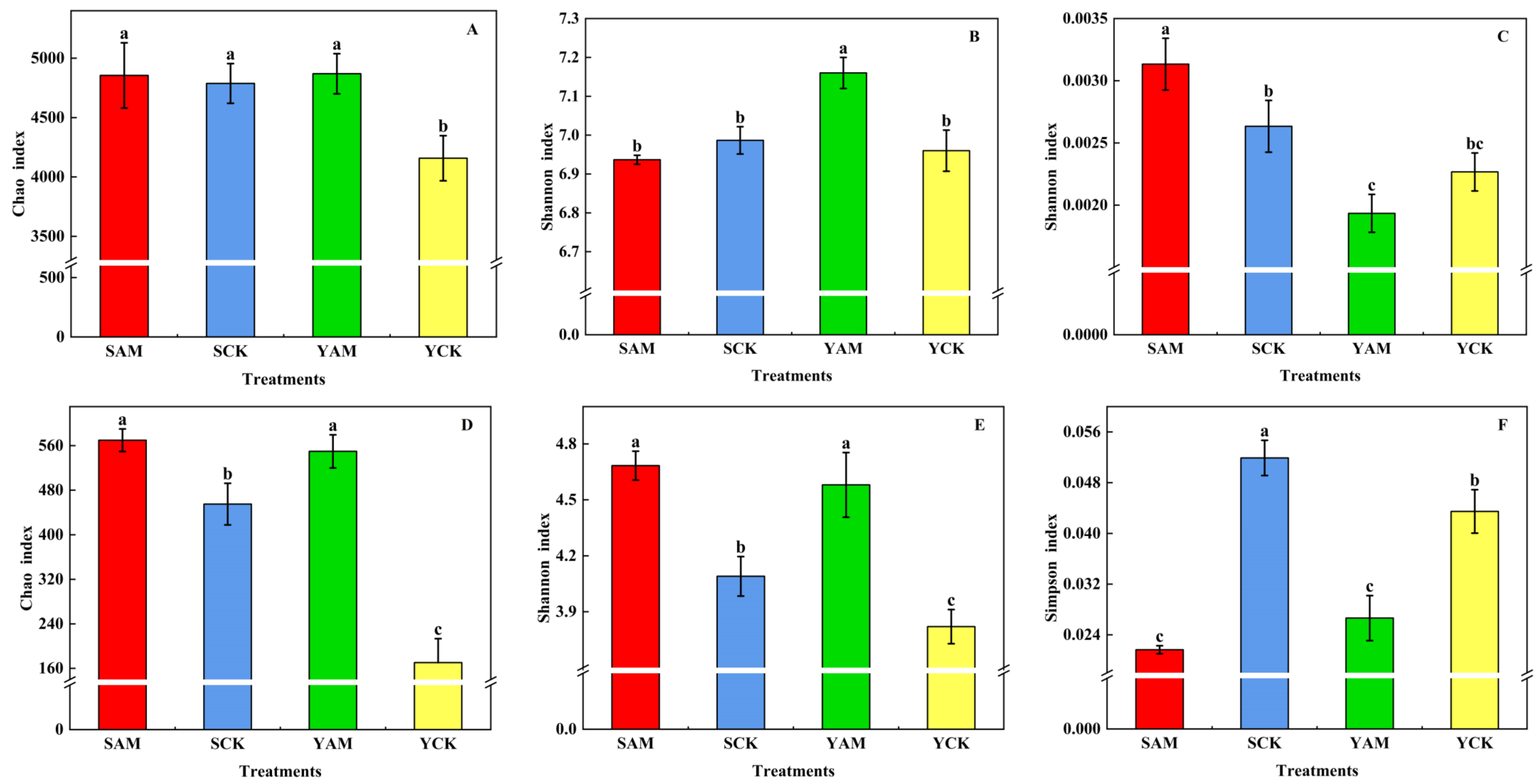
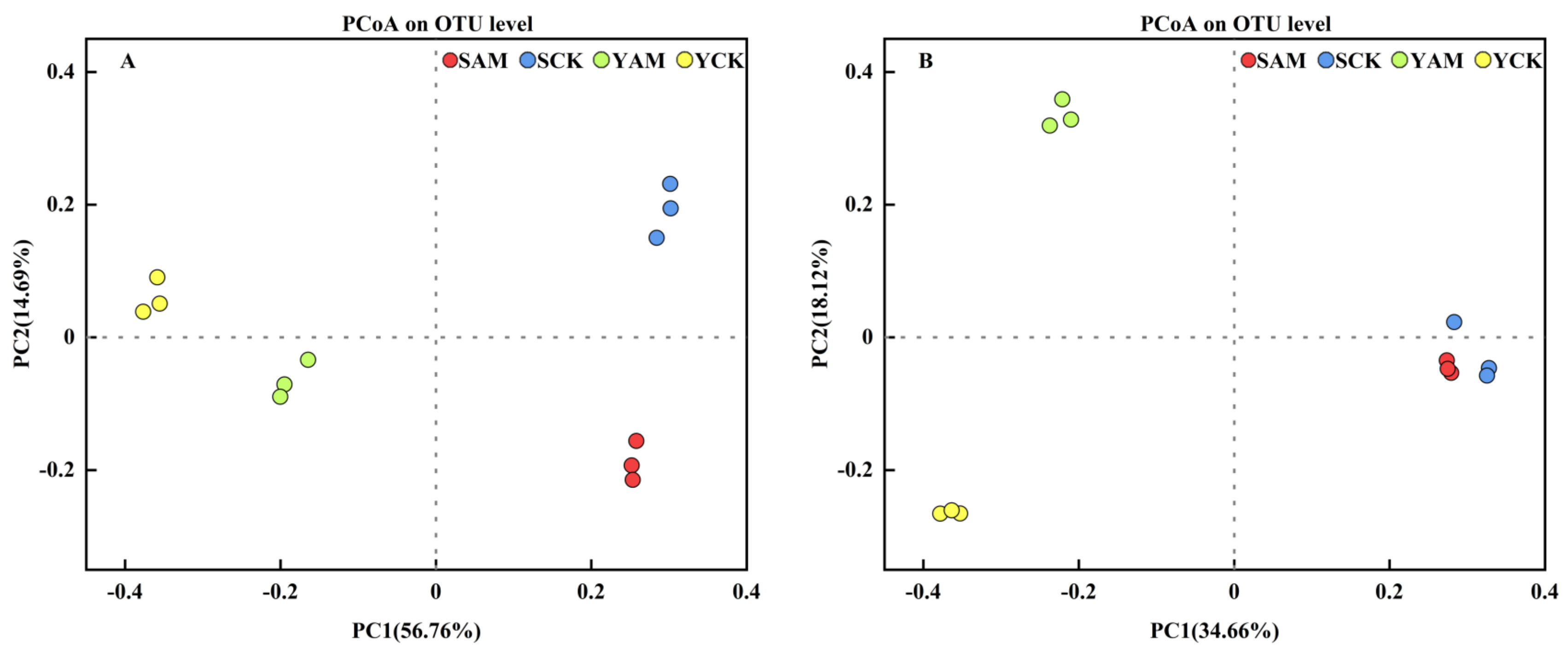
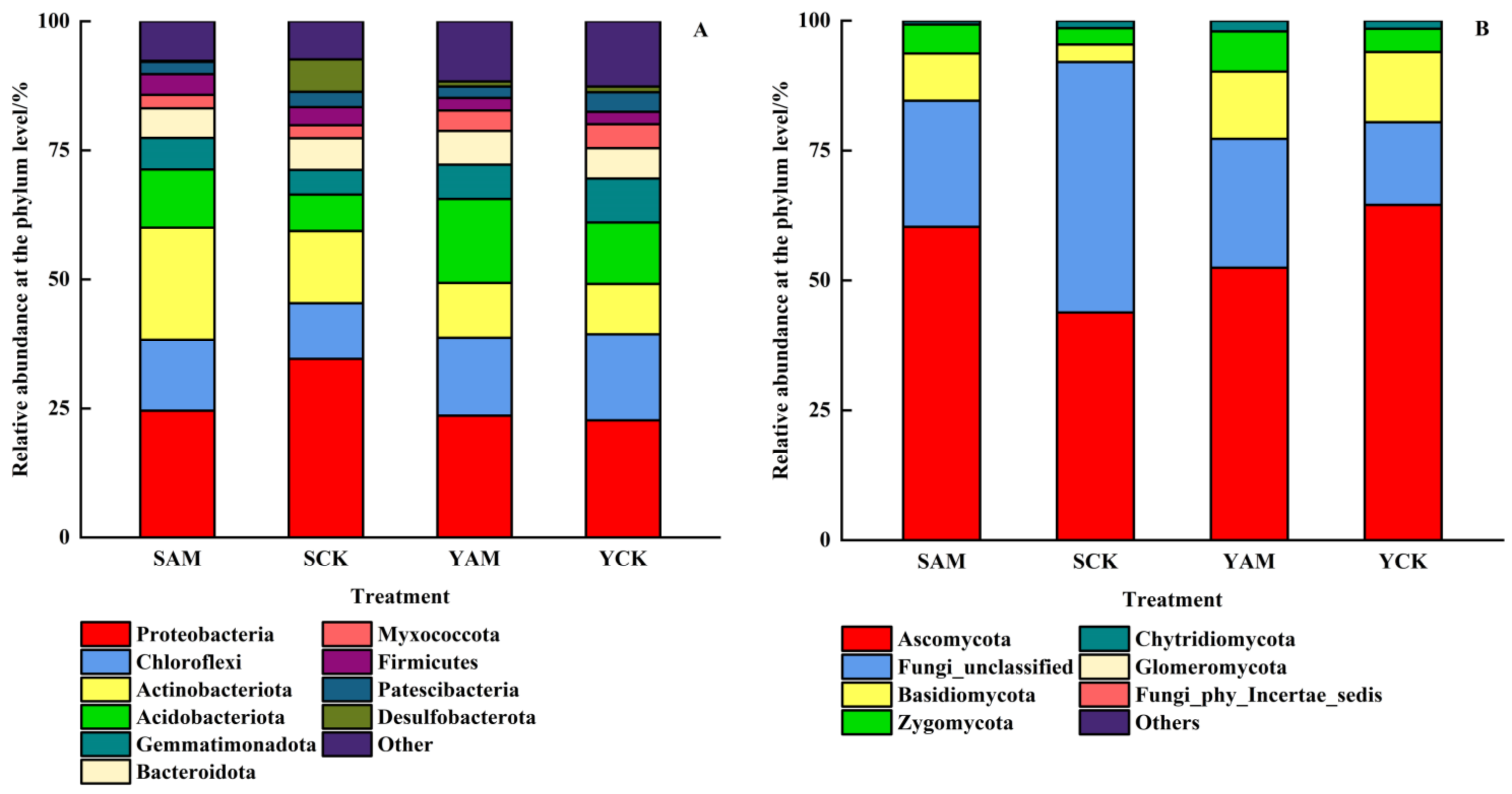
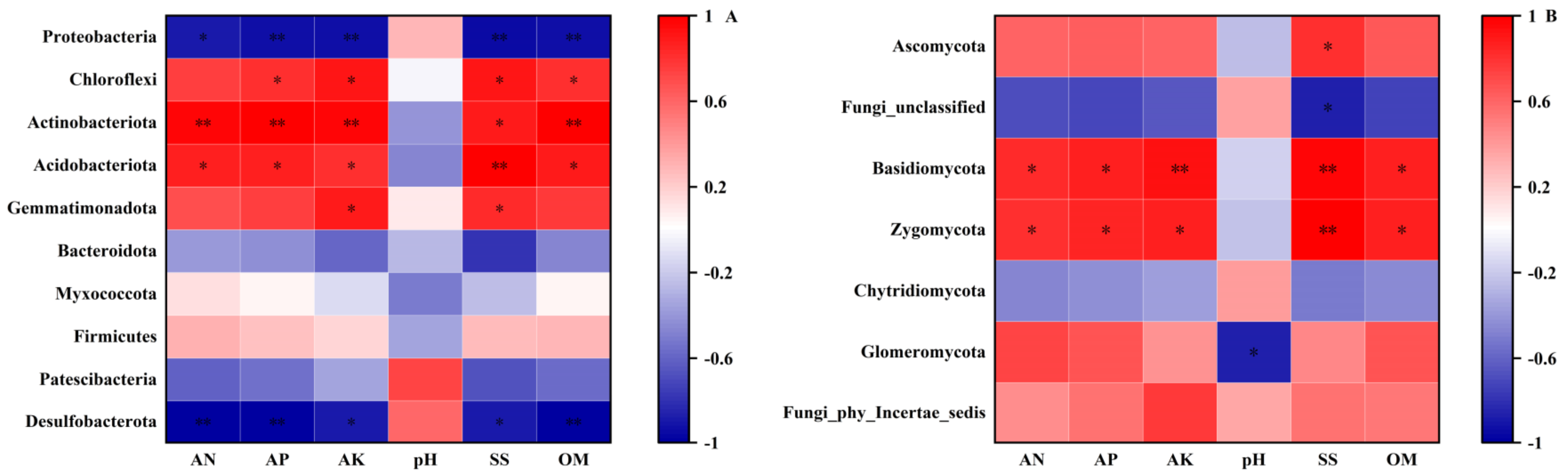
| Treatment | Total Length (cm) | Average Diameter (mm) | Surface Area (cm2) | Volume (cm3) |
|---|---|---|---|---|
| SAM | 8909.45 a | 0.76 b | 2110.03 b | 40.71 b |
| SCK | 6180.89 b | 0.87 a | 1712.03 c | 38.80 b |
| YAM | 9272.27 a | 0.80 b | 2335.28 a | 48.27 a |
| YCK | 4362.90 c | 0.80 b | 1104.59 d | 23.32 c |
| Treatment | Yield (kg/mu) | Effective Panicles Number (Number/mu) | Grain Number per Spike (Grain) | Hundred-Grain Weight (g) |
|---|---|---|---|---|
| SAM | 811.5 a | 5063 a | 534 b | 35.5 a |
| SCK | 645.2 c | 4707 b | 483 c | 33.4 ab |
| YAM | 681.4 b | 4658 b | 545 a | 32.0 bc |
| YCK | 574.4 d | 4491 c | 490 c | 30.8 c |
| Treatment | Crude Protein (mg/g) | Crude Starch (mg/g) | Crude Fat (%) |
|---|---|---|---|
| SAM | 95.16 b | 593.32 a | 4.00 ab |
| SCK | 84.89 a | 569.81 b | 3.50 c |
| YAM | 89.97 ab | 587.44 b | 4.20 a |
| YCK | 87.36 b | 567.56 ab | 3.70 bc |
| Treatment | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | pH | SS (%) | OM (%) |
|---|---|---|---|---|---|---|
| SAM | 6.48 a | 5.08 a | 100.79 b | 8.29 bc | 0.25 c | 1.60 a |
| SCK | 3.98 c | 3.52 b | 38.98 c | 8.47 ab | 0.22 d | 0.60 d |
| YAM | 5.36 ab | 4.70 a | 177.33 a | 8.23 c | 0.30 b | 1.40 b |
| YCK | 4.87 bc | 3.68 b | 105.82 b | 8.54 a | 1.39 a | 0.90 c |
| Microorganisms | Treatment | Kingdom | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|---|---|
| Bacteria | SAM | 1 | 42 | 131 | 313 | 512 | 924 | 1649 |
| SCK | 1 | 42 | 134 | 331 | 545 | 997 | 1769 | |
| YAM | 1 | 42 | 134 | 329 | 545 | 995 | 1763 | |
| YCK | 1 | 41 | 134 | 326 | 530 | 926 | 1597 | |
| Fungi | SAM | 1 | 8 | 25 | 59 | 119 | 201 | 271 |
| SCK | 1 | 7 | 25 | 56 | 106 | 183 | 244 | |
| YAM | 1 | 7 | 25 | 57 | 114 | 187 | 265 | |
| YCK | 1 | 6 | 20 | 41 | 71 | 94 | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Zhang, P.; Cao, F.; Liu, X.; Ji, M.; Xie, M. Effects of AMF on Maize Yield and Soil Microbial Community in Sandy and Saline Soils. Plants 2024, 13, 2056. https://doi.org/10.3390/plants13152056
Fan L, Zhang P, Cao F, Liu X, Ji M, Xie M. Effects of AMF on Maize Yield and Soil Microbial Community in Sandy and Saline Soils. Plants. 2024; 13(15):2056. https://doi.org/10.3390/plants13152056
Chicago/Turabian StyleFan, Li, Peng Zhang, Fuzhong Cao, Xueping Liu, Minjia Ji, and Min Xie. 2024. "Effects of AMF on Maize Yield and Soil Microbial Community in Sandy and Saline Soils" Plants 13, no. 15: 2056. https://doi.org/10.3390/plants13152056
APA StyleFan, L., Zhang, P., Cao, F., Liu, X., Ji, M., & Xie, M. (2024). Effects of AMF on Maize Yield and Soil Microbial Community in Sandy and Saline Soils. Plants, 13(15), 2056. https://doi.org/10.3390/plants13152056





