bZIP Transcription Factors: Structure, Modification, Abiotic Stress Responses and Application in Plant Improvement
Abstract
1. Introduction
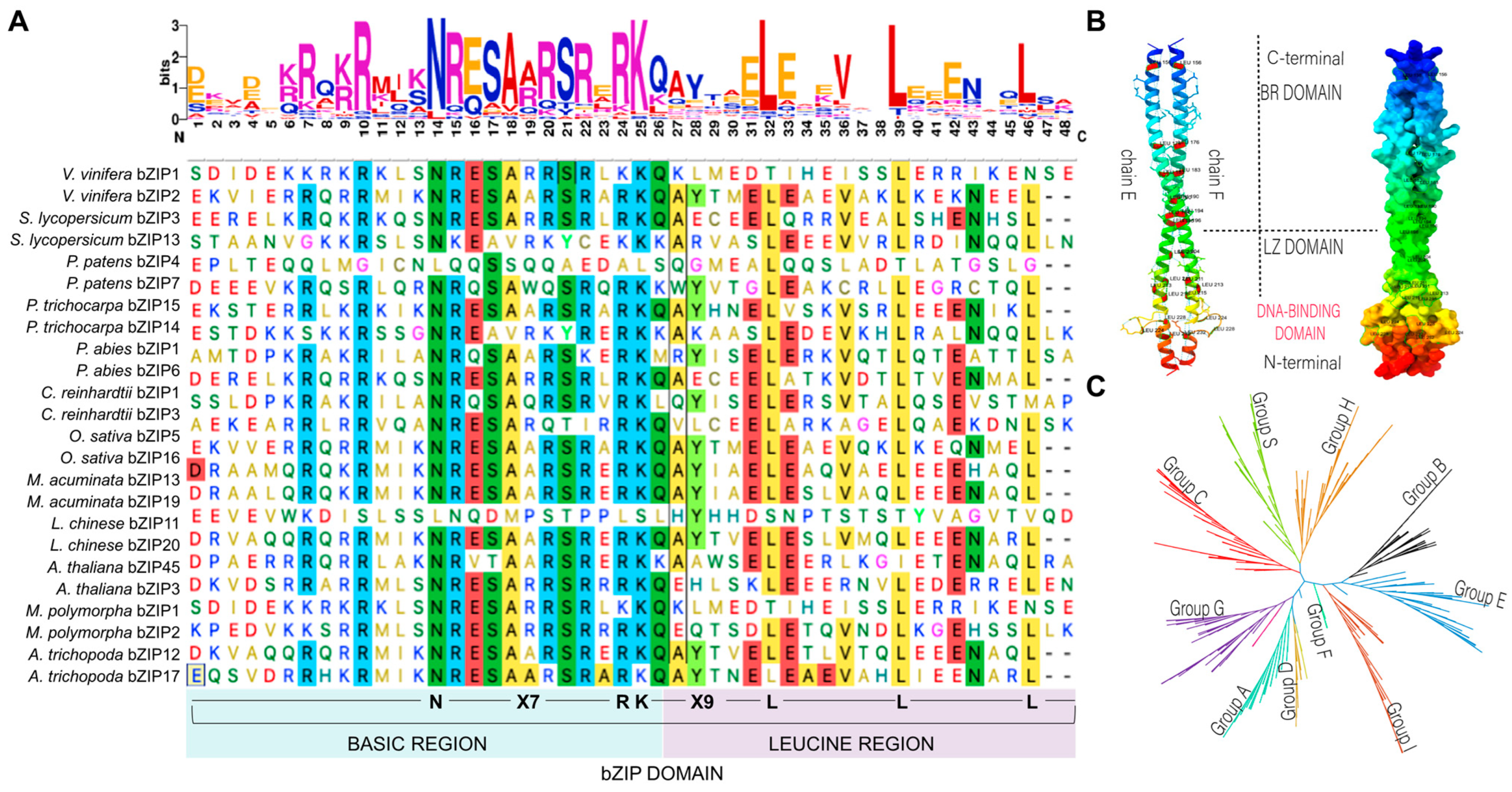
2. Structure of bZIP TFs
3. Alternative Splicing and Post-Translational Modification of bZIPs
3.1. Alternative Splicing of bZIPs
3.2. Post-Translational Modification of bZIPs
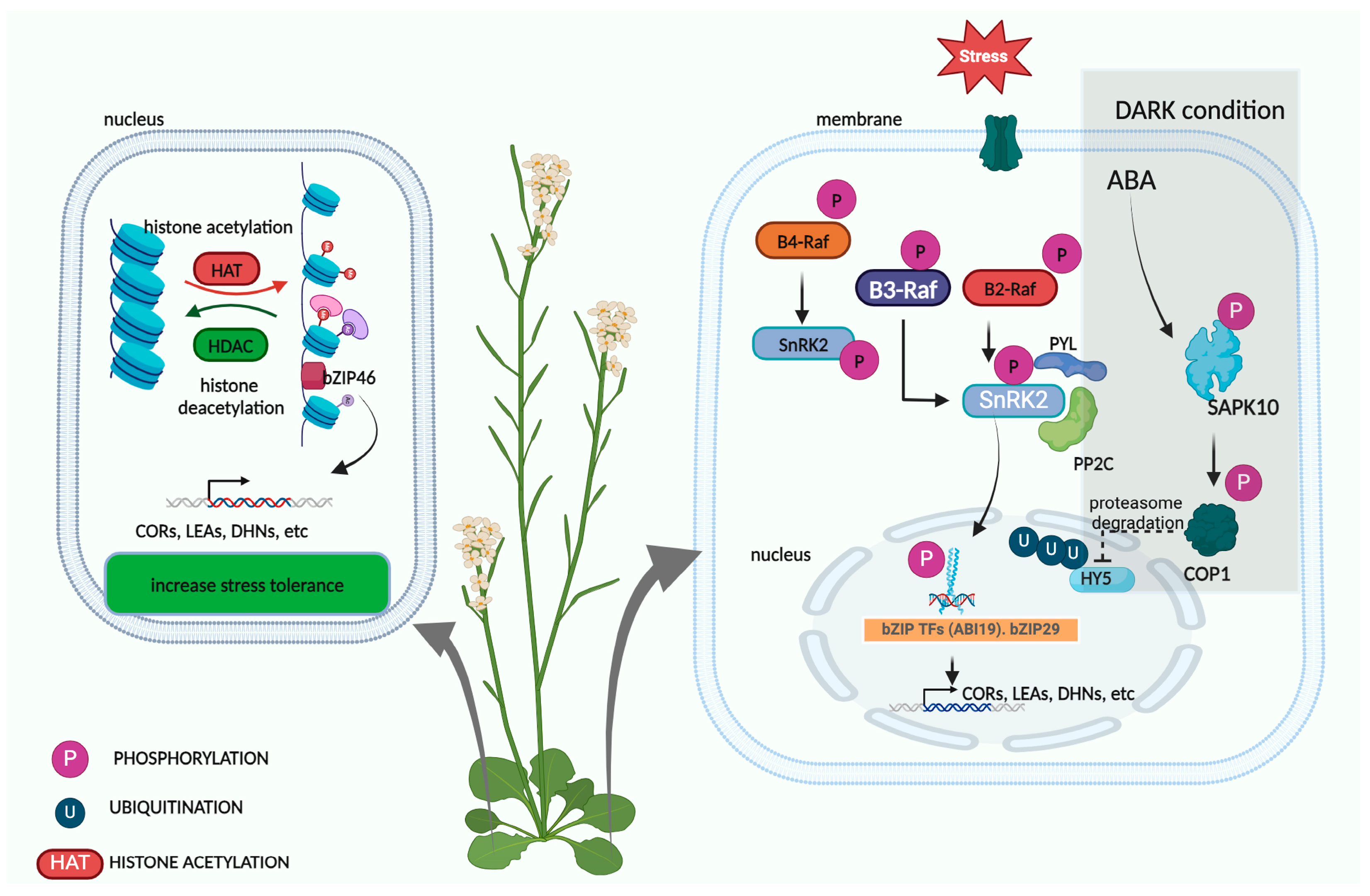
4. Regulation of Plant Response to Abiotic Stresses
4.1. Interact with Stress-Responsive Proteins
4.2. Interaction of bZIPs with Hormonal and MAPK Signaling Pathways
4.2.1. Interaction of bZIPs with Hormonal Signaling Pathways
4.2.2. Interaction of bZIPs with MAPK Signaling Pathways
5. Genetic Engineering of bZIP TFs
6. Future Perspectives, and Challenges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strader, L.; Weijers, D.; Wagner, D. Plant transcription factors—Being in the right place with the right company. Curr. Opin. Plant Biol. 2022, 65, 102136. [Google Scholar] [CrossRef]
- Gai, W.-X.; Ma, X.; Qiao, Y.-M.; Shi, B.-H.; ul Haq, S.; Li, Q.-H.; Wei, A.-M.; Liu, K.-K.; Gong, Z.-H. Characterization of the bZIP transcription factor family in pepper (Capsicum annuum L.): CabZIP25 positively modulates the salt tolerance. Front. Plant Sci. 2020, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family-an update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Lee, G.H.; Min, C.W.; Jang, J.W.; Wang, Y.; Jeon, J.-S.; Gupta, R.; Kim, S.T. Analysis of post-translational modification dynamics unveiled novel insights into Rice responses to MSP1. J. Proteom. 2023, 287, 104970. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Baek, W.; Lim, C.W.; Lee, S.C. Post-translational Modifications of bZIP Transcription Factors in Abscisic Acid Signaling and Drought Responses. Curr. Genom. 2021, 22, 4–15. [Google Scholar] [CrossRef]
- Ali, A.; Pardo, J.M.; Yun, D.-J. Desensitization of ABA-signaling: The swing from activation to degradation. Front. Plant Sci. 2020, 11, 379. [Google Scholar] [CrossRef]
- Creton, S.; Jentsch, S. SnapShot: The SUMO system. Cell 2010, 143, 848.e1. [Google Scholar] [CrossRef]
- Eom, S.H.; Lim, H.B.; Hyun, T.K. Overexpression of the Brassica rapa bZIP Transcription Factor, BrbZIP-S, Increases the Stress Tolerance in Nicotiana benthamiana. Biology 2023, 12, 517. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Cai, H.; Guo, M.; Chai, M.; She, Z.; Ye, L.; Cheng, Y.; Wang, B.; Qin, Y. The bZIP transcription factor GmbZIP15 negatively regulates salt-and drought-stress responses in soybean. Int. J. Mol. Sci. 2020, 21, 7778. [Google Scholar] [CrossRef]
- Carter, R.V. Investigating the Role of F-Group bZIP Transcription Factors in Zn Homeostasis in Barley Using CRISPR-Cas9 Gene Editing. Doctoral Dissertation, University of Southampton, Southampton, UK, 2023. [Google Scholar]
- Nguyen, N.H.; Bui, T.P.; Le, N.T.; Nguyen, C.X.; Le, M.T.T.; Dao, N.T.; Phan, Q.; Van Le, T.; To, H.M.T.; Pham, N.B. Disrupting Sc-uORFs of a transcription factor bZIP1 using CRISPR/Cas9 enhances sugar and amino acid contents in tomato (Solanum lycopersicum). Planta 2023, 257, 57. [Google Scholar] [CrossRef]
- Li, M.; Hwarari, D.; Li, Y.; Ahmad, B.; Min, T.; Zhang, W.; Wang, J.; Yang, L. The bZIP transcription factors in Liriodendron chinense: Genome-wide recognition, characteristics and cold stress response. Front. Plant Sci. 2022, 13, 1035627. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, S.; Yao, W.; Cheng, Z.; Zhou, B.; Jiang, T. Genome-wide analysis and expression profile of the bZIP gene family in poplar. BMC Plant Biol. 2021, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, Y. Chapter 18-Plant transcription factors and salt stress. In Plant Transcription Factors; Srivastava, V., Mishra, S., Mehrotra, S., Upadhyay, S.K., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 369–381. [Google Scholar]
- Liao, Y.; Zou, H.-F.; Wei, W.; Hao, Y.-J.; Tian, A.-G.; Huang, J.; Liu, Y.-F.; Zhang, J.-S.; Chen, S.-Y. Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 2008, 228, 225–240. [Google Scholar] [CrossRef]
- Yu, Y.; Qian, Y.; Jiang, M.; Xu, J.; Yang, J.; Zhang, T.; Gou, L.; Pi, E. Regulation mechanisms of plant basic leucine zippers to various abiotic stresses. Front. Plant Sci. 2020, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Baoxiang, W.; Bo, X.; Yan, L.; Jingfang, L.; Zhiguang, S.; Ming, C.; Yungao, X.; Bo, Y.; Jian, L.; Jinbo, L. A Novel mechanisms of the signaling cascade associated with the SAPK10-bZIP20-NHX1 synergistic interaction to enhance tolerance of plant to abiotic stress in rice (Oryza sativa L.). Plant Sci. 2022, 323, 111393. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ahi, E.P. The importance of alternative splicing in adaptive evolution. Mol. Ecol. 2022, 31, 1928–1938. [Google Scholar] [CrossRef]
- Mierke, C.T.; Mierke, C.T. Translation and post-translational modifications in protein biosynthesis. In Cellular Mechanics and Biophysics: Structure and Function of Basic Cellular Components Regulating Cell Mechanics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 595–665. [Google Scholar]
- Sybilska, E.; Daszkowska-Golec, A. Alternative splicing in ABA signaling during seed germination. Front. Plant Sci. 2023, 14, 1144990. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Guan, Y.; Ren, H.; Zhang, F.; Chen, F. Characterization of alternative splicing products of bZIP transcription factors OsABI5. Biochem. Biophys. Res. Commun. 2007, 360, 307–313. [Google Scholar] [CrossRef]
- Utsugi, S.; Ashikawa, I.; Nakamura, S.; Shibasaka, M. TaABI5, a wheat homolog of Arabidopsis thaliana ABA insensitive 5, controls seed germination. J. Plant Res. 2020, 133, 245–256. [Google Scholar] [CrossRef]
- Ye, L.X.; Wu, Y.M.; Zhang, J.X.; Zhang, J.X.; Zhou, H.; Zeng, R.F.; Zheng, W.X.; Qiu, M.Q.; Zhou, J.J.; Xie, Z.Z. A bZIP transcription factor (CiFD) regulates drought-and low-temperature-induced flowering by alternative splicing in citrus. J. Integr. Plant Biol. 2023, 65, 674–691. [Google Scholar] [CrossRef]
- Baoxiang, W.; Zhiguang, S.; Yan, L.; Bo, X.; Jingfang, L.; Ming, C.; Yungao, X.; Bo, Y.; Jian, L.; Jinbo, L.; et al. A pervasive phosphorylation cascade modulation of plant transcription factors in response to abiotic stress. Planta 2023, 258, 73. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Guo, L.; Wu, X.; Xiao, Q.; Wang, J.; Wang, Q.; Ma, G.; Wang, W.; Wu, Y. ABA-induced phosphorylation of basic leucine zipper 29, ABSCISIC ACID INSENSITIVE 19, and Opaque2 by SnRK2.2 enhances gene transactivation for endosperm filling in maize. Plant Cell 2022, 34, 1933–1956. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Inoue, T.; Hiraide, M.; Khatun, N.; Jahan, A.; Kuwata, K.; Katagiri, S.; Umezawa, T.; Yotsui, I.; Sakata, Y.; et al. Activation of SnRK2 by Raf-like kinase ARK represents a primary mechanism of ABA and abiotic stress responses. Plant Physiol. 2020, 185, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Hardtke, C.S.; Gohda, K.; Osterlund, M.T.; Oyama, T.; Okada, K.; Deng, X.W. HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 2000, 19, 4997–5006. [Google Scholar] [CrossRef] [PubMed]
- Morończyk, J.; Brąszewska, A.; Wójcikowska, B.; Chwiałkowska, K.; Nowak, K.; Wójcik, A.M.; Kwaśniewski, M.; Gaj, M.D. Insights into the histone acetylation-mediated regulation of the transcription factor genes that control the embryogenic transition in the somatic cells of Arabidopsis. Cells 2022, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xie, Z.; Jin, L.; Qin, T.; Zhan, C.; Huang, J. Histone deacetylase OsHDA716 represses rice chilling tolerance by deacetylating OsbZIP46 to reduce its transactivation function and protein stability. Plant Cell 2024, 36, 1913–1936. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Xu, Q.; Zhao, Y.; Zhou, D.X. Histone deacetylase HDA710 controls salt tolerance by regulating ABA signaling in rice. J. Integr. Plant Biol. 2021, 63, 451–467. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.; Shao, Z.; Chin, K.; Chakravarty, D.; Qiao, H. ENAP1 retrains seed germination via H3K9 acetylation mediated positive feedback regulation of ABI5. PLoS Genet. 2021, 17, e1009955. [Google Scholar] [CrossRef]
- Burko, Y.; Seluzicki, A.; Zander, M.; Pedmale, U.V.; Ecker, J.R.; Chory, J. Chimeric activators and repressors define HY5 activity and reveal a light-regulated feedback mechanism. Plant Cell 2020, 32, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Catalá, R.; Medina, J.; Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16475–16480. [Google Scholar] [CrossRef] [PubMed]
- Perea-Resa, C.; Rodríguez-Milla, M.A.; Iniesto, E.; Rubio, V.; Salinas, J. Prefoldins negatively regulate cold acclimation in Arabidopsis thaliana by promoting nuclear proteasome-mediated HY5 degradation. Mol. Plant 2017, 10, 791–804. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2–COP1–HY5–BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, X.; Liu, Q.; Ahammed, G.J.; Lin, R.; Wang, L.; Shao, S.; Yu, J.; Zhou, Y. The HY5 and MYB15 transcription factors positively regulate cold tolerance in tomato via the CBF pathway. Plant Cell Environ. 2020, 43, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant dehydrins: Expression, regulatory networks, and protective roles in plants challenged by abiotic stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Ma, Y.; Wang, F.; Wang, J.; Zhang, Y.; Hu, X. The bZIP transcription factor SlAREB1 regulates anthocyanin biosynthesis in response to low temperature in tomato. Plant J. 2023, 115, 205–219. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, J.; Jiang, Y.; Bao, Y.; Chen, H.; Wang, D.; Zhang, D.; Yu, J.; Cang, J. Genome-wide identification and analysis of bZIP gene family and resistance of TaABI5 (TabZIP96) under freezing stress in wheat (Triticum aestivum). Int. J. Mol. Sci. 2022, 23, 2351. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR signaling cascade and its regulation in plants responding to cold stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 234140. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Wang, X. bZIP transcription factor OsbZIP52/RISBZ5: A potential negative regulator of cold and drought stress response in rice. Planta 2012, 235, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Cheng, H.; Chiang, V.L.; Li, W.; Yang, C.; Wang, C. PtrbZIP3 transcription factor regulates drought tolerance of Populus trichocarpa. Environ. Exp. Bot. 2023, 208, 105231. [Google Scholar] [CrossRef]
- Su, H.; Cao, Y.; Ku, L.; Yao, W.; Cao, Y.; Ren, Z.; Dou, D.; Wang, H.; Ren, Z.; Liu, H.; et al. Dual functions of ZmNF-YA3 in photoperiod-dependent flowering and abiotic stress responses in maize. J. Exp. Bot. 2018, 69, 5177–5189. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, Z.; Wang, X.; Zhao, C.; Cheng, D.; Du, C.; Wang, H.; Gao, Y.; Zhang, R.; Han, J. A novel maize F-bZIP member, ZmbZIP76, functions as a positive regulator in ABA-mediated abiotic stress tolerance by binding to ACGT-containing elements. Plant Sci. 2024, 341, 111952. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, K.; Chen, S.; Li, T.; Xia, H.; Chen, L.; Liu, H.; Luo, L. A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biol. 2019, 19, 260. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Samanta, M.K.; Gayen, S.; Sen, S.K.; Maiti, M.K. Enhanced gene expression rather than natural polymorphism in coding sequence of the OsbZIP23 determines drought tolerance and yield improvement in rice genotypes. PLoS ONE 2016, 11, e0150763. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Lee, Y.H.; Song, S.I. OsbZIP42 is a positive regulator of ABA signaling and confers drought tolerance to rice. Planta 2019, 249, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Fan, R.; Huang, Y.; Jiang, X.; Wai, M.H.; Yang, Q.; Su, H.; Liu, K.; Ma, S.; Chen, Z. GmbZIP152, a soybean bZIP transcription factor, confers multiple biotic and abiotic stress responses in plant. Int. J. Mol. Sci. 2022, 23, 10935. [Google Scholar] [CrossRef]
- He, Q.; Cai, H.; Bai, M.; Zhang, M.; Chen, F.; Huang, Y.; Priyadarshani, S.V.G.N.; Chai, M.; Liu, L.; Liu, Y.; et al. A Soybean bZIP Transcription Factor GmbZIP19 Confers Multiple Biotic and Abiotic Stress Responses in Plant. Int. J. Mol. Sci. 2020, 21, 4701. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, T.-F.; Ma, J.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Wei, W.-L.; Xu, Z.-S. The soybean bZIP transcription factor gene GmbZIP2 confers drought and salt resistances in transgenic plants. Int. J. Mol. Sci. 2020, 21, 670. [Google Scholar] [CrossRef]
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.-U.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Huque, A.M.; Jung, K.W.; Kim, Y.Y.; Shin, J.S. A stress-responsive cam-binding transcription factor, BZIP4, confers abiotic stress resistance in Arabidopsis. J. Plant Biol. 2021, 64, 359–370. [Google Scholar] [CrossRef]
- Bi, C.; Yu, Y.; Dong, C.; Yang, Y.; Zhai, Y.; Du, F.; Xia, C.; Ni, Z.; Kong, X.; Zhang, L. The bZIP transcription factor TabZIP15 improves salt stress tolerance in wheat. Plant Biotechnol. J. 2021, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann-Aoyagi, M.B.; de Fátima Ruas, C.; Barbosa, E.G.G.; Braga, P.; Moraes, L.A.C.; de Oliveira, A.C.B.; Kanamori, N.; Yamaguchi-Shinozaki, K.; Nakashima, K.; Nepomuceno, A.L. Constitutive expression of Arabidopsis bZIP transcription factor AREB1 activates cross-signaling responses in soybean under drought and flooding stresses. J. Plant Physiol. 2021, 257, 153338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Long, Y.; Si, W.; Cheng, B.; Jiang, H. A novel heat shock transcription factor (ZmHsf08) negatively regulates salt and drought stress responses in maize. Int. J. Mol. Sci. 2021, 22, 11922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, L.; Wang, L.; Liu, X.; Yu, Z.; Liu, J.; Wu, W.; Ding, L.; Xia, C.; Zhang, L. TabZIP60 is involved in the regulation of ABA synthesis-mediated salt tolerance through interacting with TaCDPK30 in wheat (Triticum aestivum L.). Planta 2023, 257, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, J.; Wang, L.; Si, L.; Zheng, S.; Yang, Y.; Yang, H.; Tian, S. Wheat TabZIP8, 9, 13 participate in ABA biosynthesis in NaCl-stressed roots regulated by TaCDPK9-1. Plant Physiol. Biochem. 2020, 151, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- González Ortega-Villaizán, A.; King, E.; Patel, M.K.; Pollmann, S. Plant Hormone Crosstalk Under Abiotic Stress Conditions; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–28. [Google Scholar]
- Liu, W.; Zhang, L.; Ma, L.; Yuan, H.; Wang, A. The HY5 transcription factor negatively regulates ethylene production by inhibiting ACS1 expression under blue light conditions in pear. Hortic. Plant J. 2023, 9, 920–930. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Q.; Li, B.; Si, Y.; Wang, Y.; Sun, J.; Yuan, H.; Wang, A. LED white light-activated transcription factor MdHY5 inhibits ethylene biosynthesis during apple fruit ripening. Postharvest Biol. Technol. 2023, 202, 112372. [Google Scholar] [CrossRef]
- Wu, C.; Su, X.; Shan, W.; Chen, Y.; Yang, Y.; Wei, W.; Chen, J.; Lu, W.; Kuang, J. Banana MaWRKY49 and MaWRKY111 cooperate with MabZIP21 to activate the transcription of MaACS1 and MaACO1 during fruit ripening. Postharvest Biol. Technol. 2022, 194, 112087. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Meng, J.; Niu, L.; Pan, L.; Lu, Z.; Cui, G.; Wang, Z.; Zeng, W. Transcriptomic and Metabolic Analyses Reveal the Mechanism of Ethylene Production in Stony Hard Peach Fruit during Cold Storage. Int. J. Mol. Sci. 2021, 22, 11308. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Deng, L.; Meng, J.; Wang, Y.; Pan, L.; Niu, L.; Lu, Z.; Cui, G.; Zeng, W.; Wang, Z. Characterization and expression analysis of basic leucine zipper (bZIP) transcription factors responsive to chilling injury in peach fruit. Mol. Biol. Rep. 2023, 50, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.S.; Ali, M.; Ali Zafar, S.; Hussain, M.; Pasha, A.; Saqib Naveed, M.; Ahmad, M.; Waseem, M. Abscisic acid mediated abiotic stress tolerance in plants. Asian J. Res. Crop Sci. 2022, 7, 1–17. [Google Scholar] [CrossRef]
- Manzoor, M.A.; Manzoor, M.M.; Li, G.; Abdullah, M.; Han, W.; Wenlong, H.; Shakoor, A.; Riaz, M.W.; Rehman, S.; Cai, Y. Genome-wide identification and characterization of bZIP transcription factors and their expression profile under abiotic stresses in Chinese pear (Pyrus bretschneider i). BMC Plant Biol. 2021, 21, 413. [Google Scholar] [CrossRef]
- Yao, L.; Hao, X.; Cao, H.; Ding, C.; Yang, Y.; Wang, L.; Wang, X. ABA-dependent bZIP transcription factor, CsbZIP18, from Camellia sinensis negatively regulates freezing tolerance in Arabidopsis. Plant Cell Rep. 2020, 39, 553–565. [Google Scholar] [CrossRef]
- Song, H.; Fu, X.; Li, J.; Niu, T.; Shen, J.; Wang, X.; Li, Y.; Hou, Q.; Liu, A. Phylogenetic analysis and expression profiles of jasmonate ZIM-domain gene family provide insight into abiotic stress resistance in sunflower. Front. Plant Sci. 2022, 13, 1010404. [Google Scholar] [CrossRef]
- Ribeiro, B.; Erffelinck, M.-L.; Lacchini, E.; Ceulemans, E.; Colinas, M.; Williams, C.; Van Hamme, E.; De Clercq, R.; Perassolo, M.; Goossens, A. Interference between ER stress-related bZIP-type and jasmonate-inducible bHLH-type transcription factors in the regulation of triterpene saponin biosynthesis in Medicago truncatula. Front. Plant Sci. 2022, 13, 903793. [Google Scholar]
- Zong, W.; Tang, N.; Yang, J.; Peng, L.; Ma, S.; Xu, Y.; Li, G.; Xiong, L. Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought-resistance-related genes. Plant Physiol. 2016, 171, 2810–2825. [Google Scholar] [CrossRef]
- Liang, S.-M.; Chen, S.-C.; Liu, Z.-L.; Shan, W.; Chen, J.-Y.; Lu, W.-J.; Lakshmanan, P.; Kuang, J.-F. MabZIP74 interacts with MaMAPK11-3 to regulate the transcription of MaACO1/4 during banana fruit ripening. Postharvest Biol. Technol. 2020, 169, 111293. [Google Scholar] [CrossRef]
- Hwarari, D.; Radani, Y.; Ke, Y.; Chen, J.; Yang, L. CRISPR/Cas genome editing in plants: Mechanisms, applications, and overcoming bottlenecks. Funct. Integr. Genom. 2024, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Mo, Z.; Fan, Y.; Li, K.; Yang, M.; Li, D.; Ke, Y.; Zhang, Q.; Wang, F.; Fan, Y. Genome-wide identification and expression analysis of the bZIP transcription factor family genes in response to abiotic stress in Nicotiana tabacum L. BMC Genom. 2022, 23, 318. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Thakur, T.; Chirom, O.; Mandlik, R.; Deshmukh, R.; Salvi, P. Transcription factors as key molecular target to strengthen the drought stress tolerance in plants. Physiol. Plant. 2021, 172, 847–868. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, G.; Shen, L.; Yu, K.; Yang, W.; Li, X.; Sun, J.; Zhan, K.; Cui, D.; Liu, D. TubZIP28, a novel bZIP family transcription factor from Triticum urartu, and TabZIP28, its homologue from Triticum aestivum, enhance starch synthesis in wheat. New Phytol. 2020, 226, 1384–1398. [Google Scholar] [CrossRef]
- Li, Z.; Chao, J.; Li, X.; Li, G.; Song, D.; Guo, Y.; Wu, X.; Liu, G. Systematic analysis of the bZIP family in tobacco and functional characterization of NtbZIP62 involvement in salt stress. Agronomy 2021, 11, 148. [Google Scholar] [CrossRef]
- Ahmad, A.; Ashraf, S.; Munawar, N.; Jamil, A.; Ghaffar, A.; Shahbaz, M. CRISPR/Cas-mediated abiotic stress tolerance in crops. In CRISPR Crops: The Future of Food Security; Springer: Berlin/Heidelberg, Germany, 2021; pp. 177–211. [Google Scholar]
- Karlson, C.K.S.; Mohd-Noor, S.N.; Nolte, N.; Tan, B.C. CRISPR/dCas9-based systems: Mechanisms and applications in plant sciences. Plants 2021, 10, 2055. [Google Scholar] [CrossRef]
- Liu, X.; Bulley, S.M.; Varkonyi-Gasic, E.; Zhong, C.; Li, D. Kiwifruit bZIP transcription factor AcePosF21 elicits ascorbic acid biosynthesis during cold stress. Plant Physiol. 2023, 192, 982–999. [Google Scholar] [CrossRef]
- Baoxiang, W.; Yan, L.; Yifeng, W.; Jingfang, L.; Zhiguang, S.; Ming, C.; Yungao, X.; Bo, X.; Bo, Y.; Jian, L. OsbZIP72 is involved in transcriptional gene-regulation pathway of abscisic acid signal transduction by activating rice high-affinity potassium transporter OsHKT1; 1. Rice Sci. 2021, 28, 257–267. [Google Scholar] [CrossRef]

| Plant | Abiotic Stress | bZIP Gene | Target Downstream Genes/Pathway | Cite |
|---|---|---|---|---|
| Populus trichocarpa | Drought and salinity | PtrbZIP3 | ABA-dependent signaling | [45] |
| Zea mays | Drought and heat | ZmNF-YA3 | bHLH92, FAMA, and MYC4 | [46] |
| Salt and osmotic | ZmbZIP76 | ABA-dependent signaling, ZmNCE3/5, ZmCOR47, and ZmRD29A | [47] | |
| Oryza sativa | Drought and oxidative | OsbZIP62 | OsCL1, OsNAC10, OsDSM2, and OsSAPKs | [48] |
| Drought and yield performances | OsbZIP63 | OsRab16B, OsRab21, and OsLEA3-1 | [49] | |
| Drought | OsbZIP42 | OsLEA3, OsRab16 and ABA-dependent signaling. | [50] | |
| Drought | OsABF1 | OsCRR413-TM1, OsPP2Cs, and other OsbZIPs | [50] | |
| Salt and drought | OsbZIP20 | OsSAPK10, OsNXH1 | [19] | |
| Glycine max | Salt and heavy metal | GmbZIP152 | AtLOX6, AtACS, AtERF1, and AtABI2 | [51] |
| Drought stress sensitive | GmbZIP19 | AtABI5, AtACS6, AtLOX4, AtERF1, AtPR1, and AtWRKY26 | [52] | |
| Salt and drought | GmbZIP15 | GmSAHH1, GmWRKY12, and GmABF1 | [11] | |
| Salt and drought | GmbZIP2 | GmMYB48, GmWD40, GmDHN15, GmGST1, and GmLEA | [53] | |
| Arabidopsis thaliana | Drought, oxidative and nitro-oxidative | AtbZIP62 | AtPYRD, AtCAT2, and JA signaling pathway | [54] |
| Salt | AtbZIP4 | ABA-signaling AtCaM1, AtRD22, | [55] | |
| Triticum aestivum | Salt | TabZIP15 | TaENO-b | [56] |
| Capsicum annum | Salt | CabZIP25 | - | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Dzinyela, R.; Yang, L.; Hwarari, D. bZIP Transcription Factors: Structure, Modification, Abiotic Stress Responses and Application in Plant Improvement. Plants 2024, 13, 2058. https://doi.org/10.3390/plants13152058
Guo Z, Dzinyela R, Yang L, Hwarari D. bZIP Transcription Factors: Structure, Modification, Abiotic Stress Responses and Application in Plant Improvement. Plants. 2024; 13(15):2058. https://doi.org/10.3390/plants13152058
Chicago/Turabian StyleGuo, Zhonglong, Raphael Dzinyela, Liming Yang, and Delight Hwarari. 2024. "bZIP Transcription Factors: Structure, Modification, Abiotic Stress Responses and Application in Plant Improvement" Plants 13, no. 15: 2058. https://doi.org/10.3390/plants13152058
APA StyleGuo, Z., Dzinyela, R., Yang, L., & Hwarari, D. (2024). bZIP Transcription Factors: Structure, Modification, Abiotic Stress Responses and Application in Plant Improvement. Plants, 13(15), 2058. https://doi.org/10.3390/plants13152058







