How a Long-Term Cover Crop Cultivation Impacts Soil Phosphorus Availability in a No-Tillage System?
Abstract
:1. Introduction
2. Results
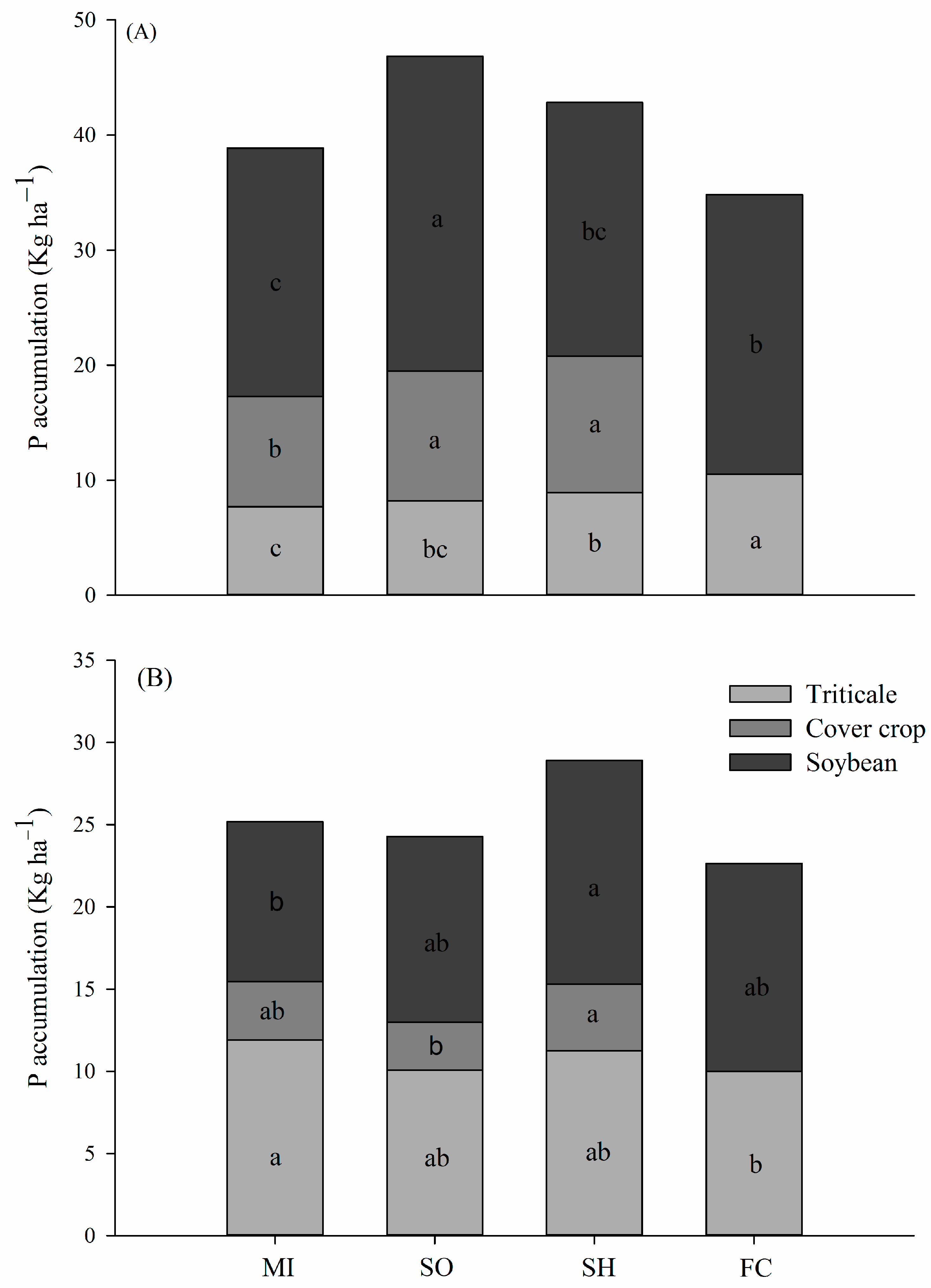
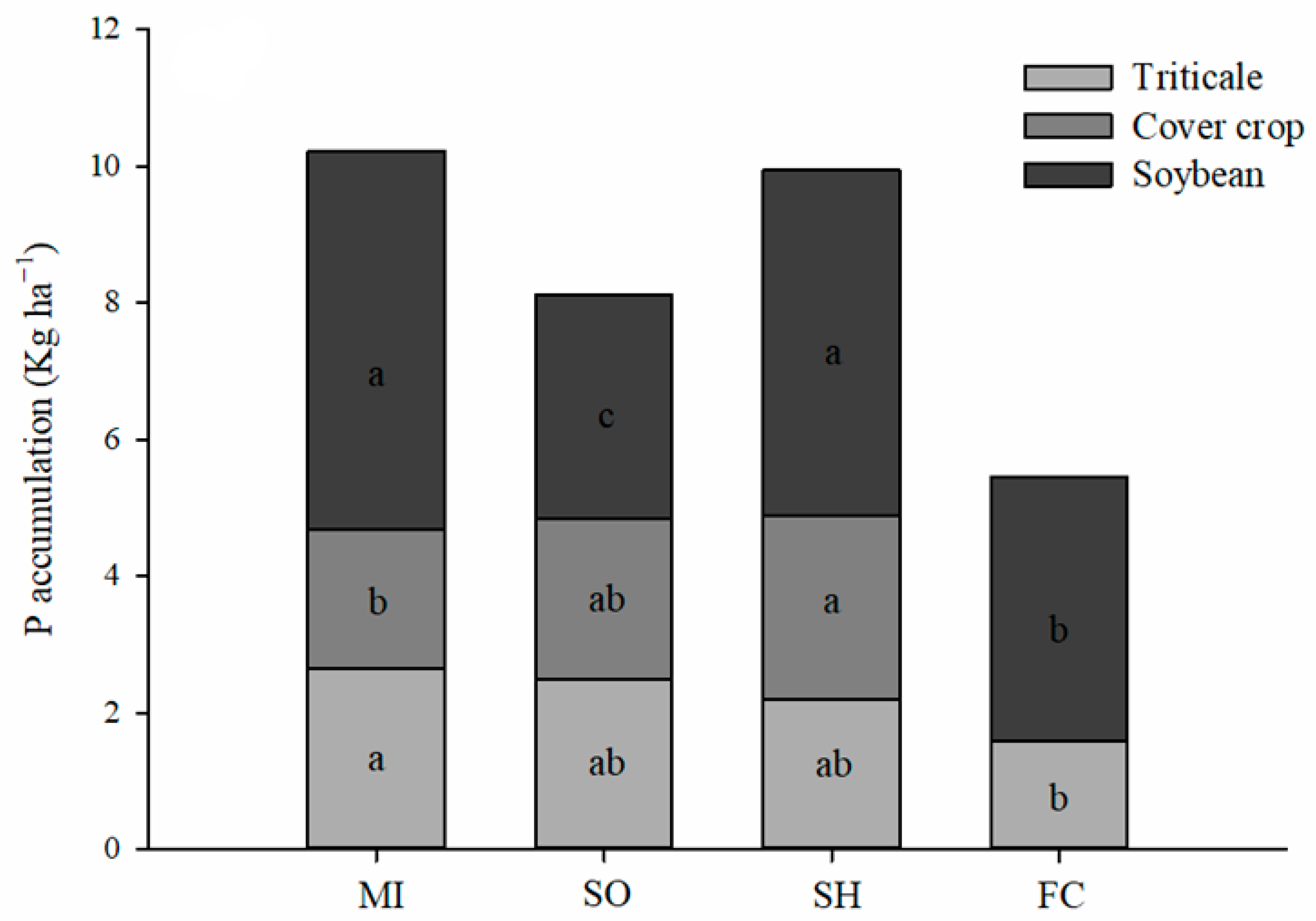
2.1. Phosphorus Accumulation in Plants
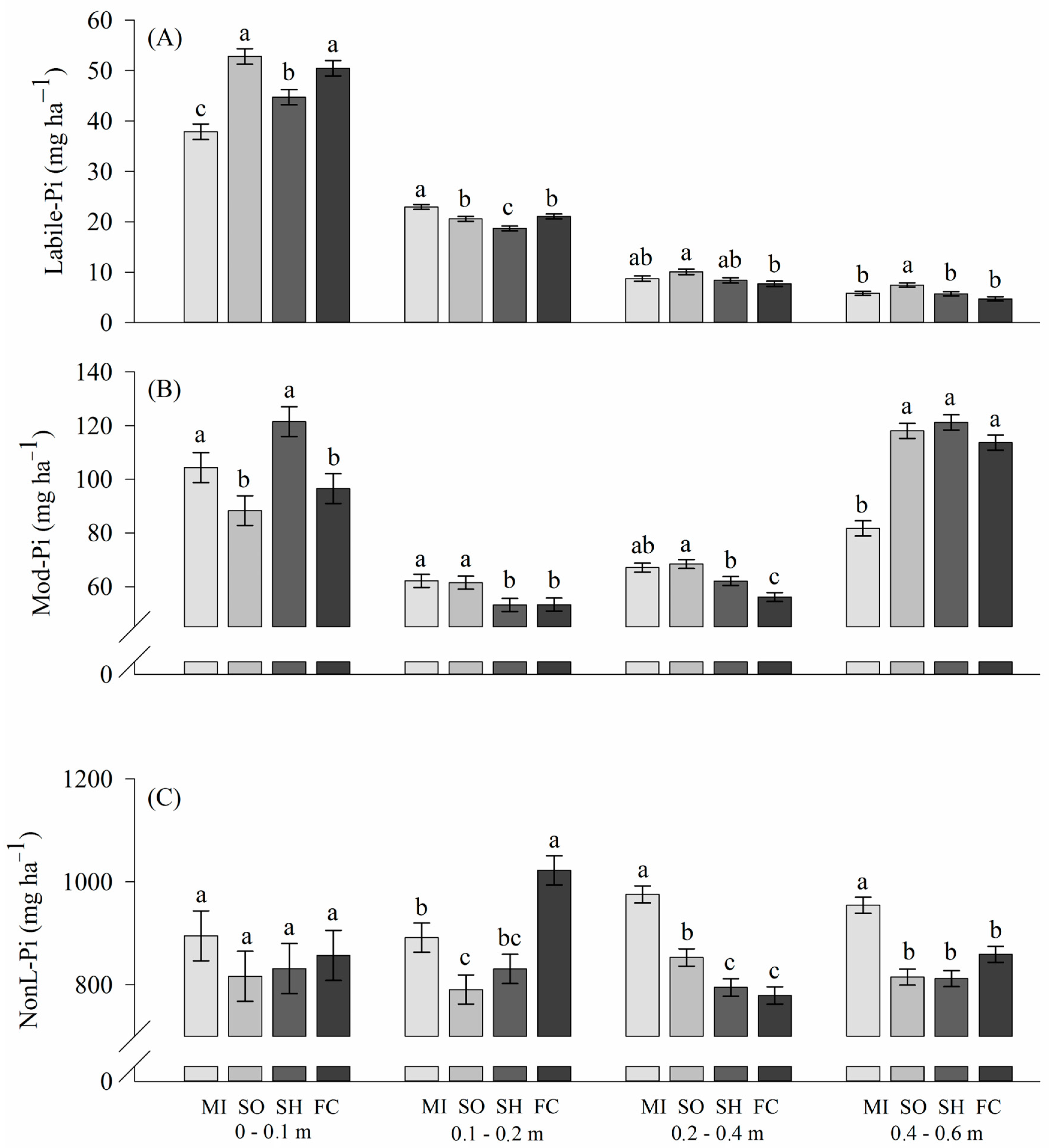
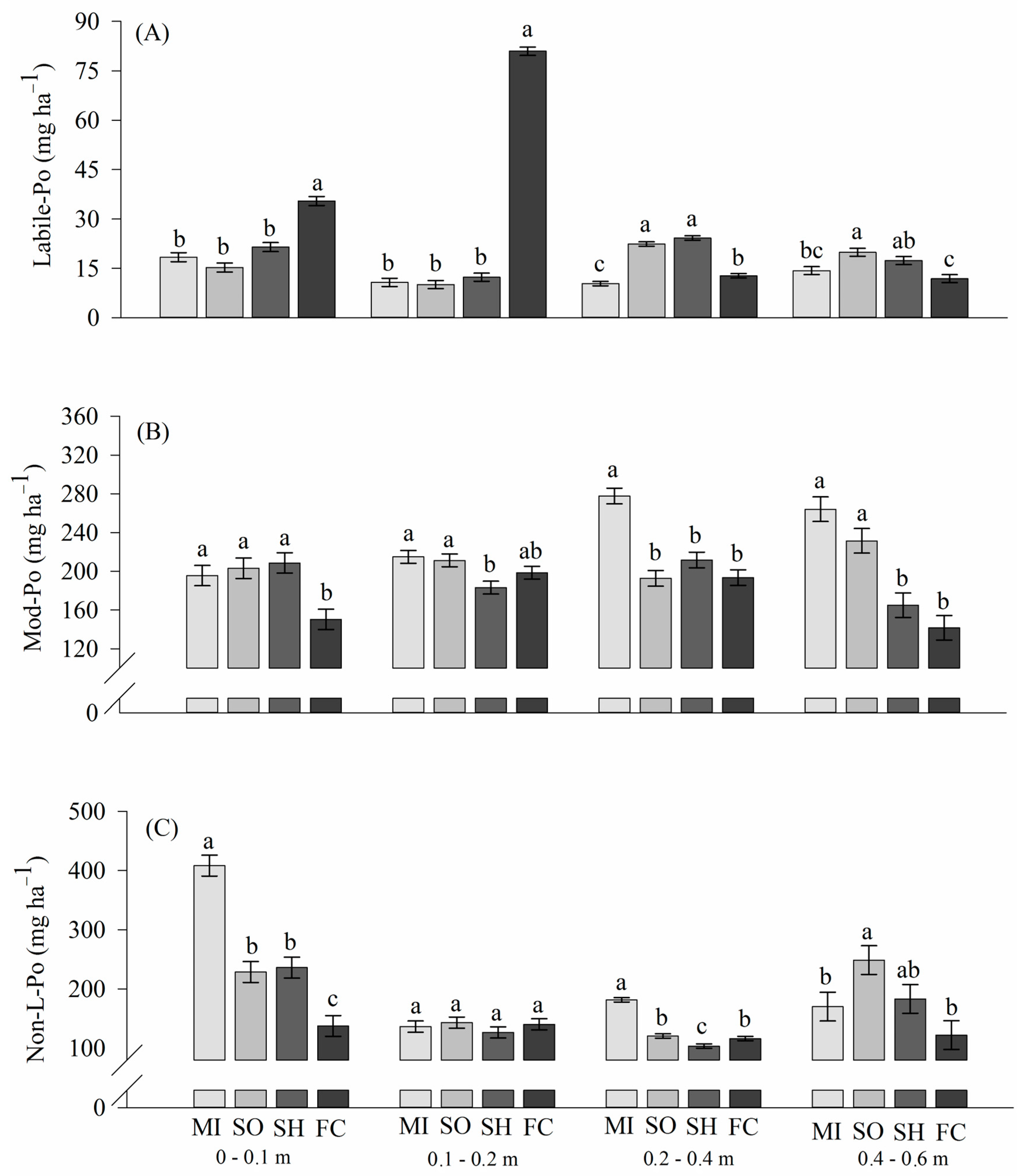
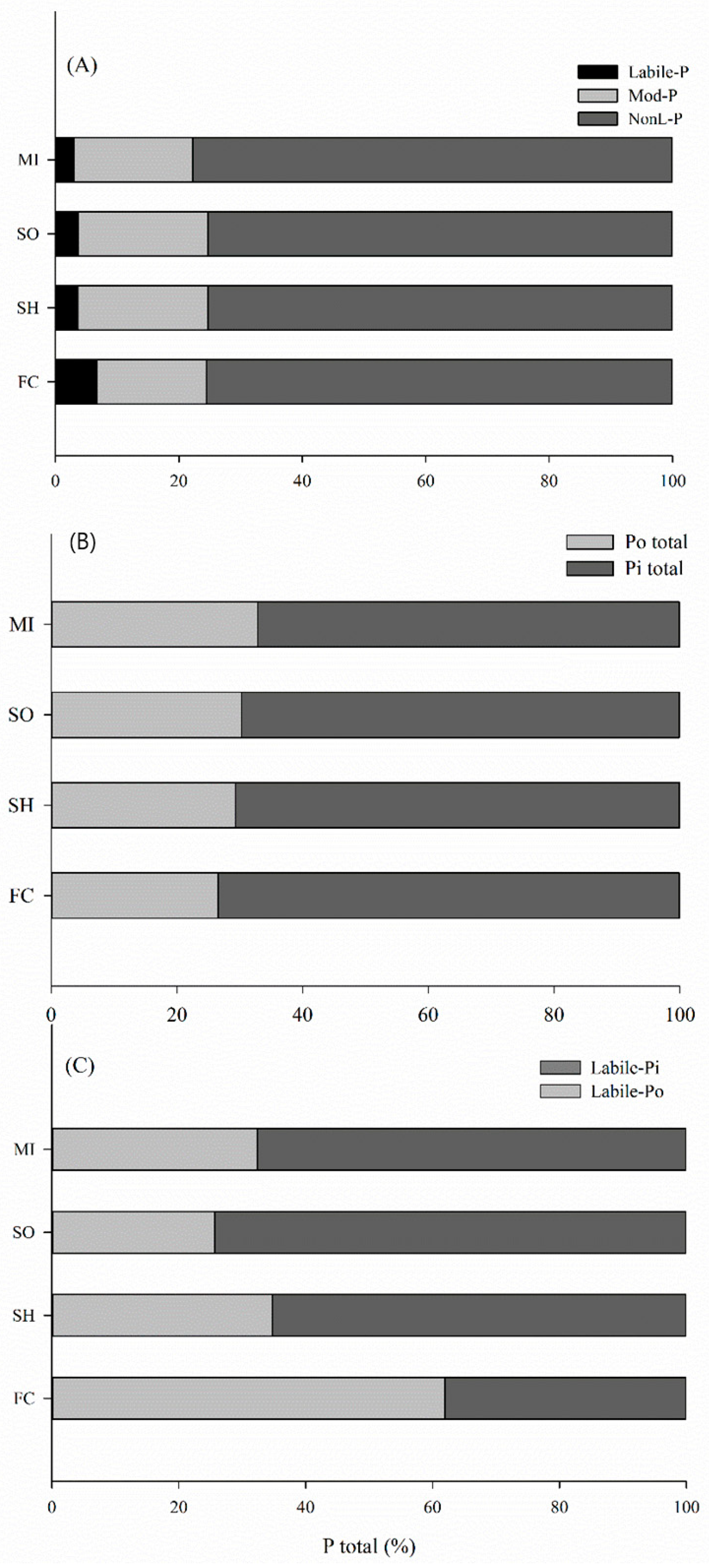
2.2. Phosphorus Lability in Soils
2.3. Distribution of Phosphorus into Soil Pools under Different Cover Crops
3. Discussion
3.1. P Accumulation by Plants
3.2. Phosphorus Lability: Inorganic Phosphorus
3.3. Phosphorus Lability: Organic Phosphorus
3.4. Distribution of P into Pools at Arable Soil Layer
4. Materials and Methods
4.1. Experimental Site
4.2. Area History and Crop Rotations
4.3. Experimental Design and Treatments
4.4. Experiment Conduction
4.5. Plant Sampling and Analyses
4.6. Soil Collections and Analyzes
Soil Phosphorus Fractionation
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alovisi, A.M.T.; Cassol, C.J.; Nascimento, J.S.; Soares, N.B.; da Silva Junior, I.R.; da Silva, R.S.; da Silva, J.A.M. Soil Factors Affecting Phosphorus Adsorption in Soils of the Cerrado, Brazil. Geoderma Reg. 2020, 22, e00298. [Google Scholar] [CrossRef]
- Rodrigues, M.; Withers, P.J.A.; Soltangheisi, A.; Vargas, V.; Holzschuh, M.; Pavinato, P.S. Tillage Systems and Cover Crops Affecting Soil Phosphorus Bioavailability in Brazilian Cerrado Oxisols. Soil. Tillage Res. 2021, 205, 104770. [Google Scholar] [CrossRef]
- Horst, W.J.; Kamh, M.; Jibrin, J.M.; Chude, V.O. Agronomic Measures for Increasing P Availability to Crops. Plant Soil. 2001, 237, 211–223. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus Activators Contribute to Legacy Phosphorus Availability in Agricultural Soils: A Review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.A.; Rodrigues, M.; Soltangheisi, A.; de Carvalho, T.S.; Guilherme, L.R.G.; Benites, V.d.M.; Gatiboni, L.C.; de Sousa, D.M.G.; Nunes, R.d.S.; Rosolem, C.A.; et al. Transitions to Sustainable Management of Phosphorus in Brazilian Agriculture. Sci. Rep. 2018, 8, 2537. [Google Scholar] [CrossRef]
- Almeida, D.S.; Rocha, K.F.; Souza, M.; Delai, L.B.; Rosolem, C.A. Soil Phosphorus Bioavailability and Soybean Grain Yield Impaired by Ruzigrass. Agron. J. 2018, 110, 654–663. [Google Scholar] [CrossRef]
- Arruda, B.; Rodrigues, M.; Gumiere, T.; Richardson, A.E.; Andreote, F.D.; Soltangheisi, A.; Gatiboni, L.C.; Pavinato, P.S. The Impact of Sugarcane Filter Cake on the Availability of P in the Rhizosphere and Associated Microbial Community Structure. Soil. Use Manag. 2019, 35, 334–345. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, J.; Xu, W.; Mou, Z. Characteristics of Inorganic Phosphate-Solubilizing Bacteria from the Sediments of a Eutrophic Lake. Int. J. Environ. Res. Public Health 2019, 16, 2141. [Google Scholar] [CrossRef] [PubMed]
- Calegari, A.; Hargrove, W.L.; Rheinheimer, D.D.S.; Ralisch, R.; Tessier, D.; Tourdonnet, S.; Fatima Guimarães, M. Impact of Long-Term No-Tillage and Cropping System Management on Soil Organic Carbon in an Oxisol: A Model for Sustainability. Agron. J. 2008, 100, 1013–1019. [Google Scholar] [CrossRef]
- da Silva, P.C.G.; Tiritan, C.S.; Echer, F.R.; Cordeiro, C.F.d.S.; Rebonatti, M.D.; dos Santos, C.H. No-Tillage and Crop Rotation Increase Crop Yields and Nitrogen Stocks in Sandy Soils under Agroclimatic Risk. Field Crops Res. 2020, 258, 107947. [Google Scholar] [CrossRef]
- Triplett, G.B.; Dick, W.A. No-Tillage Crop Production: A Revolution in Agriculture! Agron. J. 2008, 100, S-153–S-155. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy Phosphorus and No Tillage Agriculture in Tropical Oxisols of the Brazilian Savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.d.S.; de Sousa, D.M.G.; Goedert, W.J.; Vivaldi, L.J. Phosphorus Distribution in Soil as Affected by Cropping Systems and Phosphate Fertilization. Rev. Bras. Cienc. Solo 2011, 35, 877–888. [Google Scholar] [CrossRef]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden Miners—The Roles of Cover Crops and Soil Microorganisms in Phosphorus Cycling through Agroecosystems. Plant Soil. 2019, 434, 7–45. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K. Agronomy and Physiology of Tropical Cover Crops. J. Plant Nutr. 2007, 30, 1287–1339. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Nascente, A.S.; Borghi, E.; Soratto, R.P.; Martins, P.O. Improving Soil Fertility and Crop Yield in a Tropical Region with Palisadegrass Cover Crops. Agron. J. 2015, 107, 2271–2280. [Google Scholar] [CrossRef]
- Newton, P.; Civita, N.; Frankel-Goldwater, L.; Bartel, K.; Johns, C. What Is Regenerative Agriculture? A Review of Scholar and Practitioner Definitions Based on Processes and Outcomes. Front. Sustain. Food Syst. 2020, 4, 577723. [Google Scholar] [CrossRef]
- Janegitz, M.C.; Martins, A.R.H.; Rosolem, C.A. Cover Crops and Soil Phosphorus Availability. Commun. Soil. Sci. Plant Anal. 2017, 48, 1240–1246. [Google Scholar] [CrossRef]
- Da Silva, E.C.; Muraoka, T.; Franzini, V.I.; Villanueva, F.C.A.; Buzetti, S.; Moreti, D. Phosphorus Utilization by Corn as Affected by Green Manure, Nitrogen and Phosphorus Fertilizers. Pesqui. Agropecu. Bras. 2012, 47, 1150–1157. [Google Scholar] [CrossRef]
- Kuo, S.; Huang, B.; Bembenek, R. Effects of Long-Term Phosphorus Fertilization and Winter Cover Cropping on Soil Phosphorus Transformations in Less Weathered Soil. Biol. Fertil. Soils 2005, 41, 116–123. [Google Scholar] [CrossRef]
- Foltran, E.C.; Rocha, J.H.T.; Bazani, J.H.; Gonçalves, J.L.d.M.; Rodrigues, M.; Pavinato, P.; Valduga, G.R.; Erro, J.; Garcia-Mina, J.M. Phosphorus Pool Responses under Different P Inorganic Fertilizers for a Eucalyptus Plantation in a Loamy Oxisol. Ecol. Manag. 2019, 435, 170–179. [Google Scholar] [CrossRef]
- Teles, A.P.B.; Rodrigues, M.; Bejarano Herrera, W.F.; Soltangheisi, A.; Sartor, L.R.; Withers, P.J.A.; Pavinato, P.S. Do Cover Crops Change the Lability of Phosphorus in a Clayey Subtropical Soil under Different Phosphate Fertilizers? Soil. Use Manag. 2017, 33, 34–44. [Google Scholar] [CrossRef]
- Hufnagel, B.; de Sousa, S.M.; Assis, L.; Guimaraes, C.T.; Leiser, W.; Azevedo, G.C.; Negri, B.; Larson, B.G.; Shaff, J.E.; Pastina, M.M.; et al. Duplicate and Conquer: Multiple Homologs of PHOSPHORUS-STARVATION TOLERANCE1 Enhance Phosphorus Acquisition and Sorghum Performance on Low-Phosphorus Soils. Plant Physiol. 2014, 166, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Ziblim, I.A.; Paul, G.S.; Timothy, K.A. Assessing Soil Amendment Potentials of Mucuna Pruriens and Crotalaria juncea When Used as Fallow Crops. J. Soil. Sci. Environ. Manag. 2013, 4, 28–34. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary History Resolves Global Organization of Root Functional Traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, X. Bio-Tillage: A New Perspective for Sustainable Agriculture. Soil. Tillage Res. 2021, 206, 104844. [Google Scholar] [CrossRef]
- Honvault, N.; Houben, D.; Nobile, C.; Firmin, S.; Lambers, H.; Faucon, M.-P. Tradeoffs among Phosphorus-Acquisition Root Traits of Crop Species for Agroecological Intensification. Plant Soil. 2021, 461, 137–150. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for Mobilizing Recalcitrant Phosphorus from Agricultural Soils: A Review. Plant Soil. 2018, 427, 5–16. [Google Scholar] [CrossRef]
- Caniato, F.F.; Hamblin, M.T.; Guimaraes, C.T.; Zhang, Z.; Schaffert, R.E.; Kochian, L.V.; Magalhaes, J.V. Association Mapping Provides Insights into the Origin and the Fine Structure of the Sorghum Aluminum Tolerance Locus, AltSB. PLoS ONE 2014, 9, e87438. [Google Scholar] [CrossRef]
- Hu, H.Q.; He, J.Z.; Li, X.Y.; Liu, F. Effect of Several Organic Acids on Phosphate Adsorption by Variable Charge Soils of Central China. Environ. Int. 2001, 26, 353–358. [Google Scholar] [CrossRef]
- Cai, P.; Huang, Q.; Zhu, J.; Jiang, D.; Zhou, X.; Rong, X.; Liang, W. Effects of Low-Molecular-Weight Organic Ligands and Phosphate on DNA Adsorption by Soil Colloids and Minerals. Colloids Surf. B Biointerfaces 2007, 54, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Rheinheimer, D.d.S.; Fornari, M.R.; Bastos, M.C.; Fernandes, G.; Santanna, M.A.; Calegari, A.; dos Santos Canalli, L.B.; Caner, L.; Labanowski, J.; Tiecher, T. Phosphorus Distribution after Three Decades of Different Soil Management and Cover Crops in Subtropical Region. Soil. Tillage Res. 2019, 192, 33–41. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Merlin, A.; Rosolem, C.A. Organic Compounds from Plant Extracts and Their Effect on Soil Phosphorus Availability. Pesqui. Agropecu. Bras. 2008, 43, 1379–1388. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of Soil Inorganic P in the Rhizosphere as Affected by Root-Induced Chemical Changes: A Review. Plant Soil. 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Merlin, A.; Rosolem, C.A. Phosphorus Fractions in Brazilian Cerrado Soils as Affected by Tillage. Soil. Tillage Res. 2009, 105, 149–155. [Google Scholar] [CrossRef]
- Kamh, M.; Horst, W.J.; Amer, F.; Mostafa, H.; Maier, P. Mobilization of Soil and Fertilizer Phosphate by Cover Crops. Plant Soil. 1999, 211, 19–27. [Google Scholar] [CrossRef]
- Otabbong, E.; Persson, J.; Iakimenko, O.; Sadovnikova, L. The Ultuna Long-Term Soil Organic Matter Experiment. Plant Soil. 1997, 195, 17–23. [Google Scholar] [CrossRef]
- Quiquampoix, H.; Mousain, D. Enzymatic Hydrolysis of Organic Phosphorus. In Organic Phosphorus in the Environment; Turner, B.L., Frossard, E., Baldwin, D., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 89–112. [Google Scholar]
- Deiss, L.; de Moraes, A.; Dieckow, J.; Franzluebbers, A.J.; Gatiboni, L.C.; Sassaki, G.; Carvalho, P.C.F. Soil Phosphorus Compounds in Integrated Crop-Livestock Systems of Subtropical Brazil. Geoderma 2016, 274, 88–96. [Google Scholar] [CrossRef]
- Green, V.S.; Cavigelli, M.A.; Dao, T.H.; Flanagan, D.C. Soil Physical Properties and Aggregate-Associated C, N, and P Distributions in Organic and Conventional Cropping Systems. Soil. Sci. 2005, 170, 822–831. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Calonego, J.C. Phosphorus and Potassium Budget in the Soil–Plant System in Crop Rotations under No-Till. Soil. Tillage Res. 2013, 126, 127–133. [Google Scholar] [CrossRef]
- Dos Santos, H.P.; Tomm, G.O. Nutrient Availability and Organic Matter Content as Affected by Cropping Systems and Soil Management. Ciência Rural. 2003, 33, 477–486. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; Kaminski, J.; Rheinheimer, D.d.S.; Flores, J.P.C. Bioavailability of Soil Phosphorus Forms in No-Tillage System. Rev. Bras. Cienc. Solo 2007, 31, 691–699. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Foloni, J.S.S.; Tiritan, C.S. Root Growth and Nutrient Accumulation in Cover Crops as Affected by Soil Compaction. Soil. Tillage Res. 2002, 65, 109–115. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of Soil Phosphorus and Plant Adaptation Mechanisms to Phosphorus Stress for Sustainable Crop Production: A Review. J. Soil. Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Negassa, W.; Leinweber, P. How Does the Hedley Sequential Phosphorus Fractionation Reflect Impacts of Land Use and Management on Soil Phosphorus: A Review. J. Plant Nutr. Soil. Sci. 2009, 172, 305–325. [Google Scholar] [CrossRef]
- Hazra, K.K.; Swain, D.K.; Singh, S.S. The Potential of Crop Residue Recycling for Sustainable Phosphorus Management in Non-Flooded Rice-Lentil System in Alkaline Soil. Soil. Tillage Res. 2021, 213, 105147. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; McBeath, T.M.; Smernik, R.; Stacey, S.P.; Ajiboye, B.; Guppy, C. The Chemical Nature of P Accumulation in Agricultural Soils—Implications for Fertiliser Management and Design: An Australian Perspective. Plant Soil. 2011, 349, 69–87. [Google Scholar] [CrossRef]
- Nascimento, C.A.C.; Pagliari, P.H.; Faria, L.D.A.; Vitti, G.C. Phosphorus Mobility and Behavior in Soils Treated with Calcium, Ammonium, and Magnesium Phosphates. Soil. Sci. Soc. Am. J. 2018, 82, 622–631. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Management Impact and Benefit of Cover Crops on Soil Quality: A Review. Soil. Tillage Res. 2020, 204, 104717. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014; Volume 12, ISBN 0926487221. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação Do Estado Nutricional Das Plantas: Princípios e Aplicações, 2nd ed.; Potafos: Piracicaba, SP, Brazil, 1997. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil. Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Condron, L.M.; Goh, K.M. Effects of Long-Term Phosphatic Fertilizer Applications on Amounts and Forms of Phosphorus in Soils under Irrigated Pasture in New Zealand. J. Soil. Sci. 1989, 40, 383–395. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of Microbial Biomass Phosphorus in Soil. Soil. Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Dick, W.A.; Tabatabai, M.A. Determination of Orthophosphate in Aqueous Solutions Containing Labile Organic and Inorganic Phosphorus Compounds. J. Environ. Qual. 1977, 6, 82–85. [Google Scholar] [CrossRef]
- He, Z.; Honeycutt, C.W. A Modified Molybdenum Blue Method for Orthophosphate Determination Suitable for Investigating Enzymatic Hydrolysis of Organic Phosphates. Commun. Soil. Sci. Plant Anal. 2005, 36, 1373–1383. [Google Scholar] [CrossRef]
- US EPA. United States Environmental Protection Agency Method 365.3: Phosphorous, All Forms (Colorimetric, Ascorbic Acid, Two Reagent). Available online: https://www.nemi.gov/methods/method_summary/5255/ (accessed on 21 February 2023).
- do Nascimento, C.A.C.; Pagliari, P.H.; Schmitt, D.; He, Z.; Waldrip, H. Phosphorus Concentrations in Sequentially Fractionated Soil Samples as Affected by Digestion Methods. Sci. Rep. 2015, 5, 17967. [Google Scholar] [CrossRef] [PubMed]
- Dayton, E.A.; Whitacre, S.; Holloman, C. Comparison of Three Persulfate Digestion Methods for Total Phosphorus Analysis and Estimation of Suspended Sediments. Appl. Geochem. 2017, 78, 357–362. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A Literature Review and Evaluation of the. Hedley Fractionation: Applications to the Biogeochemical Cycle of Soil Phosphorus in Natural Ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
| Depth | pH | SOM | Presin | H + Al | K | Ca | Mg | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|---|---|---|
| m | CaCl2 | g dm−3 | mg dm−3 | mmolc dm−3 | g kg−1 | |||||
| 0.0–0.1 | 5.0 | 29.5 | 31.5 | 73.5 | 3.8 | 32.9 | 13.5 | 107 | 238 | 655 |
| 0.1–0.2 | 4.6 | 25.9 | 15.2 | 97.2 | 2.5 | 35.2 | 15.7 | 100 | 245 | 655 |
| 0.2–0.4 | 4.8 | 22.4 | 3.3 | 68.7 | 1.1 | 46.3 | 15.0 | 84 | 211 | 705 |
| 0.4–06 | 5.1 | 22.0 | 2.3 | 63.5 | 0.2 | 56.7 | 11.5 | 66 | 204 | 730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, H.M.F.; Calonego, J.C.; de Moraes, M.F.; Mota, L.H.d.S.d.O.; da Silva, G.F.; do Nascimento, C.A.C. How a Long-Term Cover Crop Cultivation Impacts Soil Phosphorus Availability in a No-Tillage System? Plants 2024, 13, 2057. https://doi.org/10.3390/plants13152057
Leite HMF, Calonego JC, de Moraes MF, Mota LHdSdO, da Silva GF, do Nascimento CAC. How a Long-Term Cover Crop Cultivation Impacts Soil Phosphorus Availability in a No-Tillage System? Plants. 2024; 13(15):2057. https://doi.org/10.3390/plants13152057
Chicago/Turabian StyleLeite, Hugo Mota Ferreira, Juliano Carlos Calonego, Matheus Froés de Moraes, Lydia Helena da Silva de Oliveira Mota, Gustavo Ferreira da Silva, and Carlos Antonio Costa do Nascimento. 2024. "How a Long-Term Cover Crop Cultivation Impacts Soil Phosphorus Availability in a No-Tillage System?" Plants 13, no. 15: 2057. https://doi.org/10.3390/plants13152057
APA StyleLeite, H. M. F., Calonego, J. C., de Moraes, M. F., Mota, L. H. d. S. d. O., da Silva, G. F., & do Nascimento, C. A. C. (2024). How a Long-Term Cover Crop Cultivation Impacts Soil Phosphorus Availability in a No-Tillage System? Plants, 13(15), 2057. https://doi.org/10.3390/plants13152057






